Escolar Documentos
Profissional Documentos
Cultura Documentos
Hypothalamus
Enviado por
Akbar GazaliDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Hypothalamus
Enviado por
Akbar GazaliDireitos autorais:
Formatos disponíveis
E
N D O C R I N E
S E R I E S
#5
The Hypothalamus
Mary Set& RNC, MS, ARNP
HE HYPOTHALAMUS ARISES FROM THE VENTRAL PORTION
input into the endocrine system and alters glandular function
of the diencephalon (Figure 1). Hypothalamic nuclei
and supraoptic track fibers develop by 12 to 14 weeks of gestation, with maturation of the
35 weeks. The hypothalamus
develops simultaneously with but
independently of the pituitary
gland. Located in the forebrain,
i n t h e r e g i o n o f t h e diencephalon, the hypothalamus lies
ABSTRACT
target organs of the endocrine
system. Homeostatic blood levels
The hypothalamus is an integral part of the neuroendocrine system. The anatomy, embryologic development,
and normal function of the hypothalamus are described
here. Pathophysiology of congenital abnormalities and
sent back to the hypothalamus or
the target gland itself to inhibit
hormone synthesis when adequate blood levels are reached.
horaddition, nursing implications of caring for such an infant
mones
and
the
resulting
actions
are addressed.
of the anterior pituitary hormones affect regulation of the
floor and part of the lateral wall
thyroid, adrenal, and gonadal limction as wei as growth and
&he
2).2
somatic development.3-s
the neurosecretory cells, the hypothalamus controls the
release of hormones from the anterior pituitary gland.
The integration of the neurologic and endocrine systems is
bidirectional. Not only does the nervous system affect
endocrine function, but the endocrine system can regulate
functions of the nervous system. The immune system affects
~IYIOTHALAMIC-PITIJlTAIiY
regulation of the neuroendocrine system as well. For example,
The hypothalamus acts as a control center for the autonomcytokines, substances produced by the immune system, have
\vith
been shown to act on the hypothalamus to stimulate or
various lobes of the pituitary gland by two different types of
depress hormone-releasing hormones. Cytokines include
pathways. One is a vascular link with the anterior pituitary,
interleukins, tumor necrosis factor, and interferons.
Interleukin-1 produced by macrophages act on the hypotharior pituitary hormones. The posterior pituitary is an extension
larn~~s
Acting as the core of a negative feedback network, the
hypothalamus secretes liormone~rele3sing
tither
pituit3rv hormones. Because of the close interaction be0lreen the
hypothalamus and the pituitary gland, the nervous system has
N I: 0 s .\ I
to stimulate secretion of corticotropin-releasing liormonc (CRH), which ultimately results in the release of
cortisol from the adrenal cortex. In addition, many immuno-
logically reactive cells actually secrete hormones such as
adrenocorticotropic h o r m o n e (ACTH), previously thought
to originate only in the pituitary gland. The interrelationships
N I: I % 0 II li
FIGURE 1 n External view of the brain.
(A) View of the brain at the end of the fifth week. (B) Similar view at seven weeks. (C) Median section of this brain, showing the medial surface of the
forebrain and midbrain. (D) Similar section at eight weeks. (E) Transverse section of the diencephalon, showing the epithalamus dorsally, the thalamus
laterally, and the hypothalamus ventrally.
Midbrain
Cerebral hemisphere
Forebrain
Optic cup
Olfactory bulb
\
Optic nerve
Epithalamus
Sulcus limitans
Mamillary body
Level of section E
lnfundibulum
Optic chiasma
Ependymal roof
Epithalamus
Thalamus
Hypothalamic sulcus
Hypothalamus
From: Moore KL, and Persaud TVN. 1998. The Developing Human: C/inico//y Oriented Embryology, 6th ed. Philadelphia: WB Saunders, 471. Reprinted by
permission.
FIGURE 2 n The hypothalamic reqion of the brain.
FIGURE 3 = Hypothalamic releasing factors and actions of anterior
pituitary hormones.
Mature central nervous system
Hypothalamus
Somotostatin*
GHRH
CRH
TRH
GnRH
PH
.hypothalamus
(dfencephalon)
Midbrain
(mesencephalon)
(myelencephalon)
from: Kandel ER, Schwartz JH, and Jesse1 TM. 1991. Principles of Neural
Science, 3rd ed. New York: Elsevier, 299. Reprinted by permission.
?I
Body
growth
between the immune, neurologic, and endocrine systems
affect cellular communication in such an integrated way that
these systems together are referred to as the neuro-endocrineimmune system.2>3
Adrenal
cortex
1
glucocorticoids
mineralocorticoids
sex hormones
TSH
1
FSHJA
Proloctin
1
Thyroid Ovaries Testes Ovaries Mammary
gland
gland
1
Thyroxine
I
Estrogen
I
Progesterone
HORMONE SECRETION
* Indicates action of somatostatin.
Adapted from: Meyers FH, Jawetz E, and Goldfien A. 1972. Review of
Medical Pharmacology, 3rd ed. Los Altos, California: Lange, 390.
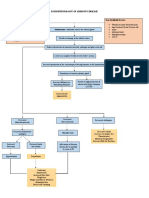
Appropriate levels of endocrine hormones are maintained
by negative feedback. Hypothalamic hormones control the
inhibition or release of hormones from the anterior pituitary
via the hypothalamic-pituitary axis (Figure 3). A hypothalamic releasing hormone stimulates the adenohypophysis (anterior pituitary) to produce a specific hormone. The hormone
released by the anterior pituitary then stimulates a specific
endocrine gland to produce another hormone, which in turn
acts on a specific target organ. The specific hormone is
released until the physiologic level is achieved. A critical or
homeostatic blood level determines when the hypothalamus
stops secreting releasing hormones. Pituitary hormones affect
the fimction of target tissues by altering cellular chemistry,
adjusting cell membrane permeability, or acting on a cell as a
whole.5
Hypothalamic hormones include growth hormone-releasing hormone (GHKH), somatostatin (SST), CRH, thyrotropin-releasing hormone (TRH), gonadotropin-releasing
hormone (GnRH), and prolactin-inhibiting hormone (PIH).
Growth hormone-re!easinH horvnone stimulates the anterior
pituitary to release growth hormone. The major target tissues
of growth hormone are the liver and adipose tissues. Growth
hormone has powerful effects on growth and metabolism,
including linear growth; stimulation of bone and cartilage
growth; stimulation of insulin-like growth factor (IGF- 1 );
increased DNA, RNA, and protein synthesis; elevation of
blood glucose levels; promotion of positive nitrogen balance;
and increased fat mobilization. Somatostatin inhibits the anterior pituitary release of growth hormone (GH), thyroid-stimulating hormone (TSH), insulin, glucagon, gastrin, and
prolactin.3,4
Corticotropin-releasing hornzone stimulates the anterior
pituitary to secrete ACTH. Adrenocorticotropic hormone
controls the function of the adrenal cortex and stimulates
release of glucocorticoids, mineralocorticoids, and some weak
androgcns.
Tbyrotvopivt-releasing horvnone stimulates the anterior pituitary to release TSH and prolactin. The major target tissue of
TSH is the thyroid gland, whereas the mammary gland is the
target tissue of prolactin.
Gonadotropin-releasing hormone stimulates the anterior
pituitary to release follicle-stimulating hormone (FSH) and
luteinizing hormone (LH), which target the ovaries and the
testes, controlling reproductive function.
Prolactin-inhibitintj hormone inhibits the release of proIactin from the anterior pituitary. Unlike the secretion of
other pituitary hormones, the secretion of prolactin is
increased in the absence of hypothalamic influences.
Dopamine, the most important PIH, suppresses all aspects of
prolactin synthesis and secretions.6
The hypothalamus also secretes the prohormones that are
responsible for the stimulation of antidiuretic hormone
(ADH, arginine) and oxytocin from the posterior pituitary.
Antidiuretic hormone is important in the regulation of plasma
osmolaliqr; it increases the permeability of the distal tubule
and collecting duct of the renal nephron, resulting in reabsorption ofwater and reduction in plasma osmolality and concentration ~furinc.~~
Both the anterior and posterior hypothalamus control
thermoregulation in response to skin receptors. The anterior
hypothalamus is temperature sensitive and controls heat loss
mechanisms. The posterior hypothalamus is the site of the
setpoint, or threshold temperature; heat production and loss
are regulated to maintain the core temperature within a range
determined by the setpoint. The posterior hypothalamus is
the central controller of responses to cold and heat stimuli,
receiving input from central and peripheral receptors. With
cold stress, the thcrmorcgulatory center acts to conserve heat
or increase heat production. Thermoregulation is more difficult in the neonate because of the thinner layer of subcutaneous fat and larger surface-to-volume ratio, especially in
preterm infants. 118 The threshold for heat production
depends more on skin temperatures in the neonate than in
adults. As a result, cold responses are related more to skin
temperature than to core temperature changes.,9
PATHOPHYSIOLOGY
The etiology of hypothalamic dysfunction in preterm and
term infants includes congenital abnormalities, intraventricular hemorrhage, bacterial meningitis, tumors, trauma, and
kernicterus. Symptoms and signs of hypothalamic dysfunction
include sexual abnormalities (hypogonadism in neonates),
diabetes insipidus (DI), somnolence, thermodysregulation,
and sphincter disturbance.
Hypothalamic injury causes decreased secretion of most
pituitary hormones, but can cause hypersecretion of horIIIOIICS normally under inhibitory control by the hypothalam u s . Impairment of inhibitory control can lead to
inappropriate ADH secretion, resulting in the syndrome of
inappropriate secretion of antidiuretic hormone (SIADH).6
Characteristic presenting signs of SIADH include weight
gain, edema, and hyponatremia with low plasma osmolality.
Urine output may be low, with high specific gravity and urine
sodium levels. Any disorder or process that interferes with
input to the hyl-othalamus-sLlcl1 as pulmonary diseases, central ner\~ous system disorders, hvpothyroidislii, or drugs that
affect the central nervous sys;em-may cause SIADH.O
Treatment often consists of tluid restriction, and diuretics ma!
be used in sonic iiistmccs.5
Congenital Malformation
Hypothalamic dysf&iction from congenital malformations
of the brain or hypothalamus is a common cause of hypopituitarism. Infants with congenital GH deficiency often have an
abnormal pituitary stalk and hypoplasia of the anterior pituitary. Holoprosencephaly, resulting from an abnormal midline
development of the embryonic forebrain, is typically associated
with hypothalamic insufficiency. Facial dysmorphism of holoprosencephaly ranges from cyclopia to hypertelorism, as well as
absence of the nasal septum, midline clefts of the palate or lip,
and sometimes a central single incisor. GH deficiency and
other pituitary hormone deficiencies may be present.
Congenital hypothyroidism occurs in 1 in 5,000 newborns. It is generally classified as primary, secondary, or tertiary. Infants with congenital hypothyroidism, whether
attributable to primary, secondary, or tertiary failure, appear
clinically normal at birth. Most are diagnosed in the first
three months of life through newborn screening programs
because of failure to thrive and grow and other problems.12
Clinical signs and symptoms of congenital hypothyroidism in
infancy include umbilical hernia, dry skin, large tongue,
hypotonia, inactivity, mottled skin, prolonged jaundice, low
birth weight, poor feeding, transient hypothermia, and large
fontanels.lJ3 Primary hypothyroidism is most often caused
by developmental defects such as ectopic thyroid, thyroid
hypoplasia, or agenesis. l4 Secondary and tertiary hypothyroidism are due to failure of secretion of TSH and TRH
from the pituitary and hypothalamus, respectively.15
Congenital hypothyroidism due to hypothalamic-pituitary
defects results in ineffective TSH stimulation of thyroid hormone secretion and can be caused by a variety of abnormalities in TSH synthesis and metabolism. These include
anomalous hypothalamic or pituitary development, isolated
or familial deficiencies in TRH or TSH secretion, or TSH
deficiency in association with other pituitary hormone deficiencies. Hypothalamic-pituitary hypothyroidism is rare. The
combined prevalence of these abnormalities is approximately
1 in 60,000 to 140,000 live births. Infants with primary
hypothyroidism have low serum T4 and high TSH concentrations in neonatal blood samples, and infants with hypothalanic-pituitary defects have low T4 and normal plasma TSH
levels. As a result, infants with TSH deficiency are not detected by most screening programs, which report as positive only
those infants with elevated plasma TSH levels. An infant with
a low free T4 should be carefully examined for evidence of
hypothyroidism, and other tests of pituitary function should
be performed. A subnormal TSH response to TRH confirms
a diagnosis of pituitary TSH deficiency; a normal or prolonged peak level of TSH atier TKH stimulation supports a
diagnosis of hypothalamic TIU3 deficiency.,
Diabetes insipidus is a disease ofantidiuretic hormone deliciency. Central DI is most often caused by a destructive lesion
;iffecting the Iieuroh~poph~selil system. Infants with central
N I: I \x o I: 1;
DI have dilute urine compared to plasma osmolality and low
to undetectable levels of plasma ADH. The symptoms resolve
after administration of ADH. Although transient DI may
follow any injury to the neurohypophysis, permanent DI
occurs only when damage is high in the pituitary stalk.6
Other disorders associated with hypothalamic hypofunction include hypothalamic dwarfism; Kallmanns syndrome,
idiopathic hypogonadotropic hypogonadism, and fertile
eunuch syndrome associated with G&H deficiency; and tertiary adrenal insufficiency. Diencephalic syndrome, a rare disorder associated with GH hypersecretion, is almost always a
result of hypothalamic neoplasm.
CASE STUDY
Baby girl (BG) C was delivered at 40 weeks gestational age
(by dates) to a 21.year-old mother whose pregnancy was
uncomplicated. Fetal tachycardia developed about an hour
before delivery. After six hours of labor, the infant was delivered vaginally with the assistance of low forceps. The infant
was dusky and required mask oxygen for approximately two
minutes. Apgars were 7 at one minute and 9 at five minutes.
Because the tachycardia continued at ten minutes of age and
BGC was noted to be very pale, she was transferred to the
NICU for fluid resuscitation for suspected hypovolemia.
A sepsis workup was performed and antibiotics started.
Tachycardia continued over the next few hours (-200 bpm).
Multiple fluid boluses were given with some improvement in
tachycardia and peripheral perfusion. The infants platelet
count was 73,000/mm3 initially, and a platelet transfusion
was given. Physical examination revealed prominent molding
of the head and a large cephalohematoma. Neurologic examination was unremarkable at admission.
Within nine hours of birth, BGC was having frequent periods of shallow respirations and apnea, resulting in desaturations and requiring almost constant stimulation. She was
intubated and placed on mechanical ventilation. Her hematocrit level dropped precipitously (from 54 mg/dl to 27
mg/dl) at about 14 hours of age, necessitating a packed red
blood cell (PRBC) transfusion. Increased prothrombin
time/partial prothrombin time (PT/lTT), decreased fibrinogcn, and fibrin split products were also noted, consistent with
disseminated intravascular coagulation (DIC). Coagulation
studies improved after further platelet, fresh frozen plasma
(FFP), and PRBC transfusions. BGC also required initiation
ofvasopressors for hypotension at 17% hours of age.
A cranial ultrasound done at 12 hours of age was normal.
The infant bcgnn exhibiting seizure activity (arching, swimming motions, and tonic positioning), as well as prolonged
clonus, at approximately 13 hours of age. She was gilzen a
loading dose of phenobarbital and, subsequently, phenytoin
to control seizures. Her neurologic status changed dramatitally; she had only occasional spontaneous movements, intet-mittent respiratory efforts, and minimal response to kyaiii.
N I. 0 K .\ I :\ I
\O I
I). so (1. $1 11 I \IliI I: ?OOO
By 24 hours of age, BGCs pupils were fixed, dilated, and
unresponsive to light, and the infant developed right-sided
exophthalmos. A follow-up cranial ultrasound on day 2 of life
revealed an area that was hyperechoic and was felt to be a
focal parenchymal hemorrhage or periventricular leukomalacia. A highly abnormal EEG on day 3 of life showed low
amplitude background and was compatible with a severe
bicortical dysfimction, carrying a poor clinical prognosis in
light of the infants clinical history. Concurrently, the infants
urine output increased and urine was very dilute. Serum sodium and serum osmolality were elevated (150 mg/dl and 3 15
mg/dl respectively), and glucose levels were increased. The
infant was diagnosed with diabetes insipidus and was treated
with desmopressin (DDAVP). Synthroid was started at 25 pg
once daily in response to abnormally low TSH and free
thyroxine levels (0.67 milliunits/ml and 0.63 ng/dl, respectively)
A computed tomography (CT) scan of the brain at one week
of age showed an extensive subdural, subarachnoid, and subgaleal hemorrhage and hypoxic degeneration to the entire cerebral area and brain stem. Magnetic resonance imaging also
showed extensive areas of hemorrhage within multiple compartments, suggestive of hypoxic/ischemic injury. The infant continued in a comatose state with no hope of improvement.
Life-sustaining treatments were discontinued after discussion
and agreement with the parents.
This case study illustrates abnormalities in hypothalamic
function that can occur as a result of trauma to the brain and,
specifically, the hypothalamus. Baby Girl C exhibited hyposecretion of thyroid hormone, as well as ADH deficiency,
resulting in central diabetes insipidus.
SUMMARY
Although the hypothalamic-pituitary axis provides oversight for the endocrine system, regulation may be dysfunctional as a result of brain insult, disease, or genetic
abnormality or in infants who are premature. Measurement of
key hormones, such as thyroid hormone or cortisol, can give
insight into possible deficiencies. If found, deficiencies of all
major hormones can be replaced. Such treatment may be critical in improving morbidity and avoiding unnecessary mortnlity in these infants.
Treatment or prevention of the etiologies responsible fol
inappropriate hormone secretion should be implemented as
soon as hypothalamic disease is diagnosed. Early detection
and treatment in some cases \vill improve long-term outcomes. In other cases, supportive care of the infant and farnil)
is the only option. (3)
N I: I \\ o I< h:
2. Toto KH. 1994. Endocrine nnd Metabolic Deranflements:
Clinical Relevance in the Critically Ill. Flower Mound, Texas:
Barbara Clark Mims Associates, 34.
3. Toto KH. 1994. Endocrine physiology: A comprehensive review.
Critical Care Nursing Clinics of North America 6(4): 637-659.
4. Ramsey I. 1986. A Synopsis of Endocrinology and Metabolism, 3rd
ed. Bristol, England: John Wright.
5. Gamblian V, et al. 1998. Assessment and management of
endocrine dysfimction. In Comprehensive Neonatal Nursing: A
Physiologic Perspective, 2nd ed., Kenner C, Lott JW, and
Flandcrmeyer AA, cds. Philadelphia: WB Saunders, 476495.
6. Reichlin S. 1992. Neuroendocrinology. In Williams Textbook of
Endocrinology, 8th ed., Wilson JD, and Foster DW, eds.
Philadelphia: WB Saunders, 165-248.
7. Hedge GA, Colby HD, and Goodman RL. 1987. Clinical
Endocrine Physiology. Philadelphia: WB Saunders.
8. Guyton AC. 1992. Function of the Human Body, 5th ed.
Philadelphia: WB Saunders, 404.
9. Mestyan J, et al. 1964. Surface temperature versus deep body
temperature and the metabolic response to cold of hypothermic
premature infants. Biology of the Neonate 1: 230.
10. Scheithauer BE, et al. 1997. Neurohypophysis and hypothalamus. In Bloodworths Endocrine Pathology, 3rd ed., Lechago J,
and Gould VE, eds. Baltimore: Lippincott Williams & Wilkins,
25-83.
11. Reiter EO, and Rosenfeld. 1992. Normal and aberrant growth.
In Williams Textbook of Endocrinology, 8th ed., Wilson fD, and
Foster DW, eds. Philadelphia: WB Saunders, 1427-1508.
12. Miculan J, et al. 1993. Congenital hypothyroidism: Diagnosis
and management. Neonatal Network 12(6): 25-34.
13. Polk DH, and Fisher DA. 1996. Thyroid disorders. In Intensive
Care of the Fetus and Neonate, Spitzer AR, ed. St. Louis: MosbyYear Book, 958-969.
14. Gamella TL, et al. Neonatology: Management, Procedures, OnCall P r o b l e m s , Diseases, a n d Drzgs, 4th ed. Stamford,
Connecticut: Appleton & Lange, 546-549.
15. Moshang T, and Thornton P.S. 1994. Endocrine disorders. In
Neonatology: Pathophysiology and Management of the Newborn,
4th ed., Avery GB, Fletcher MA, and MacDonald MG, eds.
Philadelphia: Lippincott Williams & Wilkins, 774-791,
About the Author
Mary Settle is a neonatal nurse practitioner in the NICU at Scott &
White Memorial Hospital in Temple, Texas. Her undergraduate degree
is from Lanston University, and she received her masters degree porn the
University of Maryland in 1998. Ms. Settle is a member of NANN.
For &rther information, please contact:
Mary Settle, RNC, MS, ARNP
Scott & White Memorial Hospital
Neonatal Intensive Care Unit
2401 South 31st Street
Temple, TX 76508
E-mail: msettle@mailcity.com
We Mapped Out Our
Now What About
Nursing at Parkland is a higher calling.
A place to raise your skills to new levels,
and elevate your capacity for leadership.
Youll work hard, and enjoy greater respect and
satisfaction than you ever thought possible. Youll
become the very best nurse you can be, because there
are no limits at Parkland.
Parkland offers retirement plan options, tuition
reimbursement, flexible paid time off plus medical
and dental options.
Health &Hospital System
Equal Opportunity Empkyer
NEONATXI
1-f
Nurse Recruitment
5201 Harry Hines Blvd.
Dallas, TX 75235
l-800-527-0333
FAX 2 14-590-8991
www.swmed.edu/homeqages/parkland
Você também pode gostar
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (894)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (119)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2219)
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- 68-year-old Man with Breathing Problems Due to IV Fluid OverdoseDocumento3 páginas68-year-old Man with Breathing Problems Due to IV Fluid OverdoseAnna-Marie Hanson100% (15)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- 002367889dissecting The USMLE - BookmarkedDocumento629 páginas002367889dissecting The USMLE - BookmarkedPharAway100% (6)
- Adrenal Function TestDocumento26 páginasAdrenal Function TestSaroja Veeresh83% (6)
- Problems of Choice IJSDL 3.2Documento63 páginasProblems of Choice IJSDL 3.2Edvard LjulkoAinda não há avaliações
- 34319Documento11 páginas34319Akbar GazaliAinda não há avaliações
- Self-Directed Learning Scale For Nurses-AanDocumento10 páginasSelf-Directed Learning Scale For Nurses-AanAkbar GazaliAinda não há avaliações
- Rice Spearman DissertationDocumento102 páginasRice Spearman DissertationAkbar Gazali100% (1)
- July 1991 GDocumento5 páginasJuly 1991 GAkbar GazaliAinda não há avaliações
- Neonatal Respiratory Distress 4Documento4 páginasNeonatal Respiratory Distress 4Akbar GazaliAinda não há avaliações
- Educational Strategies Associated With Development of Problem-Solving, Critical Thinking, and Self-Directed LearningDocumento12 páginasEducational Strategies Associated With Development of Problem-Solving, Critical Thinking, and Self-Directed LearningAkbar GazaliAinda não há avaliações
- Full TextDocumento116 páginasFull TextAkbar GazaliAinda não há avaliações
- Epistemological Beliefs and Self-Directed LearningDocumento138 páginasEpistemological Beliefs and Self-Directed LearningAkbar GazaliAinda não há avaliações
- Review Article: Tuberculous Meningitis: Diagnosis and Treatment OverviewDocumento10 páginasReview Article: Tuberculous Meningitis: Diagnosis and Treatment OverviewMeldaAinda não há avaliações
- Antibiotic For AURIDocumento11 páginasAntibiotic For AURIYani Dwi PratiwiAinda não há avaliações
- Growth Chart For Boys Birth To 36 MonthsDocumento2 páginasGrowth Chart For Boys Birth To 36 MonthsCarlos TejedaAinda não há avaliações
- Full TextDocumento116 páginasFull TextAkbar GazaliAinda não há avaliações
- CG 69 Full GuidelineDocumento121 páginasCG 69 Full GuidelineIras Diah DiahAinda não há avaliações
- Acute Flaccid ParalysisDocumento3 páginasAcute Flaccid ParalysisMobin Ur Rehman KhanAinda não há avaliações
- Review Article: Tuberculous Meningitis: Diagnosis and Treatment OverviewDocumento10 páginasReview Article: Tuberculous Meningitis: Diagnosis and Treatment OverviewMeldaAinda não há avaliações
- 01-04-14 KetDocumento6 páginas01-04-14 KetAkbar GazaliAinda não há avaliações
- Revisi MR Akbar 12 Mei 2014 Kala I Fase Aktif Macet + Retensio Plasenta Dan IUGRDocumento31 páginasRevisi MR Akbar 12 Mei 2014 Kala I Fase Aktif Macet + Retensio Plasenta Dan IUGRAkbar GazaliAinda não há avaliações
- Name: Mrs. E Age: 26 Yo RM: 538866 Adress: Narmada Admitted: May, 18 2012 at 02.51 Name: Mrs. E Age: 26 Yo RM: 538866 Adress: Narmada Admitted: May, 18 2012 at 02.51Documento5 páginasName: Mrs. E Age: 26 Yo RM: 538866 Adress: Narmada Admitted: May, 18 2012 at 02.51 Name: Mrs. E Age: 26 Yo RM: 538866 Adress: Narmada Admitted: May, 18 2012 at 02.51Akbar GazaliAinda não há avaliações
- Morning Report March, 24: Supervisor: Dr.H. Doddy A.K., Spog DM: Akbar, Ariq, AsriDocumento9 páginasMorning Report March, 24: Supervisor: Dr.H. Doddy A.K., Spog DM: Akbar, Ariq, AsriAkbar GazaliAinda não há avaliações
- Vol11No2 Pg68-69Documento2 páginasVol11No2 Pg68-69ikawyuAinda não há avaliações
- Supervisor: Dr. Agus Thoriq, Spog DM: AkbarDocumento31 páginasSupervisor: Dr. Agus Thoriq, Spog DM: AkbarAkbar GazaliAinda não há avaliações
- 21-03-14 KPD + Atonia UteriDocumento18 páginas21-03-14 KPD + Atonia UteriAkbar GazaliAinda não há avaliações
- 22-02-14 KPD + OligohidramnionDocumento12 páginas22-02-14 KPD + OligohidramnionAkbar GazaliAinda não há avaliações
- Supervisor: Dr. Juliawan, Spog DM: Ery, Heri, Mamat, AsriDocumento11 páginasSupervisor: Dr. Juliawan, Spog DM: Ery, Heri, Mamat, AsriAkbar GazaliAinda não há avaliações
- 28-02-14 PK I F.latenDocumento12 páginas28-02-14 PK I F.latenAkbar GazaliAinda não há avaliações
- 21-03-14 KPD + Atonia UteriDocumento18 páginas21-03-14 KPD + Atonia UteriAkbar GazaliAinda não há avaliações
- MR Jaga Sabtu 17 Mei 2014Documento10 páginasMR Jaga Sabtu 17 Mei 2014Akbar GazaliAinda não há avaliações
- Morning Report March, 24: Supervisor: Dr.H. Doddy A.K., Spog DM: Akbar, Ariq, AsriDocumento9 páginasMorning Report March, 24: Supervisor: Dr.H. Doddy A.K., Spog DM: Akbar, Ariq, AsriAkbar GazaliAinda não há avaliações
- Negative Feedback - Wikipedia, The Free Encyclopedia PDFDocumento11 páginasNegative Feedback - Wikipedia, The Free Encyclopedia PDFVanessa HollandAinda não há avaliações
- Endocrine SystemDocumento24 páginasEndocrine Systemapi-290318217Ainda não há avaliações
- 45 Lecture PresentationDocumento95 páginas45 Lecture PresentationCourtney TaylorAinda não há avaliações
- Endocrine System - outlINEDocumento4 páginasEndocrine System - outlINERalph NilloAinda não há avaliações
- Endocrinology InterpretationDocumento382 páginasEndocrinology InterpretationHernanda Adi Purwangga100% (7)
- Assessing Clients with Endocrine and Metabolic DisordersDocumento4 páginasAssessing Clients with Endocrine and Metabolic DisordersFelimon BugtongAinda não há avaliações
- New Advances in Diagnosis and Treatment of Cushing SyndromeDocumento12 páginasNew Advances in Diagnosis and Treatment of Cushing SyndromeIka AnisaAinda não há avaliações
- Endocrine SystemDocumento11 páginasEndocrine SystemPunong Grande NHS Banga NHS Annex (R XII - South Cotabato)Ainda não há avaliações
- What Is Adrenal InsufficiencyDocumento17 páginasWhat Is Adrenal InsufficiencygammuacAinda não há avaliações
- Cushing's DiseaseDocumento1 páginaCushing's DiseaseAdeline N. OmeneAinda não há avaliações
- Benefits and Harm of Systemic Steroids For ShortDocumento27 páginasBenefits and Harm of Systemic Steroids For ShortSebastian DíazAinda não há avaliações
- CH 52 Assessment and Management of Patients With Endocrine DisordersDocumento15 páginasCH 52 Assessment and Management of Patients With Endocrine Disordersfiya33Ainda não há avaliações
- Endocrine Dysfunction: Adrenal & Pituitary: Endocrine System Endocrine ReviewDocumento102 páginasEndocrine Dysfunction: Adrenal & Pituitary: Endocrine System Endocrine ReviewCalimlim KimAinda não há avaliações
- 2011 Semifinal AnswersDocumento43 páginas2011 Semifinal AnswersSamsamaAinda não há avaliações
- Adrenal Gland DisordersDocumento3 páginasAdrenal Gland DisordersJem Fabico Dee-AhrAinda não há avaliações
- Overtraining Syndrome in The Athlete: Current Clinical PracticeDocumento7 páginasOvertraining Syndrome in The Athlete: Current Clinical PracticeJosé Luis OjedaAinda não há avaliações
- Consideration of Steroids For Endodontic Pain MarshallDocumento11 páginasConsideration of Steroids For Endodontic Pain MarshallcarlafacchinAinda não há avaliações
- Impact of Stress Management on Student LearningDocumento33 páginasImpact of Stress Management on Student LearningImran100% (1)
- The Mechanisms of Postpartum DepressionDocumento70 páginasThe Mechanisms of Postpartum Depressionazniahsyam8269Ainda não há avaliações
- Clinical BiochemistryDocumento71 páginasClinical BiochemistryMuhamed ArsalanAinda não há avaliações
- Alterations in Physiology and Anatomy During Pregnancy PDFDocumento12 páginasAlterations in Physiology and Anatomy During Pregnancy PDFAsh AmeAinda não há avaliações
- PPT All Endocrine PharmacologyDocumento143 páginasPPT All Endocrine PharmacologyabenezergebrekirstosAinda não há avaliações
- The Slings and Arrows of Daily LifeDocumento17 páginasThe Slings and Arrows of Daily Lifeneurogenics_18570782Ainda não há avaliações
- American Handbook of Psychiatry - Vol7 PDFDocumento2.079 páginasAmerican Handbook of Psychiatry - Vol7 PDFLidia Maria BăloiAinda não há avaliações
- Chapter11 PDFDocumento24 páginasChapter11 PDFsgw67Ainda não há avaliações
- Pathophysiology of Addison's Disease ExplainedDocumento2 páginasPathophysiology of Addison's Disease ExplainedHanna NocumAinda não há avaliações
- Endocrine SystemDocumento3 páginasEndocrine Systemyessii_rahmawatiAinda não há avaliações