Escolar Documentos
Profissional Documentos
Cultura Documentos
335
Enviado por
ahmrakDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
335
Enviado por
ahmrakDireitos autorais:
Formatos disponíveis
The EFSA Journal (2006) 335, 1-10
Opinion of the Scientific Panel on Additives and Products or
Substances used in Animal Feed on the safety and efficacy of the
product Biomin IMB 52 a preparation of Enterococcus faecium as a
feed additive for chickens for fattening in accordance with
Regulation (EC) No 1831/2003
(Question No EFSA-Q-2005-020)
Adopted on 8 of March 2006
SUMMARY
Biomin IMB 52 is a microbial feed additive based on a single strain of Enterococcus
faecium DSM 3530. This product has been provisionally authorized at Community level
for calves. The notifier is now seeking authorization for an extension of use for chickens
for fattening.
The European Food Safety Authority (EFSA) has been requested to deliver an opinion on
the safety of Biomin IMB 52 for chickens for fattening, the consumer, the user of the
product and the environment and its efficacy, when used as a feed additive in
feedingstuff at the recommended doses (2.5 x 108 2.5 x 109 CFU kg-1).
The results of three growth trials provide evidence that inclusion of E. faecium at a
minimum dose of 5 x 108 CFU kg-1 from Biomin IMB 52 in complete feed improves
weight gain of chickens for fattening.
The FEEDAP Panel considers Biomin IMB 52 safe for the target animals. No adverse
effects were found in chickens given Biomin IMB 52 at ten-times the maximum
recommended dose over the entire production period. Microbiological analysis of the
gut flora of birds given Biomin IMB 52 at the minimum recommended dose did not
show any significant differences compared to control animals.
MIC values for all antibiotics tested were below the FEEDAP breakpoints indicating the
absence of resistance against antibiotics of human and veterinary importance. The E.
faecium DSM 3530 was also shown free from known virulence determinants as well as
of genes encoding sex pheromones.
The safety for the consumer, user and the environment of this product have been
previously addressed by SCAN with a favourable outcome. The FEEDAP Panel is not
aware of any new information that would require a revision of these conclusions.
Keywords: Enterococcus faecium, chickens for fattening, probiotic, safety assessment,
efficacy
Opinion on Biomin IMB 52 for chickens for fattening
2/10
BACKGROUND
Regulation (EC) No 1831/20031 establishes the rules governing the Community
authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that
Regulation lies down that any person seeking an authorisation for a feed additive or for
a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from the company Biomin Innovative
Animal Nutrition GmbH2 for authorisation of the product Biomin IMB 52 to be used as a
feed additive for chickens for fattening (category: zootechnical additives; functional
group: gut flora stabilisers) under the conditions described in Table 1. According to
Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the
application to the European Food Safety Authority (EFSA) as an application under Article
4.1 (authorisation of a feed additive or new use of a feed additive). EFSA received
directly from the applicant the technical dossier in support of this application. According
to Article 8 of that Regulation, EFSA, after verifying the particulars and documents
submitted by the applicant, shall undertake an assessment in order to determine
whether the feed additive complies with the conditions laid down in Article 5. The
particulars and documents in support of the application were considered valid by EFSA
as of 27 of May of 2005.
The additive Biomin IMB 52 is a preparation of Enterococcus faecium (DSM 3530). This
product was provisionally authorised (No 21) for calves up to 6 months of age
(authorisation expired on 28 February 2005).
The safety of this product for calves, consumer, user and environment was assessed by
the Scientific Committee on Animal Nutrition (SCAN) on its opinion on the use of certain
micro-organisms as additives in feedingstuffs (26 September 1997, updated 25 April
2003)3.
TERMS OF REFERENCE
According to Article 8 of Regulation (EC) No 1831/2003 EFSA shall determine whether
the feed additive complies with the conditions laid down in Article 5. Therefore, EFSA
shall deliver an opinion on the safety for the target animals, user and consumer and the
environment and the efficacy of the product Biomin IMB 52, which is a preparation of
Enterococcus faecium (DSM 3530) when used under the conditions described in Table
1.
OJ L 268, 18.10.2003, p.29.
Biomin Innovative Animal Nutrition GmbH. lndustriestrasse 21, A-3130 Herzogenburg, Austria. Company
name changed to Biomin GmbH on 28th May 2005
3 http://europa.eu.int/comm/food/fs/sc/scan/out93_en.pdf
1
2
Opinion on Biomin IMB 52 for chickens for fattening
3/10
Table 1. Register entry, as proposed by the applicant.
Additive
Registration number/EC No/No
(if appropriate)
Enterococcus faecium Biomin IMB 52
Category of additive
Zootechnical additives
Functional group of additive
Gut flora stabilisers
21
Description
Composition,
description
Chemical
formula
Method of
analysis
(if appropriate)
Purity criteria
(if appropriate)
Microbial contaminations:
Total viable count: max. 5OOOO g-1
Coliforms: max. 30 g-1
E. coli: max. 0.3 g-1
Salmonella: negative in 25g
Yeasts and molds: max. 10 g-1
Heavy metals (mg kg-1):
As 12.0
Cd 0.75
Hg 0.2
Pb 30.0
Pure culture of viable
micro-organisms (lactic
acid bacteria
Enterococcus faecium
DSM 3530 Biomin
IMB 52),
Minimum 1.0 x 1011
CFU g-1
Trade name (if appropriate)
Name of the holder of
authorisation (if appropriate)
CFU count SOP
Enterococcus faecium Biomin IMB 52
Biomin Innovative Animal Nutrition GmbH
Conditions of use
Species or
category of
animal
Maximum
Age
Chickens for
fattening
Whole period
until
slaughter
(6-7 weeks)
Minimum
content
Withdrawal
period
(if appropriate)
Maximum content
CFU kg-1 of complete feedingstuffs
2.5 x 108
2.5 x 109
None
Other provisions and additional requirements for the labelling
Specific conditions or restrictions for
use (if appropriate)
None
Specific conditions or restrictions for
handling (if appropriate)
Breathing protection during handling and safety glasses
Post market monitoring
(if appropriate)
Not applicable
Specific conditions for use in
complementary feedingstuffs
(if appropriate)
Maximum Residue Limit (MRL) (if appropriate)
Marker residue
Species or
category of animal
Target tissue(s) or
food products
Maximum content
in tissues
Opinion on Biomin IMB 52 for chickens for fattening
4/10
ASSESSMENT
1.
Introduction
The additive Biomin IMB 52, based on the strain Enterococcus faecium DSM 3530, has
been assessed for its safety by SCAN and provisionally authorised when included in feed
for calves up to 6 months of age (authorisation expired on 28 February 2005). In the
course of these previous assessments the safety for users of the additive and for
consumers of products derived from animals fed with the additive were considered. It
was also concluded that no environmental risk assessment was necessary.
In the present assessment the FEEDAP Panel has considered the efficacy and the safety
of the additive for the additional target species, chickens for fattening, focusing on:
2.
recent data on antibiotic resistance
the tolerance to an overdose of the product
the effects of the product at the recommended dose on the flora of the digestive
tract
recent data on the absence of genes encoding for virulence factors
Characterisation and use of the product
The active agent of the additive Biomin IMB 52 is the bacterial strain Enterococcus
faecium DSM 3530 deposited with DSMZ (Deutsche Sammlung fr Mikoroorganismen
and Zellkulturen) in Braunschweig, Germany. Other components are skimmed milk
powder, glucose, hydrogenated fats (food grade). Traces of fermentation substrate
(glucose, yeast extract) and traces of fermentation products (lactate) represent less than
1% in total. Quality control parameters are in place for heavy metals (cadmium, lead,
arsenic and mercury) and bacterial contaminants (coliforms, Escherichia coli,
Salmonella and yeasts and moulds).
The product is recommended for use for chickens for fattening at the recommended
dose of 2.5 x 108 2.5 x 109 CFU kg-1.
3.
Assessment of the control methods by the Community Reference Laboratory (CRL)
EFSA has verified the CRL report as it relates to the methods used for the control of the
active agent in animal feeds. The Executive Summary of the CRL report can be found in
Annex A.
4.
Efficacy
Experiment 1
A 42-day feeding trial was performed with 500 one day-old chickens (Ross 308) which
were randomly allocated to five groups: a control group without additive (Control) and
four treated groups with Biomin IMB 52 included at 1 x 108 CFU kg-1 (Group A), 2.5 x 108
CFU kg-1 (Group B), 5 x 108 CFU kg-1 (Group C) or 2.5 x 109 CFU kg-1 (Group D). Counts in
the supplemented feed were checked and found to be adequate. Chickens were offered
ad libitum a diet based on maize and soya bean meal supplemented or not with the
additive. Studied parameters included individual live weight of the animals (day 7, 14,
21, 28, 35 and 42), mean feed intake level and mortality level in each group.
For statistical comparison of the body weight gain the GLM procedure was applied. The
live weight of the treated animals from groups B, C and D was significantly higher than
the live weight of control animals (Table 2). Feed intake, feed conversion ratio and
mortality (one value per group) could not be statistically analysed; however values were
similar for all groups.
Opinion on Biomin IMB 52 for chickens for fattening
5/10
Table 2. Live weight of chickens for fattening (42 days)
Biomin IMB 52 (CFU kg-1)
Live weight (kg)
a, b:
Control
1 x 108
2.5 x 108
5 x 108
2.5 x 109
2.194a
2.186a
2.306b
2.302b
2.313b
Means not sharing a common superscript are significantly different (P0.05)
Experiment 2
A 35-day feeding trial was performed with 600 one day-old chickens (Ross 308) which
were divided in two groups (control and treated with Biomin IMB 52 at 5 x 108 CFU kg-1,
and subdivided into three subgroups of 100 birds each). Counts in the supplemented
feed were checked and found to be adequate. Chickens were offered ad libitum a diet
based on maize and soya bean meal supplemented or not with the additive. The
parameters studied included individual live weight of the animals (day 7, 14, 21, 28 and
35), feed intake level, feed conversion ration and mortality in each subgroup.
Data from each group were statistically compared by a one way variance analysis (GLM
procedure). Results concerning the whole experimental period (35 days) indicated no
significant differences between treated and control animals.
Experiment 3
A 42-day feeding trial was performed using 400 one day-old chickens. The chickens
were divided into two groups (control group and Biomin treated group at 5 x 108 CFU kg1) of 200 birds. Each group was further subdivided into four subgroups of 50 chickens
each. Counts in the supplemented feed were checked and found to be adequate.
Chickens were offered ad libitum a diet based on maize, soya bean meal and fullfat
soybean supplemented or not by the additive. The parameters studied included
individual live weight of the animals (day 7, 14, 28 and 42), feed intake level and feed
conversion ration in each subgroup (day 7, 14, 28 and 42), mortality level in subgroup
and carcass evaluation (carcass weight, breast weight, thigh weight and abdominal fat).
Results indicated a significant higher body weight for Biomin IMB 52 treated animals
when compared to control animals (2418 vs. 2321 g, P 0.05). The other zootechnical
parameters were not significantly different between the groups. Mortality was found to
be at a low level in both groups (1% and 1.5%). Regarding carcass evaluation
parameters, Biomin IMB 52 did not affect negatively the quality of animal produce.
Experiment 4
A 42-day feeding trial was performed using 400 one day-old chickens. The chickens
were divided into two groups (control group and Biomin treated group at 2.5 x 108 CFU
kg1) of 200 birds. Each group was further subdivided into four subgroups of 50 chickens
each. Microbial counts in the supplemented feed were checked and found to be
adequate. Chickens were offered a standard pelleted diet based on corn-soybean ad
libitum: a starter diet was given from day 1 to 14, a grower diet from day 15 to 28 and a
finisher diet from day 29 to 42. Water was supplied ad libitum. The parameters studies
included individual live weight of the animals (day 7, 14, 28 and 42), feed intake and
feed conversion ration in each subgroup (day 7, 14, 28 and 42), EPEF (European
Production Efficiency Factor) and mortality level. Data was analysed using the T-Test.
Results indicated a significant higher body weight and average weight gain for Biomin
IMB 52 treated animals when compared to control animals (2576 vs. 2540 g, P 0.05).
The other zootechnical parameters were not significantly different between the groups.
Mortality was found to be at a lower level in the treated group (1.5 vs. 5%).
Opinion on Biomin IMB 52 for chickens for fattening
6/10
Experiment 5
In addition, a digestibility experiment was conducted following the methodology of
Schiemann (1981) with ten chickens (21 day old) divided into two groups: control group
and a group supplemented with Biomin IMB 52 at 5 x 108 CFU kg-1 feed. The birds were
individually kept in cages with individual watering systems. The experiment was divided
into three periods: adaptation (3 days), preparation (5 days) and excrement collection (5
days). The chickens were killed at the age of 35 days in order to perform further
biochemical analysis in gut contents. The parameters studied included organic matter,
crude protein, crude fat and crude fibre analyses in excrements and in feed in order to
investigate the digestibility of the feed and biochemical analysis in gut contents (pH,
VFA, ammonia).
A significant increase of the crude protein digestibility (83.04 vs. 81.24 %) was observed
in the treated group compared to the control group. All other parameters were not
significantly influenced by the additive supplementation.
Conclusions on efficacy
These different experiments contribute to demonstrate the additive efficacy in chickens
for fattening at a minimum dose of 5 x 108 CFU kg-1, not the 2.5 x 108 CFU kg-1 dose
proposed by the Applicant.
5.
Safety for the target animals
5.1. Tolerance study in chickens for fattening
Experiment 1
A 42-day tolerance study with 200 Ross 308 one-day-old chickens randomly divided
between the control group and the tolerance group (supplemented with Biomin IMB 52
at 2 x 1010 CFU kg-1 feed) was reported. Counts in the supplemented feed were checked
and found adequate. Chickens were offered ad libitum a diet based on maize and soy
flour supplemented or not with the additive. Studied parameters included individual live
weight of the animals (day 7, 14, 21, 28, 35 and 42), mean feed intake level in each
group and mortality level in each group. For statistical analysis of the weight gain
parameters the GLM procedure was applied.
Results indicate that the live weight of the Biomin IMB 52 treated animals was
significantly higher than the live weight of control animals. However, feed intake, feed
conversion ratio and mortality (one value per group) could not be statistically analysed,
and so the experiment was considered insufficient to demonstrate the safety for the
target species. Consequently, a second tolerance study was conducted.
Experiment 2
A 42-day tolerance study was carried out with a control group of 200 Ross chickens
(four replications with 50 chickens each, mixed sex, birds kept on wood shavings) and a
group treated with Biomin IMB 52 at ten times the recommended dose (2.54 x 1010 CFU
kg-1 feed). Chickens were offered water and a standard pelleted diet based on cornsoybean ad libitum. The dose of E. faecium DSM 3530 in feed samples was confirmed
by plate counts. A starter diet was given from day 1 to 14, a grower diet from day 15 to
28 and a finisher diet from day 29 to 42.
The following performance parameters were recorded: live weight, daily weight gain,
feed intake per group, feed conversion rate, mortality. Data was analysed using the TTest.
Opinion on Biomin IMB 52 for chickens for fattening
7/10
Animals supplied with an overdose of Biomin IMB 52 showed a significantly (P<0.05)
increased overall live weight. None of the other parameters were affected by the
overdose.
The second experiment indicates that Biomin IMB 52, when administered at a ten times
overdose, is tolerated by chickens for fattening.
5.2. Antibiotic susceptibility of Enterococcus faecium DSM 3530
The susceptibility of E. faecium DSM 3530 to therapeutic antibiotics was evaluated by
using the broth micro-dilution method according to the Clinical and Laboratory Standard
Institute guidelines (NCCLS, 2003). The MIC values were determined for a subset of
fifteen clinically relevant antibiotics and compared with the microbiological breakpoints
defined by the FEEDAP Panel (EFSA, 2005). Based on the obtained data, E. faecium
DSM 3530 was found to be sensitive to a broad range of antibiotics including ampicillin,
streptomycin, kanamycin, neomycin, gentamicin, vancomycin, tetracycline,
chlortetracycline, enrofloxacin, erythromycin, quinupristin/dalfopristin, chloramphenicol,
rifampin, linezolid and to trimethoprim.
5.3. Effects of the product on the microflora of the digestive tract
A 42-day feeding trial was used to assess possible negative influences of Enterococcus
faecium DSM 3530 on intestinal microflora of chickens. At the end of the experiment
(day 42) ileal contents of 12 control and 12 treated chickens (2.5 x 108 CFU kg-1 feed)
were collected for microbial analyses: lactobacilli, enterococci, staphylococci, coliforms
and clostridia. Microbial enumerations in ileal contents from both groups were
compared by a one-way variance analysis (GLM procedure).
Clostridia could not be detected in any of the different ileal samples. No significant
differences were found for the other ileal microbial counts from animals of both groups.
Thus, Enterococcus faecium DSM 3530 does not exert any negative effect of the studied
intestinal bacterial groups.
5.4. Virulence determinants in Enterococcus faecium DSM 3530
The presence of known enterococcal virulence determinants in cells from Enterococcus
faecium DSM 3530 was examined by polymerase chain reaction (PCR) using
oligonucleotide primers as published by Eaton and Gasson (2001). Results indicated
that the Enterococcus faecium DSM 3530 strain was free of the following virulence
markers: the surface protein gene esp, the cytolysin activator cylA gene, the gelatinase
gelE gene, the cylB gene involved in transport of cytolysin, the efaAfs gene encoding an
cell wall adhesion, as well as of genes (cpd, ccf) encoding sex pheromones.
These virulence investigations provide further data which support the safety of the strain
E. faecium DSM 3530
6.
Safety for the consumer, user and the environment
The safety for the consumer, user and the environment of this product have been
previously addressed by SCAN with a favourable outcome. The FEEDAP Panel is not
aware of any new information that would require a revision of these conclusions.
7.
Post-market monitoring
Where a microbial product is based on a normal constituent of the gut microflora, and
its use does not represent a significant change in the exposure for the target animals
and the consumer, the FEEDAP Panel considers that post-market monitoring is not
necessary.
Opinion on Biomin IMB 52 for chickens for fattening
8/10
CONCLUSIONS AND RECOMMENDATIONS
CONCLUSIONS
The data provided demonstrate that the inclusion of Biomin IMB 52 at a dose of 5 x 108
CFU E. faecium kg-1 complete feed improves weight gain of chickens for fattening. The
digestibility experiment supports this positive effect.
The FEEDAP Panel considers Biomin IMB 52 safe for the target animals. No adverse
effects were found in chickens given Biomin IMB 52 at ten-times the maximum
recommended dose over the entire production period. Microbiological analysis of the
gut flora of birds given Biomin IMB 52 at the minimum recommended dose did not
show any significant differences compared to control animals.
MIC values for the tested antibiotics were below the FEEDAP breakpoints indicating the
absence of resistance against antibiotics of human and veterinary importance. The E.
faecium DSM 3530 was shown free from known virulence determinants.
The safety for the consumer, user and the environment of this product have been
previously addressed by SCAN with favourable outcome. The FEEDAP Panel is not aware
of any new information that would require a revision of these conclusions.
RECOMMENDATION
On the basis of the data provided, the FEEDAP Panel is only able to support a minimum
recommended dose of 5 x 108 CFU kg-1 complete feedingstuffs rather than the 2.5 x 108
CFU kg-1 complete feedingstuffs proposed by the applicant. The Register entry should be
modified accordingly.
DOCUMENTATION PROVIDED TO EFSA
1-
Dossier on Enterococcus faecium Biomin IMB 52. January 2005. Submitted by
Biomin Innovative Animal Nutrition GmbH.
2-
Additional data for the registration of Enterococcus faecium Biomin IMB 52 as
feed additive according to Regulation (EC) No 1831/2003. April 2005. Submitted
by Biomin Innovative Animal Nutrition GmbH.
3-
Comments on the questions of the FEEDAP Panel of the European Food Safety
Authority on the Enterococcus faecium Biomin IMB 52 dossier for chickens for
fattening. December 2005. Submitted by Biomin GmbH.
4-
Evaluation report of the Community Reference Laboratory feed additives
authorisation on the methods(s) of analysis for Biomin IMB52 for chickens for
fattening.
5-
Comments from the Member States received through the EFSAnet.
REFERENCES
Eaton, T.J. and Gasson, M.J. 2001. Molecular screening of Enterococcus virulence
determinants and potential for genetic exchange between food and medical isolates.
Appl. Environ. Microbiol. 67, 1628-1635.
EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Panel on
Additives and Products or Substances used in Animal Feed on the updating of the
criteria used in the assessment of bacteria for resistance to antibiotics of human or
veterinary
importance.
(EFSA-Q-2004-079)
http://www.efsa.eu.int/science/feedap/feedap_opinions/993_en.html
Opinion on Biomin IMB 52 for chickens for fattening
9/10
NCCLS (National committee for clinical laboratory standards). 2003. Methods for
dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved
standard sixth edition M7-A6, vol 23 N2, Wayne, Pa: National committee for clinical
laboratory standards. Villanova, PA.
Schiemann, R. 1981. Methodische Richtlinien zur Durchfhrung von Verdauungs
versuchen fr die Futterwertschatzung. Arch Tierernahrung Berlin, 31, 1-19.
SCIENTIFIC PANEL MEMBERS
Arturo Anadn, Margarita Arboix Arzo, Georges Bories, Paul Brantom, Joaquim Brufau
de Barber, Andrew Chesson, Pier Sandro Cocconcelli, Joop de Knecht, Nol Dierick,
Gerhard Flachowsky, Anders Franklin, Jrgen Gropp, Ingrid Halle, Anne-Katrine
Lundebye Haldorsen, Alberto Mantovani, Kimmo Peltonen, Guido Rychen, Pascal
Sanders, Amadeu Soares, Pieter Wester and Wilhelm Windisch
ACKNOWLEDGEMENT
The Scientific Panel on Additives and Products or Substances used in Animal Feed
wishes to thank Professor Atte von Wright for the contribution to this opinion.
Opinion on Biomin IMB 52 for chickens for fattening
10/10
ANNEX A.
Executive Summary of the Evaluation Report of the Community Reference Laboratory
Feed Additives Authorisation on the Method(s) of Analysis for Biomin IMB52 for
chickens for fattening
Biomin IMB 52 is a feed additive, consisting of the active probiotic strain Enterococcus
faecium DSM 3530 and other non-active components, belonging to zootechnical
additives, category 4.
The current application for Biomin IMB 52 seeks the following extension for its use as
feed additive in feedingstuffs when used for chicken for fattening as a gut flora
stabilizer, in the EU.
Concerning the determination of the active substance of Biomin IMB 52
(Enterococcus faecium), in the feed additive, in premixtures and in feedingstuffs, a pour
plate count method was proposed by the applicant to determine viable counts of the
probiotic bacterium of the preparation. The method is quantitative and uses KEA-Agar
(Kanamycine-Esculine-Azide Agar). The method used has a limit of quantification of 100
colony forming units (c.f.u) per gram (g) sample which is considered sufficiently
sensitive. In-house validation studies demonstrate that the method is suitable for
quantification of Enterococcus faecium taking into account validation performance
characteristics obtained. The suitability of the method has been evaluated against an
international guideline for enumeration of E. faecium in premixture and feed. The
obtained performance characteristics fulfilled the criteria defined in this guideline. A
collaborative study of the method and corresponding reproducibility data were not
provided, however, the presented method is very similar to a previously full ring trial
validated method, which uses Bile Esculine Azide (BEA) Agar for quantification of the
active substance in the feed additive, in premixtures and in feedingstuffs. The
enumeration of enterococci on BEA agar showed a relative deviation on repeatability
(RSDr) of 1.5 3.6% and a relative deviation on reproducibility (RSDR) between 2.97.4%. BEA agar was selective for enterococci in the presence of other probiotic microorganisms such as pediococci, lactobacilli and yeast. Characterisation and identification
of the microbial strain of the active substance, E. faecium IMB 52 (DSM 3530), was
carried out using appropriate methodologies.
Information on the composition of all ingredients other than the active agents, including
impurities, physical state of the product, toxins and virulence factors, antibiotic
production and resistance, stability of the additive, other physico-chemical or biological
properties and incompatibilities with other feed ingredients, have been submitted for
the purpose of this extension of the authorisation for the dossier.
In summary, and taking into account the results presented, the CRL finds that the
proposed methods fulfil the requirements for routine control methods to quantitatively
determine the colony forming units present in Biomin IMB 52 in the proposed
concentration range.
For official control we recommend a similar method which was ring-trial validated (J.
Appl. Microbiol. 2002, 93, 781-786).
On the basis of the supplied documentation, no supplementary experimental work
(testing or method validation) is required by the CRL.
Você também pode gostar
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- Oregano Oil Benefits For Infections, Fungus & More - Dr. Axe PDFDocumento7 páginasOregano Oil Benefits For Infections, Fungus & More - Dr. Axe PDFJoseph TupasAinda não há avaliações
- WHO TootlkitDocumento88 páginasWHO TootlkitVivek BasnetAinda não há avaliações
- Natural ProductsDocumento11 páginasNatural ProductsalinumlAinda não há avaliações
- The - Utics and Control of Sheep and Goat Diseases, An Issue of Veterinary Clinics, Food Animal PracticeDocumento262 páginasThe - Utics and Control of Sheep and Goat Diseases, An Issue of Veterinary Clinics, Food Animal PracticeIurydyana Villalobos0% (1)
- The Root Canal Biofilm PDFDocumento369 páginasThe Root Canal Biofilm PDFStephanny BocanegraAinda não há avaliações
- Broiler Farming Income GuideDocumento16 páginasBroiler Farming Income GuideB.p. MugunthanAinda não há avaliações
- SS II.1.1 Update On Typhoid Management - Dr. Adityo Susilo, SpPD-KPTI PDFDocumento34 páginasSS II.1.1 Update On Typhoid Management - Dr. Adityo Susilo, SpPD-KPTI PDFWisnu Yudho HAinda não há avaliações
- Franklin 2005Documento10 páginasFranklin 2005ahmrakAinda não há avaliações
- Held in Conjunction With The American Association of Avian Pathologists and The American Veterinary Medical Associatio 11PSA001Documento4 páginasHeld in Conjunction With The American Association of Avian Pathologists and The American Veterinary Medical Associatio 11PSA001ahmrakAinda não há avaliações
- FT42 Broz PDFDocumento8 páginasFT42 Broz PDFahmrakAinda não há avaliações
- 001 Boerderij Download Wp6381d01Documento2 páginas001 Boerderij Download Wp6381d01ahmrakAinda não há avaliações
- IAF - Radioökologie: 96132 SchlüsselfeldDocumento1 páginaIAF - Radioökologie: 96132 SchlüsselfeldahmrakAinda não há avaliações
- Jas 78 8 2039Documento16 páginasJas 78 8 2039ahmrakAinda não há avaliações
- Effect of Live Yeast and Mannan-Oligosaccharides On Performance of Early-Lactation Holstein Dairy CowsDocumento7 páginasEffect of Live Yeast and Mannan-Oligosaccharides On Performance of Early-Lactation Holstein Dairy CowsahmrakAinda não há avaliações
- Anesthesia-Responding To ComplicationsDocumento6 páginasAnesthesia-Responding To ComplicationsTELGE GIN 1Ainda não há avaliações
- Protease Can Alleviate Negative Effects of CoccidiosisDocumento9 páginasProtease Can Alleviate Negative Effects of CoccidiosisahmrakAinda não há avaliações
- 150 TassinariDocumento6 páginas150 TassinariahmrakAinda não há avaliações
- Zhan 2006Documento6 páginasZhan 2006ahmrakAinda não há avaliações
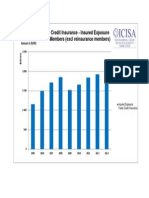
- Trade Credit Insurance - Insured Exposure ICISA Members (Excl Reinsurance Members)Documento1 páginaTrade Credit Insurance - Insured Exposure ICISA Members (Excl Reinsurance Members)ahmrakAinda não há avaliações
- Effect of Supplementation of Enzyme Complex On Growth Performance of Crossbred PigsDocumento5 páginasEffect of Supplementation of Enzyme Complex On Growth Performance of Crossbred PigsahmrakAinda não há avaliações
- At 11 P 127Documento10 páginasAt 11 P 127ahmrakAinda não há avaliações
- Anesthetic 1Documento19 páginasAnesthetic 1ahmrakAinda não há avaliações
- 31 1389780240Documento6 páginas31 1389780240ahmrakAinda não há avaliações
- Detection of Alpha ToxinDocumento1 páginaDetection of Alpha ToxinahmrakAinda não há avaliações
- Broiler Immune HealthDocumento3 páginasBroiler Immune HealthahmrakAinda não há avaliações
- Biological EvaluationDocumento1 páginaBiological EvaluationahmrakAinda não há avaliações
- LSU AgCenter - Printable Copyasd PDFDocumento2 páginasLSU AgCenter - Printable Copyasd PDFahmrakAinda não há avaliações
- Italian approach to controlling aflatoxins in milkDocumento3 páginasItalian approach to controlling aflatoxins in milkahmrakAinda não há avaliações
- Effect of Betaine Source On Nutrient Utilisation of Broilers Challenged With CoccidiaDocumento3 páginasEffect of Betaine Source On Nutrient Utilisation of Broilers Challenged With CoccidiaahmrakAinda não há avaliações
- Aflatoxin in Dairy FeedsDocumento4 páginasAflatoxin in Dairy FeedsahmrakAinda não há avaliações
- Pub 2897 Broiler PRDocumento8 páginasPub 2897 Broiler PRahmrakAinda não há avaliações
- Al 703 e 00Documento4 páginasAl 703 e 00ahmrakAinda não há avaliações
- Pro-Lyte Plus Product InfoDocumento2 páginasPro-Lyte Plus Product InfoahmrakAinda não há avaliações
- DR - Mahmoud El KelawayDocumento9 páginasDR - Mahmoud El KelawayahmrakAinda não há avaliações
- Supersect, ADWIA (Powder) : CompositionDocumento1 páginaSupersect, ADWIA (Powder) : CompositionahmrakAinda não há avaliações
- Antimicrobial ChemotherapyDocumento160 páginasAntimicrobial Chemotherapyokumu atanas0% (1)
- Micro para 15Documento21 páginasMicro para 15ROMAH JANE MENDOZAAinda não há avaliações
- Commercial Essential Oils As Potential Antimicrobials To Treat Skin DiseasesDocumento92 páginasCommercial Essential Oils As Potential Antimicrobials To Treat Skin DiseasesxiuhtlaltzinAinda não há avaliações
- Microbes: What They Do & How Antibiotics Change ThemDocumento4 páginasMicrobes: What They Do & How Antibiotics Change ThemMaheshwaran MahiAinda não há avaliações
- v1N2 P52 FA1Documento6 páginasv1N2 P52 FA1Isha JainAinda não há avaliações
- CHAPTER 9 Micro Lec TranseesDocumento4 páginasCHAPTER 9 Micro Lec TranseesDylan HimoAinda não há avaliações
- Fmicb 10 00053Documento23 páginasFmicb 10 00053hafniomonAinda não há avaliações
- Bauer 2019Documento6 páginasBauer 2019RaffaharianggaraAinda não há avaliações
- Read More About MBT Sepsityper IVD Workflow 1682516329Documento8 páginasRead More About MBT Sepsityper IVD Workflow 1682516329Jose ZelayaAinda não há avaliações
- Jurnal AntibiotikDocumento4 páginasJurnal AntibiotikYuli IndrawatiAinda não há avaliações
- Antibiotics NaturallyDocumento9 páginasAntibiotics NaturallySanjay .P.MAinda não há avaliações
- Editorial: Cellular and Molecular Mechanisms of MycobacteriumDocumento4 páginasEditorial: Cellular and Molecular Mechanisms of MycobacteriumJean.ZapataAinda não há avaliações
- Antibiotic Resistance Dissertation IdeasDocumento4 páginasAntibiotic Resistance Dissertation IdeasCollegePapersHelpUK100% (1)
- Horizon 2007-01Documento6 páginasHorizon 2007-01Lea BucadAinda não há avaliações
- Barrett Honors Thesis DefenseDocumento7 páginasBarrett Honors Thesis Defenseannashawpittsburgh100% (2)
- WHONET 4.data Analysis 1Documento17 páginasWHONET 4.data Analysis 1R.Wenceslas LendambaAinda não há avaliações
- Shona Blair PHD ThesisDocumento200 páginasShona Blair PHD ThesisJoelAinda não há avaliações
- Formulation Ang Evaluationof Herbal Hanitizer Using Psidium Guajava Leaves Extract PDFDocumento3 páginasFormulation Ang Evaluationof Herbal Hanitizer Using Psidium Guajava Leaves Extract PDFCatherine AlmarioAinda não há avaliações
- Antimicrobial Blue Light Inactivation of Pathogenic Microbes - State of The ArtDocumento44 páginasAntimicrobial Blue Light Inactivation of Pathogenic Microbes - State of The ArtJohn KibuukaAinda não há avaliações
- Monuril Vs CiproDocumento7 páginasMonuril Vs Ciproalaa morsyAinda não há avaliações
- Journal of Global Antimicrobial Resistance: Mohammad Ahmad, Asad U. KhanDocumento4 páginasJournal of Global Antimicrobial Resistance: Mohammad Ahmad, Asad U. KhanDesalegnAinda não há avaliações
- Thesis Title PDFDocumento679 páginasThesis Title PDFSabindra MaharjanAinda não há avaliações
- Antimicrobial Stewardship NICEDocumento25 páginasAntimicrobial Stewardship NICESUBHADIPAinda não há avaliações
- Info Sheet Bcid Panel Flm1 PRT 0069 03Documento2 páginasInfo Sheet Bcid Panel Flm1 PRT 0069 03SachinAinda não há avaliações