Escolar Documentos
Profissional Documentos
Cultura Documentos
Chemistry 140A Winter 2014 (K. Albizati) : Chapter 1 - Structure and Bonding
Enviado por
Michael SeoTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Chemistry 140A Winter 2014 (K. Albizati) : Chapter 1 - Structure and Bonding
Enviado por
Michael SeoDireitos autorais:
Formatos disponíveis
Chemistry 140A Winter 2014 (K.
Albizati)
What is Organic Chemistry and why is it important?
Old definition chemistry of compounds from living organisms
New definition chemistry of compounds which contain carbon
Nature has selected this element as the base element of life processes because it is able to form very strong covalent bonds to itself and to other
elements. This enables highly complicated compounds, chemical reactions and chemical reaction pathways. Our bodies are just walking bags of
chemicals inter-reacting which allow complex events, like locomotion, vision, speech and thought.
If you want to understand the field of medicine, you must understand Biochemistry and Molecular Biology. To understand Biochemistry and
Molecular Biology, you must have a firm understanding of Organic Chemistry.
Chapter 1 Structure and Bonding
Review Atoms
Most of the mass resides in the nucleus which contains protons and neutrons. The nucleus is surrounded by a cloud of electrons populating orbitals
which describe the probability of where an electron may be found. This cloud is about 2 in diameter which means atoms are about 2 in diameter.
A Molecule is made up of atoms held together by chemical bonds. Why do bonds form?
Two atoms will form a bond only if it is energetically favorable --- that is if energy is released when the bond is formed. Everything in life
tends toward lower energy states in the long run.
So, it makes sense that to break a chemical bond you must put energy into the system.
Two main causes of bonding are related to Coulombs Law:
Opposite charges attract (electrons to protons)
Like charges repel (protons repel protons but more importantly, electrons repel electrons)
1
Chemistry 140A Winter 2014 (K. Albizati)
The Two Extremes of Chemical Bonding
Recall that atoms can be viewed as a positively charged nucleus surrounded by a negatively charged electron cloud
When two atoms that can favorably form a bond approach one another from a distance the energy starts to decrease due to the favorable
attraction of one nucleus to the other atoms electrons and vice versa. At some point an optimum distance is reached with respect to the energy
of the system, i.e., the low energy point. This attraction/repulsion follows a familiar curve.
\
Energy
Absorbed
0
Energy
Released
bond length
Interatomic distance
Chemistry 140A Winter 2014 (K. Albizati)
As 2 atoms approach one another from a long distance, attractive forces start to lower the energy of the system until the atoms get to an energetically
optimal distance. If they start to get any closer, the repulsive forces between the 2 nuclei starts to become significant and the energy starts to rise
quickly. The bottom of this energy well corresponds to the bond length. The energy content of the system is at a minimum, its most favorable
state.
The picture shown earlier is a diagram of a covalent bond one in which the electrons are shared equally between the two nuclei. At the other
extreme are ionic bonds total transfer of an electron from one nucleus to another.
So.we have 2 extremes of bonding:
Covalent bonding based on the sharing of electrons between 2 nuclei
Ionic bonding based on the electrostatic attraction of a + charge to a minus charge and formed by transfer of an electron from one nucleus to
another
You have encountered ionic bonds before, as exemplified by salts like NaCl.
Organic chemistry is dominated by covalent bonding, especially between 2 carbon atoms and between carbon and hydrogen. These types of bonds
have substantial covalent character
So..what determines
A. whether two atoms will readily form a bond and
B. which of the two modes (or where in the continuum) the two atoms will exhibit in their bonding?
3
Chemistry 140A Winter 2014 (K. Albizati)
It is determined primarily by the atoms electron configuration and individual fundamental physical properties of the atoms themselves.
The Octet Rule
Look at ionic bonding first.
Review:
The elements of the periodic table are listed in order of increasing nuclear protons. In an uncharged element the # of e-s = # protons. Electrons
occupy energy levels (shells) with a fixed capacity (i.e., allowed population of e-s) corresponding to the rows in the Table. The first shell can take
2e-, the second can take 8 and the third can take 18. On the far right of the table are the Noble Gases. These are especially stable elements which
rarely take part in chemical reactions. Their outermost electron shell is filled, with 8 e-s (except for He). This is an especially stable electronic
configuration. When chemical reactions occur electrons are either transferred or shared such that the atoms involved in the new bond have an octet
of electrons around them. Electron dot structures are helpful in visualizing a chemical reaction:
+ . H
H:O:H
H .
Why does NaCl form an ionic bond and H2O forms a covalent bond? It has to do with fundamental physical properties of the individual atoms,
specifically the IONIZATION Potential (IP) and the ELECTRON AFFINITY (EA). These can be measured for every atom and one can calculate the
energetically most favorable bonding mode. However, there is a better way to predict and visualize what type of bonding will occur. First lets look
at covalent bonding a little deeper.
Chemistry 140A Winter 2014 (K. Albizati)
Methane
CH4 formed by combination of 4Hs bonded to a single carbon:
H
:
. H
H:C:H
:
H .
H
.
.
. .
C
.
.
H
Natural Gas is mostly methane.
Carbon and hydrogen tend to form covalent bonds.
Ethane
H H
. H
.
H
H:C:C:H
:
:
H
.
.
. .
C
.
:
:
H .
H
.
.
. .
C
.
.
H
H H
Carbon tends to form covalent bonds with itself.
Chemistry 140A Winter 2014 (K. Albizati)
Carbon can also form multiple bonds
Ethene
C::C
H
.
.
:C
.
.
H
H
.
.
C:
.
.
H
Each carbon is still surrounded by 8 electrons and there is a double bond between the carbons. This is also a covalent bond.
So.what atoms form covalent bonds and which ones form ionic bonds?
Well.using EAs and IPs you can calculate each kind of bond in each kind of molecule. Thats pretty boring. Or you can look at the periodic
table, knowing just one periodic trend in the Table.
Chemistry 140A Winter 2014 (K. Albizati)
ELECTRONEGATIVITY the tendency of an atom to hoard electrons or to donate electrons to other atoms. Recall that the general trends are
that electronegativity increases as you move from left to right and from bottom to top.
In quantitative terms, Table 1.2 shows relative EN values with F being the highest at a relative value of 4.0 with alkali metals at about 1. In general
An EN difference of about 0.3 or less indicates a purely covalent bond and a difference of >2 indicates a purely ionic bond.
However, qualitatively.
-
Fluorine is the most electronegative atom
The alkali metals are the least electronegative and are almost exclusively ionic bonders
Carbon and hydrogen are close in EN and almost always form covalent bonds
The main group elements almost always form covalent bonds to each other.
If the EN difference is between 0.3 and about 2 you have a POLAR COVALENT BOND. These are closer to covalent bonds than they are to ionic
bonds.
7
Chemistry 140A Winter 2014 (K. Albizati)
Example:
The carbon to oxygen bond. Carbon has a relative EN of 2.6 while O has a relative EN of 3.4. This is a polar covalent bond with the greater electron
density on oxygen and the lesser electron density on carbon. The bond is POLAR. This can be indicated in the following way:
+ C:O
Other important examples of polar covalent bonds:
C-halogen
C-N
C-S
N-O
N-S
More on the octet rule
The octet rule does not always hold, especially with charged species
Consider.
:
H:O:H
:
H
Each hydrogen brings its electron to the covalent O-H bond which means oxygen has 5 of its 6 valence electrons. The oxygen is missing an
electron which means it has what charge? +1
Note however, that the oxygen is surrounded by 8 electrons. This one follows the octet rule and is a stable species. This is called the hydronium ion,
a very common molecular species which we will see a lot. Its important in acid-base chemistry and is generally written as H3O+.
Now look at
Chemistry 140A Winter 2014 (K. Albizati)
H
:
C:H
:
H
This species has 3 Hs bonded to a single carbon. Since each of the C-H bonds is covalent each H has its required 2 e-s around it which means the
carbon has only 3 of its 4 valence electrons. If 1 electron is missing from carbon what is the charge on the carbon atom? +1 This species is the
methyl carbocation (or methyl cation). We will study such species in detail.
How about..
H
neutral or charged?
O :: C: :O
neutral or charged?
H:N:H
:C:H
:
H:S:H
neutral or charged?
Neutral or charged?
:
:
:
H:N:H
H:C:C: O:H
:
:
:
H H
neutral or charged?
: N : : : N : neutral or charged?
: C : : : O : neutral or charged?
Electron dots are boring and take too long to draw, count and visualize. We use another notation.
H H
neutral or charged?
Chemistry 140A Winter 2014 (K. Albizati)
To better represent a covalent bond we replace the two electrons with a straight line to indicate a chemical bond.
So..
H
O :: C: :O
:
H
:
H
H:N:H
becomes
becomes
H H
:
:
:
H:C:C: O:H
:
:
:
H H
H:N:H
becomes
10
: N : : : N : becomes : N
N:
becomes
becomes
H:S:H
becomes
:
H: O:H
Chemistry 140A Winter 2014 (K. Albizati)
The 3-D Shapes of Molecules
We have seen that methane can be drawn a number of ways:
H :C:H
H
CH4
or
but what is it really shaped like? Flat? Or something else?
Fundamental principal of bonding and molecular topology: Molecules adopt shapes so as to minimize electron repulsion.
Consider BeF2
:
: F
Be
: F
Be
The most favorable electronic arrangement is a linear one. Bent is not quite as good.
Consider BCl3. How do you place the Cls so as to get them as far apart as possible?
:
: Cl
Cl
:
B
: Cl :
11
Chemistry 140A Winter 2014 (K. Albizati)
A trigonal arrangement where the bonds point to the corners of a triangle is best. Flat or not?
What about methane?
H
H
Flat? Note the apparent 90 degree bond angles. The H atoms can be farther apart if you point the four bonds to the corners of a
tetrahedron. In this manner the bond angles are 109.5 degrees (greater than 90 and the electrons are as far apart as they can be. Note that this has 4
fold symmetry and that all four bonds are equivalent (same length, same bond strength). This has important implications later.
H
Look at some others
12
Chemistry 140A Winter 2014 (K. Albizati)
Nitrogen..consider the ammonium cation
H
H
should be shaped like methane..and it is indeed tetrahedral
What about ammonia?
:
N
H
like BCl3? Nope. The electron pair is like a bond. So it is tetrahedral if you consider it like this. However if you just look at
the nuclei, it is trigonal but bent.
Water?
Same idea. 2 bonds and 2 electron pairs pointed approximately to the corners of a tetrahedron. But if you just look at the nuclei, it appears bent.
13
Chemistry 140A Winter 2014 (K. Albizati)
What about CO2?
:
O
linear
How about this one?
O
H
The electrons are farthest apart in a trigonal planar arrangement
O
C
H
The bond angles are approximately 120 degrees.
14
Chemistry 140A Winter 2014 (K. Albizati)
Quantum Theory of Bonding for Organic Compounds
So exactly how are electrons arranged around a nucleus?
The movement of an electron around a nucleus is described by a series of equations that approximate the characteristics of waves. The solutions to
these equations are called atomic orbitals and they are literally the probability of finding an electron in a certain region of space around a nucleus.
The shapes of orbitals depend on the energy level of the electron.
Consider the hydrogen atom
-
It has a single electron populating a 1s orbital
S-orbitals in general are spherically symmetric (i.e., they are shaped like spheres)
There is no node
Consider a carbon atom
-
It has a 1s orbital not involved in bonding
It has a 2s orbital which is spherically symmetric which contains a node; this is involved in bonding
o Node region of space where the probability of finding the electron is zero; corresponds to where the wave function has a value of 0.
15
Chemistry 140A Winter 2014 (K. Albizati)
-
It has three 2p orbitals, also involved in bonding
o These resemble a figure 8 with + and lobes and are arrayed along the x,y and z axes with the nucleus at the o point.
Molecular orbitals (covalent bonds) are constructed from the overlap of atomic orbitals.
Consider the hydrogen molecule
The in-phase overlap of two 1s orbitals results in a bonding molecular orbital.
+
+
++
1s
1s
++
bonding MO
16
Chemistry 140A Winter 2014 (K. Albizati)
And an out-of-phase overlap resulting in an antibonding molecular orbital
node
1s
1s
antibonding MO
In general, the combination of n atomic orbitals gives rise to n molecular orbitals.
An s-orbital can overlap with a p orbital
Two ps can overlap in space in 2 ways:
Overlap of one lobe of each orbital like this is called a sigma bond.
And.
Overlap of both lobes is called a pi bond
17
Chemistry 140A Winter 2014 (K. Albizati)
Sosince the s orbital and the p orbitals look so different shape-wise and energy wise, how can all the bonds in methane be identical?
Shouldnt they be different?
Hybrid Orbitals
When atomic orbitals are combined to form molecular orbitals, a mathematical combination can take place to produce orbitals which have the
characteristics of the orbitals being combined but are neither one nor the other. This is called orbital hybridization and it is commonly invoked to
explain many types of covalent bonding.
Consider carbon it has a 2s and three 2p orbitals involved in bonding. These 4 atomic orbitals combine to form four equivalent hybrid orbitals
which have 25% s character and 75% p character. We call these sp3 hybrid orbitals
ANIMATION (See C7SP3 and C8 on laptop in V&S Animations Folder).
- sp3 orbital formation
- Methane formation
Non-bonding electron pairs can also take part in hybridization, such as in ammonia or water which are also sp3 hybridized.
18
Chemistry 140A Winter 2014 (K. Albizati)
Pi bonds and hybridization
Consider ethene:
The sigma framework of carbon consists of three sp2 hybridized orbitals in a planar trigonal arrangement. These are bonded to the hydrogen
1s orbitals in a sigma bonding arrangement and in a sigma bond arrangement between the 2 Cs. The H-C-H and H-C-C bond angles are
about 120 degrees as expected.
There is also a pi bond (remember lobe to lobe overlap?) between two carbon 2P orbitals situated above and below the plane of the nuclei.
This kind of double bond between two carbons is called an olefin or a double bond or a pi bond and is a common functional group in
organic molecules. It represents a lot of electron density and this dominates its chemical reactivity. More on this later..
Carbon can also exhibit triple bonding
Ethyne
19
Chemistry 140A Winter 2014 (K. Albizati)
-
The sigma framework of carbon consists of two sp-hybridized orbitals in a linear arrangement to minimize electron repulsion.
There is also a pi framework consisting of two pi bonds consisting of overlap of two pairs of carbon 2p orbitals at 90 degree angles to one
another.
To summarize
When carbon is bonded to:
4 other atoms sp3 hybridization
3 other atoms sp2 hybridization
2 other atoms sp hybridization
tetrahedral geometry (shape)
trigonal planar geometry
linear geometry
bond angles approx. 109 degrees
bond angles approx. 120 degrees
bond angles approx 180 degrees
Class quiz hybridization of each carbon? Geometry and bond angles?
O
H
C
C
H
C
H
H
N:
A handy table
# of Atoms Bonded
to a Carbon Atom
Hybridization of
that Carbon Atom
Geometry of that
Carbon Atom
4
3
2
sp3
sp2
sp
Tetrahedral
Trigonal planar
Linear
Bond Angles
Involving that
Carbon Atom
109
120
180
20
Chemistry 140A Winter 2014 (K. Albizati)
Drawing Organic Molecules
Several types of drawings are used to represent organic molecules
No one uses electron dot structures
For the next few weeks I will use structures with the bonds drawn out.
We will slowly transition into condensed drawings and then into line structures
Examples:
Pentane major component of gasoline
CH3CH2CH2CH2CH3
Isopropyl alcohol (isopropanol) in lots of stuff
H
OH
OH
H
CH3 CHCH3
21
Chemistry 140A Winter 2014 (K. Albizati)
Reading: Skip 1-5 (for now)
Problem Assignment: Skip 1-9, 1-10, 1-11, 1-12, 1-15, 1-18, 1-29, 1-30, 1-31, 1-32, 1-34, 1-47
22
Você também pode gostar
- UTP Student Industrial ReportDocumento50 páginasUTP Student Industrial ReportAnwar HalimAinda não há avaliações
- Ach 2101 Lesson 5Documento19 páginasAch 2101 Lesson 5Lawrence MajaliwaAinda não há avaliações
- 61 NotesDocumento133 páginas61 NotesEman NoamanAinda não há avaliações
- Ionic BondDocumento3 páginasIonic Bondayatolla ayatollaTMAinda não há avaliações
- Module 1 Lecture 2Documento31 páginasModule 1 Lecture 2Amirs AmjadAinda não há avaliações
- CH 13Documento81 páginasCH 13SylviaAinda não há avaliações
- Screenshot 2023-11-24 at 13.18.48Documento69 páginasScreenshot 2023-11-24 at 13.18.48Lana MajidAinda não há avaliações
- Brand Chemical BackgroundDocumento8 páginasBrand Chemical Backgroundninado92Ainda não há avaliações
- General IntroductionDocumento6 páginasGeneral IntroductionTolani AyoAinda não há avaliações
- TEX - CHEM 103 Organic ChemistryDocumento52 páginasTEX - CHEM 103 Organic ChemistrychioAinda não há avaliações
- Carbon and Its Compounds Part 1Documento9 páginasCarbon and Its Compounds Part 1www.luciannarikaAinda não há avaliações
- Why Water Is A DipoleDocumento50 páginasWhy Water Is A DipoleRaviverma077Ainda não há avaliações
- Week 1: Introduction To Organic Chemistry (Part 1)Documento17 páginasWeek 1: Introduction To Organic Chemistry (Part 1)Hayes VivarAinda não há avaliações
- Chemical Bonding ScriptDocumento4 páginasChemical Bonding ScriptAkhil MathewAinda não há avaliações
- Nonpolar Covalent Bonds: ChemistryDocumento9 páginasNonpolar Covalent Bonds: ChemistryRathnakrajaAinda não há avaliações
- Nonpolar Covalent Bonds: ChemistryDocumento9 páginasNonpolar Covalent Bonds: ChemistryRathnakrajaAinda não há avaliações
- Introduction of Organic ChemistryDocumento21 páginasIntroduction of Organic ChemistrySayd KamalAinda não há avaliações
- 51a Chapter 1 2014 Copy 2Documento37 páginas51a Chapter 1 2014 Copy 2Efrain AnayaAinda não há avaliações
- Lecture-Unit 4 (Chemical Bonding)Documento16 páginasLecture-Unit 4 (Chemical Bonding)Tericka JohnsonAinda não há avaliações
- Chemistry Organic tcm4-123642Documento104 páginasChemistry Organic tcm4-123642Ahmed Mohamed AwadAinda não há avaliações
- Fundamental of Organic ChemistryDocumento11 páginasFundamental of Organic ChemistryBernie Suarez100% (1)
- Ionic and Covalent BondsDocumento5 páginasIonic and Covalent BondsFern HofileñaAinda não há avaliações
- Chemistry Chapter 6 ReviewDocumento4 páginasChemistry Chapter 6 ReviewSirena GutierrezAinda não há avaliações
- Chapter 5Documento6 páginasChapter 5Rochelle Anne BandaAinda não há avaliações
- Class IX Chemistry Chapter 05Documento10 páginasClass IX Chemistry Chapter 05Sam FisherAinda não há avaliações
- Pptx5 Chemical BondingDocumento39 páginasPptx5 Chemical BondingLumbay, Jolly MaeAinda não há avaliações
- Chemistry Notes KhraDocumento24 páginasChemistry Notes KhraMohamed WageehAinda não há avaliações
- Electronegativity and Covalent BondingDocumento7 páginasElectronegativity and Covalent BondingNnamdi OnuAinda não há avaliações
- V Aro HydrocarbonsDocumento15 páginasV Aro HydrocarbonsSnehalata MishraAinda não há avaliações
- Types of Chemical BondingDocumento20 páginasTypes of Chemical BondingRSLAinda não há avaliações
- Organic Compounds Hand-Out For CHEM 2Documento11 páginasOrganic Compounds Hand-Out For CHEM 2Rafael SumayaAinda não há avaliações
- 2.electronegativity and PolarityDocumento34 páginas2.electronegativity and PolarityAtie IekahAinda não há avaliações
- Bonding Basics: Shells Atom ElectronsDocumento7 páginasBonding Basics: Shells Atom ElectronsClarice Jenn MaltoAinda não há avaliações
- CH 6 Chemical BondingDocumento14 páginasCH 6 Chemical Bondingapi-240972605Ainda não há avaliações
- Structure and Bonding: John E. McmurryDocumento44 páginasStructure and Bonding: John E. McmurryLionel MessiAinda não há avaliações
- Chapter 1 Structure & BondingDocumento44 páginasChapter 1 Structure & Bondingdead soulAinda não há avaliações
- Appendix E - Polarity WorksheetDocumento3 páginasAppendix E - Polarity WorksheetshafferjfAinda não há avaliações
- Introduction To Chemical BondingDocumento5 páginasIntroduction To Chemical BondingarjunvistaAinda não há avaliações
- Chemistry of Materials Lecture 4Documento24 páginasChemistry of Materials Lecture 4sanjunaAinda não há avaliações
- Notes1 Unit 1Documento7 páginasNotes1 Unit 1arun iyer BitcoinminerandmathematicianAinda não há avaliações
- BondingDocumento11 páginasBondingAirome CorpuzAinda não há avaliações
- Bonding III.1-7Documento7 páginasBonding III.1-7Kartik DuttaAinda não há avaliações
- Forces Between Atoms and MoleculesDocumento13 páginasForces Between Atoms and MoleculesDoc_CrocAinda não há avaliações
- The Structural Characteristics of Carbon 3Documento10 páginasThe Structural Characteristics of Carbon 3Kayelle Clyde LavarejosAinda não há avaliações
- Chemical Bonding 1Documento7 páginasChemical Bonding 1rhiannemitchbAinda não há avaliações
- General ChemistryDocumento6 páginasGeneral ChemistryJewel ValenciaAinda não há avaliações
- Chemical BondDocumento56 páginasChemical BondDzaky Zakiyal Fawwaz100% (1)
- Chemistry 9th Edition Zumdahl Solutions ManualDocumento35 páginasChemistry 9th Edition Zumdahl Solutions Manualstrewmerils1ej3n100% (14)
- Dwnload Full Chemistry 9th Edition Zumdahl Solutions Manual PDFDocumento35 páginasDwnload Full Chemistry 9th Edition Zumdahl Solutions Manual PDFelijah3oa4knight100% (13)
- Unit 1 Module 1Documento38 páginasUnit 1 Module 1Pearl NecoleAinda não há avaliações
- Chemical Bonding and Molecular StructureDocumento16 páginasChemical Bonding and Molecular StructureSancia SamAinda não há avaliações
- BondingDocumento58 páginasBondingSinfullyOffensiveAinda não há avaliações
- 2.04-2.05 Intermediate Bonding and Intermolecular ForcesDocumento16 páginas2.04-2.05 Intermediate Bonding and Intermolecular ForcesDhirenPanchaniAinda não há avaliações
- Chapter 2 Resonance and AcidDocumento4 páginasChapter 2 Resonance and AcidChakalo HapalonAinda não há avaliações
- 1.6 BondingDocumento17 páginas1.6 BondingMahmoud TahaAinda não há avaliações
- Chemical BondingDocumento132 páginasChemical BondingKowser mahmud100% (1)
- Chemical BondingDocumento11 páginasChemical BondingAmadeus BachAinda não há avaliações
- NEET Revision Notes For Chemistry Chemical Bonding and Molecular StructureDocumento21 páginasNEET Revision Notes For Chemistry Chemical Bonding and Molecular StructureTanushree DeshmukhAinda não há avaliações
- Chemistry 10th Edition Zumdahl Solutions ManualDocumento35 páginasChemistry 10th Edition Zumdahl Solutions Manualpouterhawebakefzc8eb100% (32)
- Dwnload Full Chemistry 10th Edition Zumdahl Solutions Manual PDFDocumento35 páginasDwnload Full Chemistry 10th Edition Zumdahl Solutions Manual PDFlifelike.anenstkq2h100% (11)
- Practice Makes Perfect in Chemistry: Chemical Bonding with AnswersNo EverandPractice Makes Perfect in Chemistry: Chemical Bonding with AnswersNota: 5 de 5 estrelas5/5 (1)
- Opc PPT FinalDocumento22 páginasOpc PPT FinalnischalaAinda não há avaliações
- Post Appraisal InterviewDocumento3 páginasPost Appraisal InterviewNidhi D100% (1)
- AlpaGasus: How To Train LLMs With Less Data and More AccuracyDocumento6 páginasAlpaGasus: How To Train LLMs With Less Data and More AccuracyMy SocialAinda não há avaliações
- Quantitative Methods For Economics and Business Lecture N. 5Documento20 páginasQuantitative Methods For Economics and Business Lecture N. 5ghassen msakenAinda não há avaliações
- Business Analytics Emphasis Course GuideDocumento3 páginasBusiness Analytics Emphasis Course Guidea30000496Ainda não há avaliações
- CX Programmer Operation ManualDocumento536 páginasCX Programmer Operation ManualVefik KaraegeAinda não há avaliações
- Turn Around Coordinator Job DescriptionDocumento2 páginasTurn Around Coordinator Job DescriptionMikeAinda não há avaliações
- 74HC00D 74HC00D 74HC00D 74HC00D: CMOS Digital Integrated Circuits Silicon MonolithicDocumento8 páginas74HC00D 74HC00D 74HC00D 74HC00D: CMOS Digital Integrated Circuits Silicon MonolithicAssistec TecAinda não há avaliações
- Tribal Banditry in Ottoman Ayntab (1690-1730)Documento191 páginasTribal Banditry in Ottoman Ayntab (1690-1730)Mahir DemirAinda não há avaliações
- Playful Homeschool Planner - FULLDocumento13 páginasPlayful Homeschool Planner - FULLamandalecuyer88Ainda não há avaliações
- 3 ALCE Insulators 12R03.1Documento12 páginas3 ALCE Insulators 12R03.1Amílcar Duarte100% (1)
- Guidelines For SKPMG2 TSSP - Draft For Consultation 10.10.17Documento5 páginasGuidelines For SKPMG2 TSSP - Draft For Consultation 10.10.17zqhnazAinda não há avaliações
- Acute Coronary SyndromeDocumento30 páginasAcute Coronary SyndromeEndar EszterAinda não há avaliações
- C C C C: "P P P P PDocumento25 páginasC C C C: "P P P P PShalu Dua KatyalAinda não há avaliações
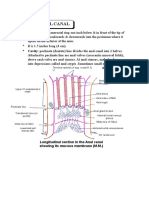
- Anatomy Anal CanalDocumento14 páginasAnatomy Anal CanalBela Ronaldoe100% (1)
- PyhookDocumento23 páginasPyhooktuan tuanAinda não há avaliações
- C - Amarjit Singh So Bhura SinghDocumento5 páginasC - Amarjit Singh So Bhura SinghRohit JindalAinda não há avaliações
- Chemistry: Crash Course For JEE Main 2020Documento18 páginasChemistry: Crash Course For JEE Main 2020Sanjeeb KumarAinda não há avaliações
- .Urp 203 Note 2022 - 1642405559000Documento6 páginas.Urp 203 Note 2022 - 1642405559000Farouk SalehAinda não há avaliações
- Hamstring - WikipediaDocumento21 páginasHamstring - WikipediaOmar MarwanAinda não há avaliações
- Chapter3 Elasticity and ForecastingDocumento25 páginasChapter3 Elasticity and ForecastingGee JoeAinda não há avaliações
- Shostack ModSec08 Experiences Threat Modeling at MicrosoftDocumento11 páginasShostack ModSec08 Experiences Threat Modeling at MicrosoftwolfenicAinda não há avaliações
- The Homework Song FunnyDocumento5 páginasThe Homework Song Funnyers57e8s100% (1)
- FAMOUS PP Past TenseDocumento21 páginasFAMOUS PP Past Tenseme me kyawAinda não há avaliações
- Proceeding of Rasce 2015Documento245 páginasProceeding of Rasce 2015Alex ChristopherAinda não há avaliações
- A Case Study Puga Geothermal System,: OF IndiaDocumento7 páginasA Case Study Puga Geothermal System,: OF IndiaPERIKALA TARUNAinda não há avaliações
- PhraseologyDocumento14 páginasPhraseologyiasminakhtar100% (1)
- 2009 2011 DS Manual - Club Car (001-061)Documento61 páginas2009 2011 DS Manual - Club Car (001-061)misaAinda não há avaliações
- V737 OverheadDocumento50 páginasV737 OverheadnewahAinda não há avaliações