Escolar Documentos
Profissional Documentos
Cultura Documentos
Callister7E - pp290 301 (The Iron Carbon System)
Enviado por
iglumacTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Callister7E - pp290 301 (The Iron Carbon System)
Enviado por
iglumacDireitos autorais:
Formatos disponíveis
1496T_c09_252-310 11/29/05 11:33 Page 290
REVISED PAGES
290 Chapter 9 / Phase Diagrams
T h e I ro n C a r b o n S ys t e m
Of all binary alloy systems, the one that is possibly the most important is that
for iron and carbon. Both steels and cast irons, primary structural materials in
every technologically advanced culture, are essentially ironcarbon alloys. This
section is devoted to a study of the phase diagram for this system and the development of several of the possible microstructures. The relationships among
heat treatment, microstructure, and mechanical properties are explored in Chapters 10 and 11.
9.18 THE IRONIRON CARBIDE (FeFe3C)
PHASE DIAGRAM
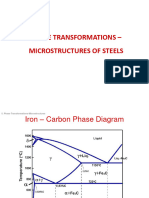
A portion of the ironcarbon phase diagram is presented in Figure 9.24. Pure iron,
upon heating, experiences two changes in crystal structure before it melts. At room
temperature the stable form, called ferrite, or a iron, has a BCC crystal structure.
Ferrite experiences a polymorphic transformation to FCC austenite, or g iron, at
9128C (16748F). This austenite persists to 13948C (25418F), at which temperature
the FCC austenite reverts back to a BCC phase known as d ferrite, which finally
ferrite
austenite
Composition (at% C)
1600
10
15
20
25
1538C
1493C
L
1400
2500
"+L
1394C
1147C
2.14
", Austenite
4.30
2000
1000
" + Fe3C
912C
800
Temperature (F)
Temperature (C)
1200
1500
+
"
727C
0.76
0.022
600
+ Fe3C
, Ferrite
Cementite (Fe3C)
400
0
(Fe)
3
4
Composition (wt% C)
1000
6.70
Figure 9.24 The ironiron carbide phase diagram. [Adapted from Binary Alloy Phase
Diagrams, 2nd edition, Vol. 1, T. B. Massalski (Editor-in-Chief), 1990. Reprinted by
permission of ASM International, Materials Park, OH.]
1496T_c09_252-310 11/29/05 11:33 Page 291
REVISED PAGES
9.18 The IronIron Carbide (FeFe3C) Phase Diagram 291
Figure 9.25
Photomicrographs of
(a) a ferrite (903)
and (b) austenite
(3253). (Copyright
1971 by United
States Steel
Corporation.)
(a)
cementite
(b)
melts at 15388C (28008F). All these changes are apparent along the left vertical axis
of the phase diagram.1
The composition axis in Figure 9.24 extends only to 6.70 wt% C; at this concentration the intermediate compound iron carbide, or cementite (Fe3C), is formed, which
is represented by a vertical line on the phase diagram. Thus, the ironcarbon system
may be divided into two parts: an iron-rich portion, as in Figure 9.24, and the other
(not shown) for compositions between 6.70 and 100 wt% C (pure graphite). In practice, all steels and cast irons have carbon contents less than 6.70 wt% C; therefore,
we consider only the ironiron carbide system. Figure 9.24 would be more appropriately labeled the FeFe3C phase diagram, since Fe3C is now considered to be a component. Convention and convenience dictate that composition still be expressed in
wt% C rather than wt% Fe3C; 6.70 wt% C corresponds to 100 wt% Fe3C.
Carbon is an interstitial impurity in iron and forms a solid solution with each
of a and d ferrites, and also with austenite, as indicated by the a, d, and g singlephase fields in Figure 9.24. In the BCC a ferrite, only small concentrations of carbon are soluble; the maximum solubility is 0.022 wt% at 7278C (13418F). The limited
solubility is explained by the shape and size of the BCC interstitial positions, which
make it difficult to accommodate the carbon atoms. Even though present in relatively low concentrations, carbon significantly influences the mechanical properties
of ferrite. This particular ironcarbon phase is relatively soft, may be made magnetic at temperatures below 7688C (14148F), and has a density of 7.88 g/cm3. Figure 9.25a is a photomicrograph of a ferrite.
The reader may wonder why no b phase is found on the FeFe3C phase diagram, Figure
9.24 (consistent with the a, b, g, etc. labeling scheme described previously). Early investigators observed that the ferromagnetic behavior of iron disappears at 7688C and attributed this
phenomenon to a phase transformation; the b label was assigned to the high-temperature
phase. Later it was discovered that this loss of magnetism did not result from a phase transformation (see Section 20.6) and, therefore, the presumed b phase did not exist.
1496T_c09_252-310 11/29/05 11:33 Page 292
REVISED PAGES
292 Chapter 9 / Phase Diagrams
The austenite, or g phase of iron, when alloyed with carbon alone, is not stable
below 7278C (13418F), as indicated in Figure 9.24. The maximum solubility of carbon
in austenite, 2.14 wt%, occurs at 11478C (20978F). This solubility is approximately
100 times greater than the maximum for BCC ferrite, since the FCC interstitial
positions are larger (see the results of Problem 4.5), and, therefore, the strains imposed on the surrounding iron atoms are much lower. As the discussions that follow demonstrate, phase transformations involving austenite are very important in
the heat treating of steels. In passing, it should be mentioned that austenite is nonmagnetic. Figure 9.25b shows a photomicrograph of this austenite phase.
The d ferrite is virtually the same as a ferrite, except for the range of temperatures over which each exists. Since the d ferrite is stable only at relatively high
temperatures, it is of no technological importance and is not discussed further.
Cementite (Fe3C) forms when the solubility limit of carbon in a ferrite is exceeded below 7278C (13418F) (for compositions within the a 1 Fe3C phase region).
As indicated in Figure 9.24, Fe3C will also coexist with the g phase between 727
and 11478C (1341 and 20978F). Mechanically, cementite is very hard and brittle; the
strength of some steels is greatly enhanced by its presence.
Strictly speaking, cementite is only metastable; that is, it will remain as a compound indefinitely at room temperature. However, if heated to between 650 and 7008C
(1200 and 13008F) for several years, it will gradually change or transform into a iron
and carbon, in the form of graphite, which will remain upon subsequent cooling to
room temperature. Thus, the phase diagram in Figure 9.24 is not a true equilibrium
one because cementite is not an equilibrium compound. However, inasmuch as the decomposition rate of cementite is extremely sluggish, virtually all the carbon in steel
will be as Fe3C instead of graphite, and the ironiron carbide phase diagram is, for all
practical purposes, valid.As will be seen in Section 11.2, addition of silicon to cast irons
greatly accelerates this cementite decomposition reaction to form graphite.
The two-phase regions are labeled in Figure 9.24. It may be noted that one eutectic exists for the ironiron carbide system, at 4.30 wt% C and 11478C (20978F);
for this eutectic reaction,
Eutectic reaction for
the iron-iron carbide
system
cooling
L g 1 Fe3C
heating
(9.18)
the liquid solidifies to form austenite and cementite phases. Of course, subsequent
cooling to room temperature will promote additional phase changes.
It may be noted that a eutectoid invariant point exists at a composition of 0.76 wt%
C and a temperature of 7278C (13418F).This eutectoid reaction may be represented by
Eutectoid reaction
for the iron-iron
carbide system
cooling
g10.76 wt% C2 a10.022 wt% C2 1 Fe3C 16.7 wt%C2
heating
(9.19)
or, upon cooling, the solid g phase is transformed into a iron and cementite. (Eutectoid phase transformations were addressed in Section 9.14.) The eutectoid phase
changes described by Equation 9.19 are very important, being fundamental to the
heat treatment of steels, as explained in subsequent discussions.
Ferrous alloys are those in which iron is the prime component, but carbon as
well as other alloying elements may be present. In the classification scheme of ferrous alloys based on carbon content, there are three types: iron, steel, and cast iron.
Commercially pure iron contains less than 0.008 wt% C and, from the phase diagram, is composed almost exclusively of the ferrite phase at room temperature. The
1496T_c09_252-310 11/29/05 11:33 Page 293
REVISED PAGES
9.19 Development of Microstructure in IronCarbon Alloys 293
ironcarbon alloys that contain between 0.008 and 2.14 wt% C are classified as
steels. In most steels the microstructure consists of both a and Fe3C phases. Upon
cooling to room temperature, an alloy within this composition range must pass
through at least a portion of the g-phase field; distinctive microstructures are subsequently produced, as discussed below. Although a steel alloy may contain as much
as 2.14 wt% C, in practice, carbon concentrations rarely exceed 1.0 wt%. The properties and various classifications of steels are treated in Section 11.2. Cast irons are
classified as ferrous alloys that contain between 2.14 and 6.70 wt% C. However,
commercial cast irons normally contain less than 4.5 wt% C. These alloys are discussed further also in Section 11.2.
9.19 DEVELOPMENT OF MICROSTRUCTURE IN
IRONCARBON ALLOYS
Several of the various microstructures that may be produced in steel alloys and their
relationships to the ironiron carbon phase diagram are now discussed, and it is
shown that the microstructure that develops depends on both the carbon content
and heat treatment. This discussion is confined to very slow cooling of steel alloys,
in which equilibrium is continuously maintained. A more detailed exploration of
the influence of heat treatment on microstructure, and ultimately on the mechanical properties of steels, is contained in Chapter 10.
Phase changes that occur upon passing from the g region into the a 1 Fe3C
phase field (Figure 9.24) are relatively complex and similar to those described for
the eutectic systems in Section 9.12. Consider, for example, an alloy of eutectoid
composition (0.76 wt% C) as it is cooled from a temperature within the g phase region, say, 8008Cthat is, beginning at point a in Figure 9.26 and moving down the
1100
1000
+ Fe3C
900
Temperature (C)
800
+
727C
b
700
600
Fe3C
+ Fe3C
500
x9
400
1.0
Composition (wt% C)
2.0
Figure 9.26 Schematic
representations of the
microstructures for an
ironcarbon alloy of eutectoid
composition (0.76 wt% C) above
and below the eutectoid
temperature.
1496T_c09_252-310 11/29/05 11:33 Page 294
REVISED PAGES
294 Chapter 9 / Phase Diagrams
Figure 9.27 Photomicrograph of a eutectoid
steel showing the pearlite microstructure
consisting of alternating layers of a ferrite
(the light phase) and Fe3C (thin layers most
of which appear dark). 5003. (Reproduced
with permission from Metals Handbook,
9th edition, Vol. 9, Metallography and
Microstructures, American Society for
Metals, Materials Park, OH, 1985.)
pearlite
vertical line xx. Initially, the alloy is composed entirely of the austenite phase having
a composition of 0.76 wt% C and corresponding microstructure, also indicated in
Figure 9.26. As the alloy is cooled, there will occur no changes until the eutectoid
temperature (7278C) is reached. Upon crossing this temperature to point b, the
austenite transforms according to Equation 9.19.
The microstructure for this eutectoid steel that is slowly cooled through the
eutectoid temperature consists of alternating layers or lamellae of the two phases
(a and Fe3C) that form simultaneously during the transformation. In this case, the
relative layer thickness is approximately 8 to 1. This microstructure, represented
schematically in Figure 9.26, point b, is called pearlite because it has the appearance of mother of pearl when viewed under the microscope at low magnifications.
Figure 9.27 is a photomicrograph of a eutectoid steel showing the pearlite. The
pearlite exists as grains, often termed colonies; within each colony the layers
are oriented in essentially the same direction, which varies from one colony to
another. The thick light layers are the ferrite phase, and the cementite phase appears as thin lamellae most of which appear dark. Many cementite layers are so
thin that adjacent phase boundaries are so close together that they are indistinguishable at this magnification, and, therefore, appear dark. Mechanically, pearlite
has properties intermediate between the soft, ductile ferrite and the hard, brittle
cementite.
The alternating a and Fe3C layers in pearlite form as such for the same reason that the eutectic structure (Figures 9.13 and 9.14) formsbecause the composition of the parent phase [in this case austenite (0.76 wt% C)] is different
from either of the product phases [ferrite (0.022 wt% C) and cementite (6.7 wt%
C)], and the phase transformation requires that there be a redistribution of the
carbon by diffusion. Figure 9.28 illustrates schematically microstructural changes
that accompany this eutectoid reaction; here the directions of carbon diffusion
are indicated by arrows. Carbon atoms diffuse away from the 0.022 wt% ferrite
regions and to the 6.7 wt% cementite layers, as the pearlite extends from the
grain boundary into the unreacted austenite grain. The layered pearlite forms because carbon atoms need diffuse only minimal distances with the formation of
this structure.
Furthermore, subsequent cooling of the pearlite from point b in Figure 9.26 will
produce relatively insignificant microstructural changes.
1496T_c09_252-310 12/21/05 7:56 Page 295
2nd REVISE PAGES
9.19 Development of Microstructure in IronCarbon Alloys 295
Figure 9.28 Schematic
representation of the
formation of pearlite from
austenite; direction of carbon
diffusion indicated by arrows.
Austenite grain
boundary
Ferrite ( )
Austenite
(! )
Ferrite ( )
Austenite
(! )
Ferrite ( )
Cementite
(Fe3C)
Growth direction
of pearlite
Ferrite ( )
Carbon diffusion
Hypoeutectoid Alloys
1100
!
!
!
1000
!
! + Fe3C
M
900
!
c
Temperature (C)
hypoeutectoid alloy
Microstructures for ironiron carbide alloys having other than the eutectoid composition are now explored; these are analogous to the fourth case described in
Section 9.12 and illustrated in Figure 9.16 for the eutectic system. Consider a composition C0 to the left of the eutectoid, between 0.022 and 0.76 wt% C; this is termed
a hypoeutectoid (less than eutectoid) alloy. Cooling an alloy of this composition is
represented by moving down the vertical line yy in Figure 9.29. At about 8758C,
point c, the microstructure will consist entirely of grains of the g phase, as shown
800
d
N
Te
e
O
700
Pearlite
600
Fe3C
Proeutectoid
Eutectoid
+ Fe3C
500
y9
400
0
1.0
C0
Composition (wt% C)
2.0
Figure 9.29 Schematic
representations of the
microstructures for an
ironcarbon alloy of
hypoeutectoid composition C0
(containing less than 0.76 wt%
C) as it is cooled from within
the austenite phase region to
below the eutectoid
temperature.
1496T_c09_252-310 11/29/05 11:33 Page 296
REVISED PAGES
296 Chapter 9 / Phase Diagrams
proeutectoid ferrite
Figure 9.30
Photomicrograph
of a 0.38 wt% C
steel having a
microstructure
consisting of pearlite
and proeutectoid
ferrite. 6353.
(Photomicrograph
courtesy of Republic
Steel Corporation.)
schematically in the figure. In cooling to point d, about 7758C, which is within the
a 1 g phase region, both these phases will coexist as in the schematic microstructure. Most of the small a particles will form along the original g grain boundaries.
The compositions of both a and g phases may be determined using the appropriate
tie line; these compositions correspond, respectively, to about 0.020 and 0.40 wt% C.
While cooling an alloy through the a 1 g phase region, the composition of the
ferrite phase changes with temperature along the a 2 1a 1 g2 phase boundary, line
MN, becoming slightly richer in carbon. On the other hand, the change in composition of the austenite is more dramatic, proceeding along the 1a 1 g2 2 g boundary,
line MO, as the temperature is reduced.
Cooling from point d to e, just above the eutectoid but still in the a 1 g region,
will produce an increased fraction of the a phase and a microstructure similar to
that also shown: the a particles will have grown larger. At this point, the compositions of the a and g phases are determined by constructing a tie line at the temperature Te; the a phase will contain 0.022 wt% C, while the g phase will be of the
eutectoid composition, 0.76 wt% C.
As the temperature is lowered just below the eutectoid, to point f, all the g
phase that was present at temperature Te (and having the eutectoid composition)
will transform to pearlite, according to the reaction in Equation 9.19. There will be
virtually no change in the a phase that existed at point e in crossing the eutectoid
temperatureit will normally be present as a continuous matrix phase surrounding the isolated pearlite colonies. The microstructure at point f will appear as the
corresponding schematic inset of Figure 9.29. Thus the ferrite phase will be present
both in the pearlite and also as the phase that formed while cooling through the
a 1 g phase region. The ferrite that is present in the pearlite is called eutectoid
ferrite, whereas the other, that formed above Te, is termed proeutectoid (meaning
pre- or before eutectoid) ferrite, as labeled in Figure 9.29. Figure 9.30 is a photomicrograph of a 0.38 wt% C steel; large, white regions correspond to the proeutectoid ferrite. For pearlite, the spacing between the a and Fe3C layers varies
from grain to grain; some of the pearlite appears dark because the many closespaced layers are unresolved at the magnification of the photomicrograph. The
Proeutectoid
ferrite
Pearlite
1496T_c09_252-310 11/29/05 11:33 Page 297
REVISED PAGES
9.19 Development of Microstructure in IronCarbon Alloys 297
chapter-opening photograph for this chapter is a scanning electron micrograph of
a hypoeutectoid (0.44 wt% C) steel in which may also be seen both pearlite and
proeutectoid ferrite, only at a higher magnification. Note also that two microconstituents are present in these micrographsproeutectoid ferrite and pearlite
which will appear in all hypoeutectoid ironcarbon alloys that are slowly cooled to
a temperature below the eutectoid.
The relative amounts of the proeutectoid a and pearlite may be determined in
a manner similar to that described in Section 9.12 for primary and eutectic microconstituents. We use the lever rule in conjunction with a tie line that extends from
the a 2 (a 1 Fe3C) phase boundary (0.022 wt% C) to the eutectoid composition
(0.76 wt% C), inasmuch as pearlite is the transformation product of austenite having
this composition. For example, let us consider an alloy of composition C0 in Figure 9.31. Thus, the fraction of pearlite, Wp, may be determined according to
Lever rule
expression for
computation of
pearlite mass
fraction
(composition C0,
Figure 9.31)
Wp 5
T
T1U
C0 2 0.022
C0 2 0.022
5
0.76 2 0.022
0.74
(9.20)
Furthermore, the fraction of proeutectoid a, Wa, is computed as follows:
Lever rule
expression for
computation of
proeutectoid ferrite
mass fraction
Wa 5
U
T1U
0.76 2 C0
0.76 2 C0
5
0.76 2 0.022
0.74
(9.21)
Of course, fractions of both total a (eutectoid and proeutectoid) and cementite are
determined using the lever rule and a tie line that extends across the entirety of the
a 1 Fe3C phase region, from 0.022 to 6.7 wt% C.
+ Fe3C
Temperature
Figure 9.31 A
portion of the
FeFe3C phase
diagram used in
computations for
relative amounts
of proeutectoid
and pearlite
microconstituents for
hypoeutectoid (C0)
and hypereutectoid
(C1) compositions.
X
+ Fe3C
6.70
0.022 C90
0.76
C91
Composition (wt% C)
1496T_c09_252-310 12/21/05 7:56 Page 298
2nd REVISE PAGES
298 Chapter 9 / Phase Diagrams
1100
Figure 9.32 Schematic
representations of the
microstructures for an
ironcarbon alloy of
hypereutectoid composition
C1 (containing between 0.76
and 2.14 wt% C), as it is
cooled from within the
austenite phase region to
below the eutectoid
temperature.
P
! + Fe3C
1000
!
!
!
900
Fe3C
Temperature (C)
!
!
800
+!
O
700
i
Pearlite
600
Proeutectoid
Eutectoid Fe3C
Fe3C
500
400
+ Fe3C
z'
0
1.0
2.0
C1
Composition (wt% C)
Hypereutectoid Alloys
hypereutectoid
alloy
proeutectoid
cementite
Analogous transformations and microstructures result for hypereutectoid alloys,
those containing between 0.76 and 2.14 wt% C, which are cooled from temperatures
within the g phase field. Consider an alloy of composition C1 in Figure 9.32 that,
upon cooling, moves down the line zz. At point g only the g phase will be present
with a composition of C1; the microstructure will appear as shown, having only g
grains. Upon cooling into the g 1 Fe3C phase fieldsay, to point hthe cementite
phase will begin to form along the initial g grain boundaries, similar to the a phase
in Figure 9.29, point d. This cementite is called proeutectoid cementitethat which
forms before the eutectoid reaction. Of course, the cementite composition remains
constant (6.70 wt% C) as the temperature changes. However, the composition of
the austenite phase will move along line PO toward the eutectoid. As the temperature is lowered through the eutectoid to point i, all remaining austenite of eutectoid composition is converted into pearlite; thus, the resulting microstructure consists of pearlite and proeutectoid cementite as microconstituents (Figure 9.32). In
the photomicrograph of a 1.4 wt% C steel (Figure 9.33), note that the proeutectoid
cementite appears light. Since it has much the same appearance as proeutectoid ferrite (Figure 9.30), there is some difficulty in distinguishing between hypoeutectoid
and hypereutectoid steels on the basis of microstructure.
Relative amounts of both pearlite and proeutectoid Fe3C microconstituents may
be computed for hypereutectoid steel alloys in a manner analogous to that for
hypoeutectoid materials; the appropriate tie line extends between 0.76 and 6.70
1496T_c09_252-310 11/29/05 11:33 Page 299
REVISED PAGES
9.19 Development of Microstructure in IronCarbon Alloys 299
Figure 9.33
Photomicrograph
of a 1.4 wt% C
steel having a
microstructure
consisting of a white
proeutectoid
cementite network
surrounding the
pearlite colonies.
10003. (Copyright
1971 by United
States Steel
Corporation.)
Proeutectoid
cementite
Pearlite
wt% C. Thus, for an alloy having composition C1 in Figure 9.31, fractions of pearlite
Wp and proeutectoid cementite WFe3C are determined from the following lever rule
expressions:
6.70 2 C1
6.70 2 C1
X
5
5
Wp 5
(9.22)
V1X
6.70 2 0.76
5.94
and
C1 2 0.76
C1 2 0.76
V
(9.23)
5
5
WFe3C 5
V1X
6.70 2 0.76
5.94
Concept Check 9.8
Briefly explain why a proeutectoid phase (ferrite or cementite) forms along austenite grain boundaries. Hint: Consult Section 4.6.
[The answer may be found at www.wiley.com/college/callister (Student Companion Site).]
EXAMPLE PROBLEM 9.4
Determination of Relative Amounts of Ferrite, Cementite,
and Pearlite Microconstituents
For a 99.65 wt% Fe0.35 wt% C alloy at a temperature just below the eutectoid, determine the following:
(a) The fractions of total ferrite and cementite phases
(b) The fractions of the proeutectoid ferrite and pearlite
(c) The fraction of eutectoid ferrite
1496T_c09_252-310 11/29/05 11:33 Page 300
REVISED PAGES
300 Chapter 9 / Phase Diagrams
Solution
(a) This part of the problem is solved by application of the lever rule expressions employing a tie line that extends all the way across the a 1 Fe3C
phase field. Thus, C0 is 0.35 wt% C, and
Wa 5
6.70 2 0.35
5 0.95
6.70 2 0.022
and
WFe3C 5
0.35 2 0.022
5 0.05
6.70 2 0.022
(b) The fractions of proeutectoid ferrite and pearlite are determined by using the lever rule and a tie line that extends only to the eutectoid composition (i.e., Equations 9.20 and 9.21). Or
Wp 5
0.35 2 0.022
5 0.44
0.76 2 0.022
Wa 5
0.76 2 0.35
5 0.56
0.76 2 0.022
and
(c) All ferrite is either as proeutectoid or eutectoid (in the pearlite). Therefore, the sum of these two ferrite fractions will equal the fraction of total ferrite; that is,
Wa 1 Wae 5 Wa
where Wae denotes the fraction of the total alloy that is eutectoid ferrite.Values
for Wa and Wa were determined in parts (a) and (b) as 0.95 and 0.56,
respectively. Therefore,
Wae 5 Wa 2 Wa 5 0.95 2 0.56 5 0.39
Nonequilibrium Cooling
In this discussion on the microstructural development of ironcarbon alloys it has
been assumed that, upon cooling, conditions of metastable equilibrium2 have been
continuously maintained; that is, sufficient time has been allowed at each new temperature for any necessary adjustment in phase compositions and relative amounts
as predicted from the FeFe3C phase diagram. In most situations these cooling rates
are impractically slow and really unnecessary; in fact, on many occasions nonequilibrium conditions are desirable. Two nonequilibrium effects of practical importance
are (1) the occurrence of phase changes or transformations at temperatures other
than those predicted by phase boundary lines on the phase diagram, and (2) the
2
The term metastable equilibrium is used in this discussion inasmuch as Fe3C is only a
metastable compound.
1496T_c09_252-310 11/29/05 11:33 Page 301
REVISED PAGES
9.20 The Influence of Other Alloying Elements 301
Eutectoid temperature (C)
Mo
1200
2200
Si
2000
1000
1800
Cr
1600
800
1400
Mn
Eutectoid temperature (F)
2400
Ti
Figure 9.34 The dependence of
eutectoid temperature on alloy
concentration for several alloying
elements in steel. (From Edgar C.
Bain, Functions of the Alloying
Elements in Steel, American Society
for Metals, 1939, p. 127.)
1200
600
Ni
0
10
12
1000
14
Concentration of alloying elements (wt%)
existence at room temperature of nonequilibrium phases that do not appear on the
phase diagram. Both are discussed in the next chapter.
9.20 THE INFLUENCE OF OTHER ALLOYING
ELEMENTS
Additions of other alloying elements (Cr, Ni, Ti, etc.) bring about rather dramatic
changes in the binary ironiron carbide phase diagram, Figure 9.24. The extent
of these alterations of the positions of phase boundaries and the shapes of the
phase fields depends on the particular alloying element and its concentration.
One of the important changes is the shift in position of the eutectoid with respect to temperature and to carbon concentration. These effects are illustrated
in Figures 9.34 and 9.35, which plot the eutectoid temperature and eutectoid
composition (in wt% C) as a function of concentration for several other alloying elements. Thus, other alloy additions alter not only the temperature of the
eutectoid reaction but also the relative fractions of pearlite and the proeutectoid
phase that form. Steels are normally alloyed for other reasons, howeverusually
either to improve their corrosion resistance or to render them amenable to heat
treatment (see Section 11.8).
Figure 9.35 The dependence of eutectoid
composition (wt% C) on alloy concentration
for several alloying elements in steel. (From
Edgar C. Bain, Functions of the Alloying
Elements in Steel, American Society for Metals,
1939, p. 127.)
Eutectoid composition (wt% C)
0.8
Ni
0.6
Cr
0.4
Si
Mo
0.2
Ti
Mn
2
4
6
8
10 12 14
Concentration of alloying elements (wt%)
Você também pode gostar
- Iron-IronCarbide Phase DiagramDocumento3 páginasIron-IronCarbide Phase Diagramumangmodi32Ainda não há avaliações
- Iron Carbon Equilibrium DiagramDocumento11 páginasIron Carbon Equilibrium Diagramganesh82Ainda não há avaliações
- Weldability of Metals - NPTELDocumento18 páginasWeldability of Metals - NPTELKaushal Gandhi0% (1)
- Chapter 5, THE IRON-CARBON EQUILIBRIUM DIAGRAMDocumento13 páginasChapter 5, THE IRON-CARBON EQUILIBRIUM DIAGRAMPAUL NDIRITUAinda não há avaliações
- Handout Chapter 5 Iron Carbon SystemDocumento7 páginasHandout Chapter 5 Iron Carbon SystemBikram MuduliAinda não há avaliações
- Capili Jefferson 10Documento5 páginasCapili Jefferson 10Christian Al EncarnacionAinda não há avaliações
- University of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedDocumento13 páginasUniversity of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedAris BulaongAinda não há avaliações
- Foundations of Materials Science and Engineering 5th Edition Smith Solutions ManualDocumento79 páginasFoundations of Materials Science and Engineering 5th Edition Smith Solutions Manualdextrermachete4amgqgAinda não há avaliações
- Iron Carbon Part1 PDFDocumento33 páginasIron Carbon Part1 PDFErick HoganAinda não há avaliações
- Foundations of Materials Science and Engineering 5th Edition Smith Solutions ManualDocumento39 páginasFoundations of Materials Science and Engineering 5th Edition Smith Solutions Manualcacoonnymphaea6wgyct100% (13)
- Welding Technology English (Cont.)Documento143 páginasWelding Technology English (Cont.)Long Beautéophile100% (1)
- Lron-Carbon: Iron - RonDocumento1 páginaLron-Carbon: Iron - RonSreejith NairAinda não há avaliações
- Lec 7 Fe C DiagramDocumento45 páginasLec 7 Fe C DiagramAdnan MehmoodAinda não há avaliações
- Iron Carbon Equillibrium Diagram GandhidhamDocumento22 páginasIron Carbon Equillibrium Diagram Gandhidhamcal2_uniAinda não há avaliações
- Iron Carbon DiagramDocumento10 páginasIron Carbon DiagramsivakumarAinda não há avaliações
- MSM GTU Study Material E-Notes Unit-5 23112020052908AMDocumento14 páginasMSM GTU Study Material E-Notes Unit-5 23112020052908AMVijayAinda não há avaliações
- Principles of Heat Treating of SteelsDocumento30 páginasPrinciples of Heat Treating of Steelssatish_trivediAinda não há avaliações
- The Microstructural Nature of Carbon Steels Phase DiagramDocumento4 páginasThe Microstructural Nature of Carbon Steels Phase Diagramamitkharb111195Ainda não há avaliações
- Chapter 2 - TTA - TTT - DiagramsDocumento13 páginasChapter 2 - TTA - TTT - DiagramsPrasad Mhatre100% (1)
- Iron Carbon Phase DiagramDocumento4 páginasIron Carbon Phase DiagramMizanur RahmanAinda não há avaliações
- Iron Carbon Phase Diagram TEDDocumento6 páginasIron Carbon Phase Diagram TEDAnonymous dh6DITAinda não há avaliações
- Fe-Fe3C Phase Diagram and MicrostructuresDocumento42 páginasFe-Fe3C Phase Diagram and MicrostructuresTalaat Ahmed Mohamed El-Benawy100% (2)
- MEC 414 - Iron Phase Diagram Experiment 2Documento7 páginasMEC 414 - Iron Phase Diagram Experiment 2boatcomAinda não há avaliações
- M. Tech. (FFT) Technology of Ferrous Casting Phase DiagramDocumento7 páginasM. Tech. (FFT) Technology of Ferrous Casting Phase DiagramRajulapati Sunil KumarAinda não há avaliações
- Iron Carbon Equilibrium DiagramDocumento4 páginasIron Carbon Equilibrium DiagramParameshwari PrabakarAinda não há avaliações
- Iron-Carbon Phase Diagram Explained BrieflyDocumento4 páginasIron-Carbon Phase Diagram Explained BrieflyZicoAinda não há avaliações
- The IronCarbide DiagramDocumento11 páginasThe IronCarbide DiagramshajjikhalidAinda não há avaliações
- Engineering Material-II: Iron Carbide Phase DiagramDocumento16 páginasEngineering Material-II: Iron Carbide Phase DiagramAla ZiAinda não há avaliações
- Structure and Properties of Plain Carbon SteelDocumento4 páginasStructure and Properties of Plain Carbon Steelsatish_trivediAinda não há avaliações
- Iron - Carbon SystemDocumento21 páginasIron - Carbon SystemYavana KeerthiAinda não há avaliações
- Iron Carbon Diagram (ChE Handbook)Documento21 páginasIron Carbon Diagram (ChE Handbook)Mohamed Ismail100% (1)
- Metallury of SteelsDocumento10 páginasMetallury of SteelsDalitso MwanzaAinda não há avaliações
- EMAT 10 (2k21)Documento41 páginasEMAT 10 (2k21)Kumail AbbasAinda não há avaliações
- Dokumen - Tips - Iron Iron Carbide Phase Diagram 58ac3a092bd8dDocumento16 páginasDokumen - Tips - Iron Iron Carbide Phase Diagram 58ac3a092bd8dAfrizal Adithya PAinda não há avaliações
- Phase Diagram - : Dr. Aneela Wakeel 02-01-2018Documento42 páginasPhase Diagram - : Dr. Aneela Wakeel 02-01-2018Hassan KhanAinda não há avaliações
- Introduction To Fe-C Equilibrium Phase Diagram: Chia-Chang ShihDocumento42 páginasIntroduction To Fe-C Equilibrium Phase Diagram: Chia-Chang ShihTuấnPhạmAinda não há avaliações
- Lesson 5 - Fe-C Diagram - Rev. 0Documento11 páginasLesson 5 - Fe-C Diagram - Rev. 0Arga SetyaAinda não há avaliações
- Phase Transformation in Metals: Dr. Aneela WakeelDocumento29 páginasPhase Transformation in Metals: Dr. Aneela WakeelmazharAinda não há avaliações
- Composition of Plain Carbon Steel Carbon Steel or Plain-Carbon Steel, Is A Metal Alloy. It Is A Combination ofDocumento14 páginasComposition of Plain Carbon Steel Carbon Steel or Plain-Carbon Steel, Is A Metal Alloy. It Is A Combination ofkayodeAinda não há avaliações
- The Effects of Alloying Elements On SteelsDocumento36 páginasThe Effects of Alloying Elements On SteelsRahul PandeyAinda não há avaliações
- Phase Diagrams:: The Iron-Iron Carbide (Fe-Fe3C) Diagram or Iron-Carbon (Fe-C) Equilibrium DiagramDocumento46 páginasPhase Diagrams:: The Iron-Iron Carbide (Fe-Fe3C) Diagram or Iron-Carbon (Fe-C) Equilibrium DiagramUsman FarooqAinda não há avaliações
- Fe-C Equilibrium Diagram PhasesDocumento2 páginasFe-C Equilibrium Diagram Phasesروشان فاطمة روشانAinda não há avaliações
- Iron-Carbon DiagramDocumento3 páginasIron-Carbon DiagramnaniAinda não há avaliações
- 05 - MetE 414-Phase Transformations-Microstructures of Steels-Fall 2023Documento70 páginas05 - MetE 414-Phase Transformations-Microstructures of Steels-Fall 2023egesenturk2000Ainda não há avaliações
- Iron Carbon Phase DiagramDocumento7 páginasIron Carbon Phase Diagrampratap biswasAinda não há avaliações
- Fe-C Phase Diagram ExplainedDocumento5 páginasFe-C Phase Diagram Explainedحسين كاظم ياسينAinda não há avaliações
- Iron-Carbide DiagramDocumento6 páginasIron-Carbide DiagramAbhijit GhanwatAinda não há avaliações
- Iron Carbon Note 1 2023Documento23 páginasIron Carbon Note 1 2023gerrard samuelAinda não há avaliações
- TTT Curves 1Documento101 páginasTTT Curves 1ibrahimAinda não há avaliações
- Cast Steel: The Iron-Carbon Equilibrium Diagram: AbstractDocumento5 páginasCast Steel: The Iron-Carbon Equilibrium Diagram: Abstractchacha4500Ainda não há avaliações
- 05 Heat Treatments To Produce Ferrite and PerliteDocumento23 páginas05 Heat Treatments To Produce Ferrite and PerliteRicardo Fidel Duarte SánchezAinda não há avaliações
- Iron Carbon Phase DiagramDocumento4 páginasIron Carbon Phase DiagramFuhad HasanAinda não há avaliações
- Ch-27.3 Iron Carbon Equilibrium DiagramDocumento58 páginasCh-27.3 Iron Carbon Equilibrium DiagramasjfgauojfgfAinda não há avaliações
- Review MetalDocumento38 páginasReview MetalnisannisaAinda não há avaliações
- 3 Iron Carbon DiaDocumento21 páginas3 Iron Carbon DiaChhavi SharmaAinda não há avaliações
- Constr Materials B PDFDocumento72 páginasConstr Materials B PDFAgniva DuttaAinda não há avaliações
- Iron-Iron Carbide DiagramDocumento10 páginasIron-Iron Carbide DiagrammusabAinda não há avaliações
- Ch-27.5 Iron Carbon Equilibrium DiagramDocumento52 páginasCh-27.5 Iron Carbon Equilibrium DiagramManojAinda não há avaliações
- HM403 Mobile Heat Treatment UnitDocumento2 páginasHM403 Mobile Heat Treatment UnitAnand SankarAinda não há avaliações
- Aeb4101 Engineering and Design: Module - 3Documento14 páginasAeb4101 Engineering and Design: Module - 3saiAinda não há avaliações
- Thermowell Material Selection Guide: Heat TreatingDocumento11 páginasThermowell Material Selection Guide: Heat TreatingTelugu MoviesAinda não há avaliações
- Metallurgy in The Automotive IndustryDocumento29 páginasMetallurgy in The Automotive IndustryOlivier BaertAinda não há avaliações
- Double Bainitic Step Full (B)Documento5 páginasDouble Bainitic Step Full (B)HICHAM SBAITIAinda não há avaliações
- Question Bank-ME 303 2017-18Documento18 páginasQuestion Bank-ME 303 2017-18Saikat BanerjeeAinda não há avaliações
- Casting Terminology GuideDocumento12 páginasCasting Terminology GuideNonameAinda não há avaliações
- Understanding Phase TransformationsDocumento30 páginasUnderstanding Phase TransformationswinnieAinda não há avaliações
- Corporate A4 Brochure CompressedDocumento20 páginasCorporate A4 Brochure CompressedMarcel HidajatAinda não há avaliações
- Bw140e - Welding in Tool MakingDocumento24 páginasBw140e - Welding in Tool MakingAlvaro A. Kalle GonzalesAinda não há avaliações
- Annealing processes for hypoeutectoid and hypereutectoid steelDocumento1 páginaAnnealing processes for hypoeutectoid and hypereutectoid steelAhmad Helmi AdnanAinda não há avaliações
- 20603E01, Guidelines For Material Selection in O&G Processing Facilities - Jan 2011 PDFDocumento63 páginas20603E01, Guidelines For Material Selection in O&G Processing Facilities - Jan 2011 PDFanghel_florin82Ainda não há avaliações
- Ferrotherm 4742Documento2 páginasFerrotherm 4742Özlem KarataşAinda não há avaliações
- 14.materials Science and Engineering PDFDocumento18 páginas14.materials Science and Engineering PDFs_manikandanAinda não há avaliações
- LMA AUG 2013 Lowres-Esub noPWDocumento100 páginasLMA AUG 2013 Lowres-Esub noPWMichael KimAinda não há avaliações
- Solution Manual Callister 8th EditionDocumento15 páginasSolution Manual Callister 8th EditionAbdur Rozaq25% (8)
- Stainless Steel List of StandardsDocumento12 páginasStainless Steel List of StandardsSuresh Kumar100% (1)
- YSS Cold Work Tool Steel GuideDocumento6 páginasYSS Cold Work Tool Steel GuidetaknevAinda não há avaliações
- Metal and Metal Alloys NotesDocumento18 páginasMetal and Metal Alloys Notesrutuja75% (4)
- Heat Treatment of Aluminum AlloysDocumento9 páginasHeat Treatment of Aluminum AlloysUmar Shaukat100% (1)
- A941-13b Standard Terminology Relating To Steel, Stainless Steel, Related Alloys, and FerroalloysDocumento8 páginasA941-13b Standard Terminology Relating To Steel, Stainless Steel, Related Alloys, and FerroalloysChuthaAinda não há avaliações
- and 68070 S2012 Final v2 No AnswersDocumento42 páginasand 68070 S2012 Final v2 No AnswersZadrin TuckerAinda não há avaliações
- Understanding Chain Grades and InspectionDocumento7 páginasUnderstanding Chain Grades and InspectionWade SperryAinda não há avaliações
- Asme Section II A Sa-105 Sa-105mDocumento6 páginasAsme Section II A Sa-105 Sa-105mAnonymous GhPzn1xAinda não há avaliações
- Vibe Steel & Engineering Co. ProfileDocumento19 páginasVibe Steel & Engineering Co. ProfileMirza Shaizad BegAinda não há avaliações
- ASTM A192, ASME SA192 Seamless Boiler and Superheater TubesDocumento5 páginasASTM A192, ASME SA192 Seamless Boiler and Superheater TubesSambandam ElangovanAinda não há avaliações
- 76 202Documento8 páginas76 202wawanAinda não há avaliações
- F541 PDFDocumento5 páginasF541 PDFfrengki jmAinda não há avaliações
- Heat Treating FurnacesDocumento63 páginasHeat Treating FurnacesNawaz KhanAinda não há avaliações
- Charpy TestsDocumento10 páginasCharpy Testssuresh himathAinda não há avaliações