Escolar Documentos
Profissional Documentos
Cultura Documentos
Uric Acid and CVD
Enviado por
Sukma EffendyTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Uric Acid and CVD
Enviado por
Sukma EffendyDireitos autorais:
Formatos disponíveis
Q J Med 2000; 93:707713
Review
QJM
Uric acid as a risk factor for cardiovascular disease
W.S. WARING, D.J. WEBB and S.R.J. MAXWELL
From the Clinical Pharmacology Unit and Research Centre, Department of
Medical Sciences, University of Edinburgh, Edinburgh, UK
Introduction
Over recent years there has been renewed debate
about the nature of the association between raised
serum uric acid concentration and cardiovascular
disease.1 Several large studies have identified the
value, in populations, of serum uric acid concentration in predicting the risk of cardiovascular events,
such as myocardial infarction. This has directed
research towards the potential mechanisms by
which uric acid might have direct or indirect effects
on the cardiovascular system. It has been difficult to
identify the specific role of elevated serum uric acid
because of its association with established cardiovascular risk factors such as hypertension, diabetes
mellitus, hyperlipidaemia and obesity.2,3 Indeed, it
is not even clear at this stage whether uric acid has
a damaging or protective effect in these circumstances. Increased understanding of the mechanisms
underlying these associations may allow a clearer
interpretation of the importance of elevated serum
uric acid concentrations, and the potential value of
specific urate-lowering treatment on cardiovascular
disease.
Uric acid synthesis
Purines arise from metabolism of dietary and endogenous nucleic acids, and are degraded ultimately
to uric acid in man, through the action of the
enzyme xanthine oxidase (Figure 1). Uric acid is
a weak acid (pKa 5.8), distributed throughout the
extracellular fluid compartment as sodium urate,
and cleared from the plasma by glomerular
filtration.4 Around 90% of filtered uric acid is
reabsorbed from the proximal renal tubule,
while active secretion into the distal tubule by an
ATPase-dependent mechanism contributes to
overall clearance.5 Serum uric acid concentration
within the population has a Gaussian distribution,
with a typical reference range (95% CI) of
120420 mmol/l. For an individual, urate concentration is determined by a combination of the
rate of purine metabolism (both endogenous and
exogenous) and the efficiency of renal clearance.
Purine metabolism is influenced by dietary, as well
as genetic factors regulating cell turnover. Uric acid
is sparingly soluble in aqueous media, and persistent exposure to high serum levels predisposes to
urate crystal deposition within soft tissues.4 All
species apart from man and higher apes express
urate oxidase, an enzyme responsible for further
metabolism of uric acid to allantoin (a more soluble waste product) prior to excretion.6 In man, the
urate oxidase gene located on chromosome 1 is not
expressed due to two non-sense mutations.7 Loss of
uric oxidase activity appears to have developed
under evolutionary pressure,7 suggesting that higher
serum uric acid concentrations, or reduced urate
oxidase may confer important advantages in man.
Uric acid as a risk factor for
cardiovascular disease
An epidemiological link between elevated serum
uric acid and an increased cardiovascular risk has
Address correspondence to Dr W.S. Waring, Clinical Pharmacology Unit, University of Edinburgh, Western General
Hospital, Crewe Road, Edinburgh EH4 2LH. e-mail: s.waring@ed.ac.uk
Association of Physicians 2000
708
W.S. Waring et al.
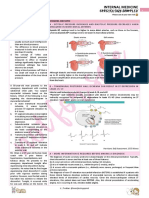
Figure 1. Adenosine release is increased during hypoxia, and its local vasodilatating action may preserve blood flow and
prevent tissue ischaemia. Uric acid is the end-product of purine metabolism in man, whereas other species express uric
oxidase, responsible for conversion of uric acid to more soluble excretory products.
been recognized for many years.8,9 Observational
studies show that serum uric acid concentrations
are higher in patients with established coronary
heart disease compared with healthy controls.10
Elevated serum uric acid concentrations are also
found in healthy offspring of parents with coronary
artery disease, indicating a possible causal relationship.2 However, hyperuricaemia is also associated
with possible confounding factors including elevated serum triglyceride and cholesterol concentrations, blood glucose, fasting and post-carbohydrate
plasma insulin concentrations, waist-hip ratio
and body mass index.3,8,11,12 About one quarter
of hypertensive patients have co-existent hyperuricaemia13 and, interestingly, asymptomatic
hyperuricaemia predicts future development of
hypertension, irrespective of renal function.14
Among patients with established hypertension,
elevated serum uric acid concentration has been
associated with a significantly increased cardiovascular risk during a mean 6.6-year follow-up
period.15 The proportional hazard ratio for one SD
elevation of uric acid (29.2 mmol/l) was 1.22
(95%CI 1.111.35), which was higher than for
one S.D. elevation of blood glucose (1.10, 95%CI
1.021.19), cholesterol (1.18, 95%CI 1.091.29) or
systolic blood pressure (1.09, 95%CI 1.001.19).
Thiazide diuretics confer unequivocal benefits
in treatment of hypertensive patients, and cause
a significant reduction in cardiovascular and allcause mortality.16 Persistence of the relationship
between elevated serum uric acid concentration
and increased cardiovascular risk among thiazidetreated patients has prompted speculation that uric
acid elevation may attenuate some of their potential
benefits.17 Indeed, the US National Health and
Nutrition Survey (NHANES) III showed that ageadjusted rates of myocardial infarction and stroke
Uric acid and cardiovascular disease
are higher across increasing serum uric acid
quartiles among male and female hypertensive
patients.18
Some studies have suggested that the importance
of uric acid may be independent of confounding
risk factors. Multivariate analysis of data from the
MONICA cohort of 1044 males showed a significant association between raised serum uric acid
and cardiovascular mortality, independent of body
mass index, serum cholesterol concentration, hypertension, diuretic use, alcohol intake and smoking
habits.19 Comparison of those individuals within the
highest serum uric acid quartile (0373 mmol/l)
versus those in the lowest quartile ((319 mmol/l)
gave an adjusted risk of myocardial infarction of
1.7 (95%CI 0.83.3) and cardiovascular death of
2.2 (95%CI 1.04.8). The Gothenburg prospective
study of 1462 women aged 38 to 60 years also
found a significant relationship between serum
uric acid concentration and total mortality during
12-year follow-up, which was independent of body
mass index, serum lipid concentrations, smoking
habit, blood pressure and age.20 Uric acid also
has a predictive role in high-risk patient groups.
For instance, diabetes mellitus is a very powerful
risk factor for cardiovascular disease, and a prospective study of 1017 non-insulin-dependent
patients showed that serum uric acid concentration
)295 mmol/l conferred a hazard ratio of 1.91
(95%CI 1.242.94) of fatal or non-fatal stroke
during 7-year follow-up.21
In contrast to these findings, several studies have
suggested that the relationship between elevated
serum uric acid and cardiovascular risk does not
persist after correcting for other risk factors. The
British Regional Heart Study of 7688 men aged
40 to 59 years showed a significant association
between elevated serum uric acid and fatal and
non-fatal coronary disease over a mean 16.8
years.22 However, this relationship disappeared
after correcting for other risk factors, particularly
serum cholesterol concentration. The Coronary
Drug Project Research Group studied 2789 men,
aged 30 to 64 years, and found that the association
between increased cardiovascular risk and elevated
serum uric acid concentration was not significant
after consideration of other risk factors, and when
thiazide diuretic use was considered.23 Similar findings have been reported from the Social Insurance
Institution of Finland Study24 and Framingham
Heart Study.25
The Atherosclerosis Risk in Communities study
of 11 488 healthy men and women showed an
apparent association between serum uric acid
concentration and early carotid artery atherosclerosis, which was dependent of other coronary risk
factors.26 Similarly, the Honolulu Heart Program
709
found that elevated serum uric acid was not an
independent risk factor for the presence at autopsy
of aortic or coronary atherosclerosis in Japanese
men.27
In summary, although there is overwhelming
evidence that elevated serum uric acid concentrations are strongly associated with increased cardiovascular risk and poor outcome, prospective
population studies are often confounded by
co-existent risk factors. It remains unclear whether
uric acid is an independent predictor of poor cardiovascular outcome. To unravel this association, it is
important to understand the mechanisms by which
hyperuricaemia relates to other risk factors, vascular
dysfunction and cardiovascular disease. We shall
now consider these relationships in more detail.
Uric acid as a marker of
subclinical ischaemia
Adenosine is synthesized and released by cardiac
and vascular myocytes. Binding to specific adenosine receptors causes relaxation of vascular smooth
muscle and arteriolar vasodilatation.28 Adenosine
makes a small contribution to normal resting vascular tone, since competitive antagonism at the
adenosine receptor by methylxanthines, such as
theophylline, reduce blood flow response to
ischaemia in the forearm vascular bed.29 Under
conditions of hypoxia and tissue ischaemia, vascular
adenosine synthesis and release are upregulated,
causing significantly increased circulating concentrations.30 Cardiac and visceral ischaemia promote
generation of adenosine, which may serve as an
important regulatory mechanism for restoring
blood flow and limiting the ischaemia31 (Figure 1).
Adenosine synthesized locally by vascular smooth
muscle in cardiac tissue is rapidly degraded by the
endothelium to uric acid, which undergoes rapid
efflux to the vascular lumen due to low intracellular
pH and negative membrane potential.32 Xanthine
oxidase activity33 and uric acid synthesis34 are
increased in vivo under ischaemic conditions, and
therefore elevated serum uric acid may act as
a marker of underlying tissue ischaemia. In the
human coronary circulation, hypoxia, caused by
transient coronary artery occlusion, leads to an
increase in the local circulating concentration of
uric acid.35 Study of tourniquet-induced lower limb
exsanguination in patients undergoing surgery
shows a five-fold increase in systemic vascular
xanthine oxidase activity during reperfusion, and
a significant elevation of serum uric acid, which
persists for at least 2 h.36 These findings are also
consistent with the inverse relation between baseline serum uric acid concentration and maximal
710
W.S. Waring et al.
lower limb blood flow in patients with cardiac
failure, where higher concentrations could predict
subclinical ischaemia.37 In conclusion therefore,
elevated serum uric acid may be a marker of local
or systemic tissue ischaemia and provides one
possible explanation for a non-causal associative
link between hyperuricaemia and cardiovascular
disease.
Uric acid as a marker of
insulin resistance
Insulin resistance syndromes result in attenuation
of insulin-mediated glucose utilization and confer
a substantial increase in cardiovascular risk,38
through activation of several pathways including
the sympathetic nervous system.39 Elevated serum
uric acid is a consistent feature of the insulin
resistance syndromes, which are also characterized
by elevated plasma insulin level (fasting and postcarbohydrate), blood glucose concentration, and
serum triglyceride concentration, and raised body
mass index and waist-hip ratio.2,3 Insulin has
a physiological action on renal tubules, causing
reduced sodium and uric acid clearance.40 Despite
blunting of the action of insulin on glucose metabolism, sensitivity to the renal effects persists.40,41
Because plasma insulin concentration is characteristically elevated, hyperuricaemia may arise as
a consequence of enhanced renal insulin activity.
Elevated serum uric acid concentrations predict
subsequent development of diabetes mellitus42
and hypertension,14 even in the presence of
normal creatinine clearance and plasma glucose
concentrations, and therefore may be a subtle, early
marker of peripheral insulin resistance syndromes.
Thus a link between elevated serum uric acid
concentration and cardiovascular disease may arise
through its non-causal relationship with insulin
resistance syndromes, where cardiovascular risk is
mediated by other factors.
Direct impact of uric acid on
vascular function
The endothelium plays a central role in maintaining
vascular tone through synthesis and release of nitric
oxide, a potent vasodilator.43 Reduction of nitric
oxide bioavailability is an important early step
in the development of atherosclerosis.44 So-called
endothelial dysfunction, associated with impaired
endothelium-dependent vasodilatation may arise
from excessive free radical activity, which disrupts
synthesis and accelerates degradation of nitric
oxide.45 Thus increased oxidative stress appears to
have an important role in development and progression of atherosclerosis and is a characteristic
finding associated with its major risk factors, such
as diabetes mellitus, hypertension, hypercholesterolaemia and smoking.44,46 Serum uric acid possesses antioxidant properties, and contributes about
60% of free radical scavenging activity in human
serum.47,48 Uric acid interacts with peroxynitrite to
form a stable nitric oxide donor, thus promoting
vasodilatation and reducing the potential for
peroxynitrite-induced oxidative damage.49 Thus,
uric acid could be expected to protect against
oxidative stresses.
However, uric acid has been found to promote
low-density lipoprotein (LDL) oxidation in vitro,
a key step in the progression of atherosclerosis,50,51
and these effects are inhibited by vitamin C52
indicating an important interaction between aqueous anti-oxidants. Uric acid can also stimulate
granulocyte adherence to the endothelium,53 and
peroxide and superoxide free radical liberation.53,54
Therefore uric acid may have a deleterious effect on
the endothelium through leukocyte activation and,
interestingly, a consistent relationship has been
noted between elevated serum uric acid concentration and circulating inflammatory markers.5557
Uric acid traverses dysfunctional endothelial cells
and accumulates as crystal within atherosclerotic
plaques.32,58 These crystals may contribute to local
inflammation and plaque progression, and we
speculate that crystal accumulation may be
greater in patients with elevated serum uric acid
concentration.
Thus, while uric acid appears to make a significant contribution to serum anti-oxidant capacity, it
could also lead directly or indirectly to vascular
injury. It is interesting to note that treatment of
chronic cardiac failure patients with allopurinol
(a xanthine oxidase inhibitor) restored endothelial
function.59 This effect may have been due to an
increase in recorded serum antioxidant capacity,59
although the effect of uric acid was not considered.
Study of the direct effects of uric acid on vascular
function has been hampered by its poor solubility,
although this has been overcome recently.60 Direct
study of the actions of uric acid on endothelial function, platelet aggregation, vessel wall elasticity and
autonomic cardiovascular regulation is required
so that its effects on the cardiovascular system and
in cardiovascular disease can be determined.
Conclusions
Raised serum uric acid concentrations are a powerful predictor of cardiovascular risk and poor
Uric acid and cardiovascular disease
outcome, although the underlying mechanisms
remain unclear. Several potential explanations
have been put forward to explain the apparent
association between hyperuricaemia and cardiovascular risk. Studies have demonstrated mechanisms by which uric acid could be directly injurious
to the endothelium and to cardiovascular function.
Paradoxically, uric acid elevation could be
expected to confer protective anti-oxidant effects
in the cardiovascular system, but these potential
benefits may be obscured by detrimental effects
elsewhere. The effects of raising or lowering serum
uric acid on endothelial function, autonomic
regulation and progression of atherosclerosis
require direct investigation, in order to understand
a possible dual action in the cardiovascular system.
Identifying the mechanisms by which uric acid
interacts with cardiovascular regulation will give us
greater understanding of the role of hyperuricaemia
for individual patients and allow a more rational
approach to treatments that modify serum uric acid
concentration.
Acknowledgements
This work has been supported by an endowment
grant from the Faculty of Medicine of The
University of Edinburgh (E80870). Dr W.S. Waring
is in receipt of a Bristol Myer-Squibb Cardiovascular
Research Fellowship. Professor D.J. Webb is currently supported by a Research Leave Fellowship
from the Wellcome Trust (WT 0526330).
711
7. Wu X, Muzny DM, Lee CC, Casker CT. Two independent
mutational events in the loss of urate oxidase. J Mol Evol
1992; 34:7884.
8. Kohn PM, Prozan GB. Hyperuricemia: relationship to
hypercholesterolemia and acute myocardial infarction.
JAMA 1959; 170:190915.
9. Beard JT. Serum uric acid and coronary heart disease. Am
Heart J 1983; 106:397400.
10. Torun M, Yardim S, Simsek B, Burgaz S. Serum uric acid
levels in cardiovascular diseases. J Clin Pharm Ther 1998;
23:259.
11. Lee J, Sparrow D, Vokonas PS, Landsberg L, Weiss ST. Uric
acid and coronary heart disease risk: evidence for a role of
uric acid in the obesity-insulin resistance syndrome. The
Normative Aging Study. Am J Epidemiol 1995; 142:28894.
12. Patten RL, Hewitt D, Waldman GT, Jones G, Little JA.
Associations of plasma high-density lipoprotein cholesterol
with clinical chemistry data. Circulation 1980;
62:IV31IV41.
13. Tykarski A. Evaluation of renal handling of uric acid in
essential hypertension: hyperuricemia related to decreased
urate secretion. Nephron 1991; 59:3648.
14. Selby JV, Friedman GD, Quesenberry CPJ. Precursors of
essential hypertension: pulmonary function, heart rate, uric
acid, serum cholesterol, and other serum chemistries. Am J
Epidemiol 1990; 131:101727.
15. Alderman MH, Cohen H, Madhavan S, Kivlighn S. Serum
uric acid and cardiovascular events in successfully treated
hypertensive patients. Hypertens 1999; 34:14450.
16. SHEP Cooperative Research Group. Prevention of stroke by
anti-hypertensive drug treatment in older persons with
isolated systolic hypertension: final results of the Systolic
Hypertension in the Elderly Program (SHEP). Lancet 1991;
265:325564.
17. Alderman MH, Cohen H, Kinlign S, Madhavan S. Does
treatment-mediated increase in serum uric acid reduce the
cardioprotective effect of diuretics in hypertensive patients?
Am J Hypertens 1998; 2:16A.
18. Ward H. Uric acid as an independent risk factor in the
treatment of hypertension. Lancet 1998, 352:6701.
References
1. Dobson A. Is raised serum uric acid a cause of cardiovascular
disease or death? Lancet 1999; 354:1578.
2. Bonora E, Targher G, Zenere MB, Saggiani F, Cacciatori V,
Tosi F, Travia D, Zenti MG, Branzi P, Santi L, Muggeo M.
Relationship of uric acid concentration to cardiovascular risk
factors in young men. Role of obesity and central fat
distribution. The Verona Young Men Atherosclerosis Risk
Factors Study. Int J Obes Relat Metab Disord 1996;
20:97580.
19. Liese AD, Hense HW, Lowel H, Doring A, Tietze M, Keil U.
Association of serum uric acid with all-cause and cardiovascular disease mortality and incident myocardial
infarction in the MONICA Augsburg cohort. World Health
Organization Monitoring Trends and Determinants in
Cardiovascular Diseases. Epidemiology 1999; 10:3917.
20. Bengtsson C, Lapidus L, Stendahl C, Waldenstrom J.
Hyperuricaemia and risk of cardiovascular disease and
overall death. A 12-year follow-up of participants in the
population study of women in Gothenburg, Sweden. Acta
Med Scand 1988; 224:54955.
3. Agamah ES, Srinivasan SR, Webber LS, Berenson GS. Serum
uric acid and its relation to cardiovascular disease risk factors
in children and young adults from a biracial community: the
Bogalusa Heart Study. J Lab Clin Med 1991; 118:2419.
21. Lehto S, Niskanen L, Ronnemaa T, Laakso M. Serum uric
acid is a strong predictor of stroke in patients with
non-insulin-dependent diabetes mellitus. Stroke 1998;
29:6359.
4. Emmerson BT. The management of gout. N Engl J Med 1996;
334:44551.
22. Wannamethee SG, Shaper AG, Whincup PH. Serum urate
and the risk of major coronary heart disease events. Heart
1997; 78:14753.
5. Steele TH. Hyperuricemic nephropathies. Nephron 1999;
81(Suppl. 1):459.
6. Yeldandi AV, Patel YD, Liao M, Kao FT, Rao MS, Reddy JK, Le
Beau MM. Localization of the human urate oxidase gene
(UOX) to 1p22. Cytogenet Cell Genet 1992; 61:1212.
23. The Coronary Drug Project Research Group. Serum
uric acid: its association with other risk factors and with
mortality in coronary heart disease. J Chron Dis 1976;
29:55769.
712
W.S. Waring et al.
24. Reunanen A, Takkunen H, Knekt P, Aromaa A.
Hyperuricemia as a risk factor for cardiovascular mortality.
Acta Med Scand Suppl 1982; 668:4959.
25. Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric
acid and risk for cardiovascular disease and death: the
Framingham Heart Study. Ann Intern Med 1999; 131:713.
26. Iribarren C, Folsom AR, Eckfeldt JH, McGovern PG, Nieto FJ.
Correlates of uric acid and its association with asymptomatic
carotid atherosclerosis: the ARIC Study. Atherosclerosis Risk
in Communities. Ann Epidemiol 1996; 6:33140.
27. Reed DM, MacLean CJ, Hayashi T. Predictors of atherosclerosis in the Honolulu Heart Program I. Biologic, dietary
and lifestyle characteristics. Am J Epidemiol 1987;
126:21425.
insulin on renal sodium and uric acid handling in essential
hypertension. Am J Hypertens 1996; 9:74652.
41. DeFronzo RA. The effect of insulin on renal sodium
metabolism. A review with clinical implications.
Diabetologia 1981; 21:16571.
42. Perry IJ, Wannamethee SG, Walker MK, Thomson AG,
Whincup PH, Shaper AG. Prospective study of risk factors for
development of non-insulin dependent diabetes in middle
aged British men. BMJ 1995; 310:5604.
43. Webb DJ. The pharmacology of human blood vessels
in vivo. J Vasc Res 1995; 32:215.
44. Vallance P. Nitric oxide in the human cardiovascular
system- SKB lecture 1997. Br J Clin Pharmacol 1998;
45:4339.
28. Berne M. The role of adenosine in the regulation of coronary
blood flow. Circulation Res 1980; 47:80713.
45. Hoeschen RJ. Oxidative stress and cardiovascular disease.
Can J Cardiol 1997; 13:10215.
29. Costa F, Sulur P, Angel M, Cavalcante J, Haile V, Christman
B, Biaggioni I. Intravascular source of adenosine during
forearm ischemia in humans. Implications for reactive
hyperemia. Hypertens 1999; 33:14537.
46. Ferro CJ, Webb DJ. Endothelial dysfunction and
hypertension. Drugs 1997, 53(Suppl. 1): 3041.
30. Raatikainen MJ, Peuhkurinen KJ, Hassinen IE. Contribution
of endothelium and cardiomyocytes to hypoxia-induced
adenosine release. J Mol Cell Cardiol 1994; 26:106980.
31. Fredholm BB, Sollevi A. Cardiovascular effects of adenosine.
Clin Physiol 1986; 6:121.
32. Kroll K, Bukowski TR, Schwartz LM, Knoepfler D,
Bassingthwaighte JB. Capillary endothelial transport of uric
acid in guinea pig heart. Am J Physiol 1992,
262:H420-H431.
33. Castelli P, Condemi AM, Brambillasca C, Fundaro P, Botta
M, Lemma M, Vanelli P, Santoli C, Gatti S, Riva E.
Improvement of cardiac function by allopurinol in patients
undergoing cardiac surgery. J Cardiovasc Pharmacol 1995;
25:11925.
34. Kogure K, Ishizaki M, Nemoto M, Kuwano H, Tatemoto K,
Maruyama Y, Ikarashi Y, Makuuchi M. Evaluation of serum
uric acid changes in different forms of hepatic vascular
inflow occlusion in human liver surgeries. Life Sci 1999;
64:30513.
35. De Scheerder I, van de Kraay AM, Lamers JM, Koster JF,
de Jong JW, Serruys PW. Myocardial malondialdehyde and
uric acid release after short-lasting coronary occlusions
during coronary angioplasty: potential mechanisms for free
radical generation. Am J Cardiol 1991; 68:3925.
36. Mathru M, Dries DJ, Barnes L, Tonino P, Sukhani R, Rooney
MW. Tourniquet-induced exsanguination in patients
requiring lower limb surgery. An ischemia-reperfusion
model of oxidant and antioxidant metabolism.
Anesthesiology 1996; 84:1422.
37. Anker SD, Leyva F, Poole-Wilson PA, Kox WJ, Stevenson JC,
Coats AJ. Relation between serum uric acid and lower
limb blood flow in patients with chronic heart failure. Heart
1997; 78:3943.
38. Cleland SJ, Petrie JR, Ueda S, Elliott HL, Connell JM. Insulin
as a vascular hormone: implications for the pathophysiology
of cardiovascular disease. Clin Exp Pharmacol Physiol 1998;
25:17584.
39. Modan M, Halkin H. Hyperinsulinemia or increased
sympathetic drive as links for obesity and hypertension.
Diabetes Care 1991; 14:47087.
40. Muscelli E, Natali A, Bianchi S, Bigazzi R, Galvan AQ,
Sironi AM, Frascerra S, Ciociaro D, Ferrannini E. Effect of
47. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric
acid provides an antioxidant defense in humans against
oxidant- and radical-caused aging and cancer: a hypothesis.
Proc Natl Acad Sci USA 1981; 78:685862.
48. Maxwell SR, Thomason H, Sandler D, LeGuen C, Baxter
MA, Thorpe GH, Jones AF, Barnett AH. Antioxidant status in
patients with uncomplicated insulin-dependent and
non-insulin-dependent diabetes mellitus. Eur J Clin Invest
1997; 27:48490.
49. Skinner KA, White CR, Patel R, Tan S, Barnes S, Kirk M,
Darley-Usmar V, Parks DA. Nitrosation of uric acid by
peroxynitrite. Formation of a vasoactive nitric oxide donor.
J Biol Chem 1998; 273:244917.
50. Bagnati M, Perugini C, Cau C, Bordone R, Albano E,
Bellomo G. When and why a water-soluble antioxidant
becomes pro-oxidant during copper-induced low-density
lipoprotein oxidation: a study using uric acid. Biochem J
1999; 340:14352.
51. Schlotte V, Sevanian A, Hochstein P, Weithmann KU. Effect
of uric acid and chemical analogues on oxidation of human
low density lipoprotein in vitro. Free Rad Biol Med 1998;
25:83947.
52. Abuja PM. Ascorbate prevents prooxidant effects of urate in
oxidation of human low density lipoprotein. FEBS Lett 1999;
446:3058.
53. Boogaerts MA, Hammerschmidt DE, Roelant C, Verwilghen
RL, Jacob HS. Mechanisms of vascular damage in gout and
oxalosis: crystal induced, granulocyte mediated, endothelial
injury. Thromb Haemost 1983; 50:57680.
54. Falasca GF, Ramachandrula A, Kelley KA, O'Connor CR,
Reginato AJ. Superoxide anion production and phagocytosis
of crystals by cultured endothelial cells. Arthritis Rheum
1993; 36:10516.
55. Duff GW, Atkins E, Malawista SE. The fever of gout: urate
crystals activate endogenous pyrogen production from
human and rabbit mononuclear phagocytes. Trans Assoc
Am Physicians 1983; 96:23445.
56. Hutton CW, Collins AJ, Chambers RE, Whicher J, Dieppe
PA. Systemic response to local urate crystal induced
inflammation in man: a possible model to study the acute
phase response. Ann Rheum Dis 1985; 44:5336.
57. Leyva F, Anker SD, Godsland IF, Teixeira M, Hellewell PG,
Kox WJ, Poole-Wilson PA, Coats AJ. Uric acid in chronic
heart failure: a marker of chronic inflammation. Eur Heart J
1998; 19:181422.
Uric acid and cardiovascular disease
58. Patetsios P, Rodino W, Wisselink W, Bryan D, Kirwin JD,
Panetta TF. Identification of uric acid in aortic aneurysms
and atherosclerotic artery. Ann N Y Acad Sci 1996;
800:2435.
59. Farquharson CAJ, Butler R, Hill A, Belch JJ,
Struthers AD. Allopurinol improves endothelial
713
dysfunction in patients with chronic heart failure.
Scott Med J 1999; 44:24.
60. Waring WS, Webb DJ, Maxwell SRJ. Effect of local
hyperuricaemia on endothelial function in the human
forearm vascular bed. Br J Clin Pharmacol 2000; 49:511.
Você também pode gostar
- A6 MMWR Update On IGRA in Diagnosis of TB 2010Documento28 páginasA6 MMWR Update On IGRA in Diagnosis of TB 2010Rhea DerijeAinda não há avaliações
- Tanpa JudulDocumento689 páginasTanpa JudulSukma Effendy100% (3)
- Cpns Soal2Documento25 páginasCpns Soal2Sukma EffendyAinda não há avaliações
- 2010 Research Quantitative Analysis of Urine Based Assay For Detection of LAM in Patient With TB PDFDocumento3 páginas2010 Research Quantitative Analysis of Urine Based Assay For Detection of LAM in Patient With TB PDFSukma EffendyAinda não há avaliações
- 2010 Systematic Review Interferon Gamma Release Assays For Tuberculosis Screening of Healthcare WorkersDocumento9 páginas2010 Systematic Review Interferon Gamma Release Assays For Tuberculosis Screening of Healthcare WorkersSukma EffendyAinda não há avaliações
- 1999, JCP REVIEW The Molecular Basis of Disorders of Red Cell EnzymesDocumento4 páginas1999, JCP REVIEW The Molecular Basis of Disorders of Red Cell EnzymesSukma EffendyAinda não há avaliações
- MRSA Recommendations: Members of The Task ForceDocumento13 páginasMRSA Recommendations: Members of The Task ForceNasrullah Samejo 2Ainda não há avaliações
- 032-Roubort-Advantages and Integration of Multi-Vendor LIS EnvironmentsDocumento59 páginas032-Roubort-Advantages and Integration of Multi-Vendor LIS EnvironmentsSukma EffendyAinda não há avaliações
- 032-Roubort-Advantages and Integration of Multi-Vendor LIS EnvironmentsDocumento59 páginas032-Roubort-Advantages and Integration of Multi-Vendor LIS EnvironmentsSukma EffendyAinda não há avaliações
- MRSA Infections: Jama Patient PageDocumento1 páginaMRSA Infections: Jama Patient PageSukma EffendyAinda não há avaliações
- Building A Smart Laboratory: An Introduction To The Integrated LabDocumento36 páginasBuilding A Smart Laboratory: An Introduction To The Integrated LabSukma EffendyAinda não há avaliações
- 032-Roubort-Advantages and Integration of Multi-Vendor LIS EnvironmentsDocumento59 páginas032-Roubort-Advantages and Integration of Multi-Vendor LIS EnvironmentsSukma EffendyAinda não há avaliações
- 032-Roubort-Advantages and Integration of Multi-Vendor LIS EnvironmentsDocumento59 páginas032-Roubort-Advantages and Integration of Multi-Vendor LIS EnvironmentsSukma EffendyAinda não há avaliações
- 02 RX Monaco (RX5000)Documento1 página02 RX Monaco (RX5000)Sukma EffendyAinda não há avaliações
- An Overview of The Pulmonary SystemDocumento9 páginasAn Overview of The Pulmonary SystemSukma EffendyAinda não há avaliações
- Study of Lipid Peroxide and Lipid Profile in Diabetes Mellitus PDFDocumento5 páginasStudy of Lipid Peroxide and Lipid Profile in Diabetes Mellitus PDFSukma EffendyAinda não há avaliações
- In Flight ArabicDocumento12 páginasIn Flight ArabicSukma EffendyAinda não há avaliações
- Surveys Manual Glossary PDFDocumento57 páginasSurveys Manual Glossary PDFSukma EffendyAinda não há avaliações
- National Geographic 2010-05Documento164 páginasNational Geographic 2010-05arsivlik12100% (1)
- Anemia MonographDocumento140 páginasAnemia MonographZaveri Hemant GirishkumarAinda não há avaliações
- In Flight ArabicDocumento12 páginasIn Flight ArabicSukma EffendyAinda não há avaliações
- DM Lipid Profile With LDL PDFDocumento1 páginaDM Lipid Profile With LDL PDFSukma EffendyAinda não há avaliações
- Validated Sandwich ELISA For The Quantification of Von Willebrand Fact - PDF - 3236 PDFDocumento9 páginasValidated Sandwich ELISA For The Quantification of Von Willebrand Fact - PDF - 3236 PDFSukma EffendyAinda não há avaliações
- DM Lipid Profile With LDL PDFDocumento1 páginaDM Lipid Profile With LDL PDFSukma EffendyAinda não há avaliações
- A Study of Oxidative Stress, Antioxidant Status and Lipid Profile in Diabetic Patient in The Western Region of Nepal PDFDocumento7 páginasA Study of Oxidative Stress, Antioxidant Status and Lipid Profile in Diabetic Patient in The Western Region of Nepal PDFSukma EffendyAinda não há avaliações
- Serial NumberDocumento1 páginaSerial NumberSukma EffendyAinda não há avaliações
- Detection of Mycobacterium Tuberculosis in Blood Using The Xpert MTBRIF AssayDocumento28 páginasDetection of Mycobacterium Tuberculosis in Blood Using The Xpert MTBRIF AssaySukma EffendyAinda não há avaliações
- Vascular Permeability ICAM-1: Role in Inflammation and in The Regulation ofDocumento3 páginasVascular Permeability ICAM-1: Role in Inflammation and in The Regulation ofSukma EffendyAinda não há avaliações
- Detection of VWF PDFDocumento11 páginasDetection of VWF PDFSukma EffendyAinda não há avaliações
- Evaluation of The GeneXpert MTBRIF Assay For Rapid Diagnosis TB ElimDocumento5 páginasEvaluation of The GeneXpert MTBRIF Assay For Rapid Diagnosis TB ElimSukma EffendyAinda não há avaliações
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (120)
- Deep Learning-Based ElectrocarDocumento9 páginasDeep Learning-Based ElectrocarAiraa ShaneAinda não há avaliações
- Wrist Pulsating Laser HOEKO - 350 (Semiconductor Device For Laser TherapyDocumento30 páginasWrist Pulsating Laser HOEKO - 350 (Semiconductor Device For Laser TherapysumarumAinda não há avaliações
- 6 Minute Walk Test ATS GuidelinesDocumento7 páginas6 Minute Walk Test ATS GuidelinescpradheepAinda não há avaliações
- Comparison of Inflammatory Markers in HTNDocumento6 páginasComparison of Inflammatory Markers in HTNDan JohnstonAinda não há avaliações
- Occupational Stress: A Comparative Study Among Doctors of Hospitals of HaryanaDocumento12 páginasOccupational Stress: A Comparative Study Among Doctors of Hospitals of Haryanashanu kumarAinda não há avaliações
- A Comprehensive Review On Metabolic SyndromeDocumento36 páginasA Comprehensive Review On Metabolic SyndromekotraeAinda não há avaliações
- AR 26 April Efusi PleuraDocumento18 páginasAR 26 April Efusi PleuraShaastieAinda não há avaliações
- GORDON's AssessmentDocumento3 páginasGORDON's AssessmentCharles Lester Adalim83% (6)
- Lesson Plan On Hypertension 1Documento11 páginasLesson Plan On Hypertension 1Samiran Kumar DasAinda não há avaliações
- Prelox - A Patented Sexual Pleasure Enhancer - Fsponline-Recommends - Co.ukDocumento18 páginasPrelox - A Patented Sexual Pleasure Enhancer - Fsponline-Recommends - Co.ukmihaiacAinda não há avaliações
- Drug Study ICUDocumento15 páginasDrug Study ICUJulie Nambatac100% (1)
- MEDICAL - APPLICATIONS - OF - YOGA of DR NAGARATNA PDFDocumento86 páginasMEDICAL - APPLICATIONS - OF - YOGA of DR NAGARATNA PDFKIRAN GARGYAAinda não há avaliações
- Board Reveiw Electrolyte Acid Base MCQ and AnswerDocumento15 páginasBoard Reveiw Electrolyte Acid Base MCQ and AnswerEma100% (8)
- RITE Glossary of Diseases With Specific Objectives 2021 (With ToC)Documento106 páginasRITE Glossary of Diseases With Specific Objectives 2021 (With ToC)Karl PinedaAinda não há avaliações
- PHA Individual Health Profile & Assessment Form As of Oct.09, 2017Documento8 páginasPHA Individual Health Profile & Assessment Form As of Oct.09, 2017Eden VblagasyAinda não há avaliações
- Soft Gelatin Capsules Oral Solution Injection: SandimmuneDocumento21 páginasSoft Gelatin Capsules Oral Solution Injection: Sandimmuneit4728Ainda não há avaliações
- Physical Fitness PPT 2023Documento124 páginasPhysical Fitness PPT 2023Nati MsbAinda não há avaliações
- Presented By: Jagdish Sambad M.SC - Nursing, Ikdrc College of NursingDocumento80 páginasPresented By: Jagdish Sambad M.SC - Nursing, Ikdrc College of NursingAdia MasooraAinda não há avaliações
- Bisoprolol and Hypertension: Effects On Sexual Functioning in MenDocumento8 páginasBisoprolol and Hypertension: Effects On Sexual Functioning in MenZahid MahmoodAinda não há avaliações
- NCP Preeclampsia and EclampsiaDocumento14 páginasNCP Preeclampsia and EclampsiaBiway RegalaAinda não há avaliações
- Adoukonou - Lacroix - Vascular Disorder in Tropical HealthDocumento10 páginasAdoukonou - Lacroix - Vascular Disorder in Tropical HealthCorine HouehanouAinda não há avaliações
- Pathophysiology of Cardiogenic Pulmonary EdemaDocumento13 páginasPathophysiology of Cardiogenic Pulmonary EdemaIrina DuceacAinda não há avaliações
- Case Based Discussion: Advisor: Dr. H. M. Saugi Abduh, SP - PD, KKV, FINASIMDocumento33 páginasCase Based Discussion: Advisor: Dr. H. M. Saugi Abduh, SP - PD, KKV, FINASIMIlhamAinda não há avaliações
- Risk Factors For Hypertension Among Young Adults (18-35) YearsDocumento8 páginasRisk Factors For Hypertension Among Young Adults (18-35) YearsAndhika DAinda não há avaliações
- Product Osma Sven Did As 4985415945639977094Documento161 páginasProduct Osma Sven Did As 4985415945639977094Daniel RiveroAinda não há avaliações
- Case Study 1Documento6 páginasCase Study 1rajambal_ayyasamy0% (1)
- IM Cardiology Samplex All-In - LDocumento35 páginasIM Cardiology Samplex All-In - LDeepbluexAinda não há avaliações
- Preeclampsia: Gestational HypertensionDocumento10 páginasPreeclampsia: Gestational HypertensionDr-Saja O. DmourAinda não há avaliações
- Acid UricDocumento3 páginasAcid UricCosmin AndreiAinda não há avaliações
- Needs AssessmentDocumento24 páginasNeeds Assessmentapi-295271022Ainda não há avaliações