Escolar Documentos
Profissional Documentos
Cultura Documentos
3,4-DHPEA-EA From Olea Europaea L. Is Effectiveagainst Standard and Clinical Isolates of Staphylococcus SP .
Enviado por
Domitian PascaTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
3,4-DHPEA-EA From Olea Europaea L. Is Effectiveagainst Standard and Clinical Isolates of Staphylococcus SP .
Enviado por
Domitian PascaDireitos autorais:
Formatos disponíveis
Bisignano et al.
Annals of Clinical Microbiology and Antimicrobials 2014, 13:24
http://www.ann-clinmicrob.com/content/13/1/24
RESEARCH
Open Access
3,4-DHPEA-EA from Olea Europaea L. is effective
against standard and clinical isolates of
Staphylococcus sp.
Carlo Bisignano1, Angela Filocamo2, Giovanna Ginestra2, Salvatore V Giofre2, Michele Navarra2,
Roberto Romeo2 and Giuseppina Mandalari2*
Abstract
Background: The aim of the present work was to evaluate the antibacterial effect of 3,4-DHPEA-EA
(methyl-4-(2-(3,4-dihydroxyphenethoxy)-2-oxoethyl)-3-formyl-2-methyl-3,4-dihydro-2H-pyran-5-carboxylate), a
derivate of oleuropein, against a range of Gram-positive bacteria, including ATCC strains, food and clinical isolates.
Methods: The minimum inhibitory concentrations (MICs) of 3,4-DHPEA-EA were determined by the broth
microdilution method and the Bioscreen C.
Results: 3,4-DHPEA-EA was effective against ATCC and clinical isolates of Staphylococcus aureus (MIC values between
125 and 250 g/ml) and ATCC and clinical isolates of Staphylococcus epidermidis (MIC values between 7.81 and
62.5 g/ml). No significant differences were observed between the two solvents (methanol and DMSO) used to
dissolve 3,4-DHPEA-EA.
Conclusions: The results obtained could be used to develop novel therapies for the treatment of skin infections.
Further studies need to be performed to elucidate the formation of 3,4-DHPEA-EA by acid hydrolysis of
oleuropein in the human stomach.
Keywords: Olea europaea L, 3,4-DHPEA-EA, Antimicrobial, Staphylococci
Background
The potential beneficial effects of biophenols from olives
(Olea europaea L.) has been observed in several studies,
with antioxidant, anti-inflammatory and antimicrobial
activities attributed to olive oil [1-3]. Although the
health effects of olive oil were traditionally attributed to
oleic acid, more recent knowledge has shown that the
phenolic fraction plays a crucial role in the reported benefits [4]. Polyphenols that reach the large bowel can beneficially modulate the gut microbial ecosystem increasing the
number of Bifidobacterium spp., Lactobacillus spp. and
Enterococcus spp. which are known for their antiinflammatory, immunoregulatory and cholesterol lowering properties through production of short chain fatty
acids [5,6]. The most biologically relevant compounds
* Correspondence: gmandalari@unime.it
2
Dipartimento di Scienze del Farmaco e Prodotti per la Salute, University of
Messina, Viale Annunziata, 98100 Messina, Italy
Full list of author information is available at the end of the article
contributing to the sensory and nutritional aspects of olives
and olive oil are oleuropein, hydroxytyrosol, quercetin,
ferulic acid, caffeic acid, p-hydroxybenzoic acid, protocatechuic acid, 3,4-dyhydroxyphenylacetic acid (3,4-DHPA),
homovanillic acid and vanilethanediol, whose metabolic
and transcriptional profiling has been recently evaluated
during fruit development [7]. Oleuropein, present in large
quantities in olive tree leaves and in low amounts in extravirgin olive oil, is known to be responsible for the bitter
taste of the oil. We have previously demonstrated that
complexes of olive biophenols with -cyclodextrin were effective decreasing the oil bitterness and preserving from
decomposition during storage [8]. The acid-hydrolysis of
oleuropein in the stomach generates the formation of
a number of metabolites whose distribution and concentration is dependent on the acidity of the gastric
compartment [9]. The dyaldehydes originated by the
cleavage of the -glycosidic bond are unstable in the lipid/
water interface and are converted into the metabolite
2014 Bisignano et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative
Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and
reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain
Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article,
unless otherwise stated.
Bisignano et al. Annals of Clinical Microbiology and Antimicrobials 2014, 13:24
http://www.ann-clinmicrob.com/content/13/1/24
known as transposed secoiridoid or dihydropyranic form
(3,4-DHPEA-EA) [10].
We have previously demonstrated that aliphatic aldehydes from Olea europeaea L. were active against human intestinal and respiratory tract infection strains,
whereas Mycoplasma spp. were sensitive to oleuropein
[11,12]. Other studies have also reported an antibacterial
and antifungal action of both olive leaves and olive
glutaraldehyde-like compounds [13,14]. A range of microbial gastrointestinal pathogens, including Escherichia
coli and Helicobacter pylori, and viruses such as parainfluenza type 3 virus were sensitive to olive oil phenolic
compounds [15-17].
The aim of the present work was to investigate the effectiveness of 3,4-DHPEA-EA against a range of Grampositive bacteria which included ATCC strains and food
and clinical isolates.
Materials and methods
3,4-DHPEA-EA
3,4-DHPEA-EA (methyl-4-(2-(3,4-dihydroxyphenethoxy)2-oxoethyl)-3-formyl-2-methyl-3,4-dihydro-2H-pyran-5carboxylate) was obtained by enzymatic hydrolysis of
oleuropein, as a molecular evolution consequence of the
hemiacetal functionality of the aglycon 2, formed by glycosidic bond cleavage (Figure 1). The lipidic/water interface
promotes the rapid rearrangement of the intermediate
oleuropeinenol 3 into the final stable biomolecule, the
transposed secoiridoid 4, within 5 min [18].
Thus, to a solution of 100 mg of oleuropein, previously
extracted from olive leaves, endogenous -glucosidase
was added at 40C for 6 h in 20 ml of a H2O/CHCl3 1:1
mixture, The mixture was evaporated and purified using
Waters XTerra C18 column on a Varian HPLC system
(H2O/MeCN H2O/MeCN gradient) with a flow rate of
2 ml/min.
Methyl-4-(2-(3,4-dihydroxyphenethoxy)-2-oxoethyl)-3formyl-2-methyl-3,4-dihydro-2H-pyran -5-carboxylate was
obtained as a yellow oil (20% yield). 1H and 13C NMR spectra were in agreement with literature data [10].
Page 2 of 4
Staphylococcus aureus ATCC 51153, Staphylococcus aureus
ATCC 6538P, Staphylococcus aureus ATCC 43300,
Staphylococcus epidermidis ATCC 49134, Staphylococcus epidermidis ATCC 35984, Staphylococcus epidermidis ATCC 12228, Streptococcus pneumoniae ATCC
6003, Streptococcus pyogenes ATCC 19615, Streptococcus
pyogenes ATCC 10782, Listeria monocytogenes ATCC
7644, Listeria monocytogenes ATCC 1392, Enterococcus
hirae ATCC 10541, Moraxella catarrhalis ATCC 8176, 10
food isolates of L. monocytogenes belonging to serotypes
1/2a (7 strains) and 1/2b (3 strains), 14 clinical isolates
of S. aureus obtained from specimens of skin infections
and surgical infections, 13 clinical isolates of S. pneumoniae obtained from hospitalized patients, 14 clinical
isolates of S. pyogenes obtained from hospitalized patients,
16 clinical isolates of M. catarrhalis obtained from ocular
and respiratory tract infections, 13 clinical isolates from
S. epidermidis obtained from orthopedic protesis, 13 isolates of E. fecium and 15 isolates from E. faecalis from
urinary tract infections.
Cultures for antimicrobial activity tests were grown either in Mueller Hinton Broth (MHB, Oxoid, CM0405,
S. aureus, S. epidermidis, L. monocytogenes, E. hirae,
E. fecium, E. faecalis) or Brain Heart Infusion (BHI,
Difco) at 37C (24 h). For solid media 1.5% (w/v) agar
(Difco) was added.
Antimicrobial testing
The minimum inhibitory concentrations (MICs) of 3,4DHPEA-EA solubilized in either methanol or dimethyl sulfoxide (DMSO) were determined by the broth microdilution
method, according to CLSI [19]. The MICs were also performed in the Bioscreen C (Labsystems Oy, Helsinki,
Finland) for all strains as previously reported [20].
All experiments were performed in triplicate on three
independent days. A number of positive and negative controls with selected antibiotics (ampicillin and ciprofloxacin)
and solvents (methanol, DMSO) were included in each
assay.
Results
Microbial strains and culture conditions
Minimum inhibitory concentrations
The following Gram-positive strains were used for the antimicrobial testing and were obtained from the University
of Messinas in-house culture collection (Messina, Italy):
The MIC values of 3,4-DHPEA-EA against the ATCC
strains tested are shown in Table 1. Results of negative
controls indicated the complete absence of inhibition of
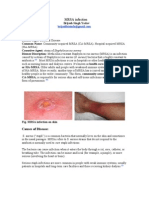
Figure 1 Synthesis of 3,4-DHPEA-EA from oleuropein.
Bisignano et al. Annals of Clinical Microbiology and Antimicrobials 2014, 13:24
http://www.ann-clinmicrob.com/content/13/1/24
resistant. Higher MIC values were obtained with S. aureus
compared to S. epidermidis (Table 2).
Table 1 Minimum inhibitory concentration (MIC) of
3,4-DHPEA-EA against ATCC Gram-positive bacteria
Strain
3,4-DHPEA-EA
S. aureus ATCC 51153
125
S. aureus ATCC 6538P
125
S. aureus ATCC 43300
250
S. epidermidis ATCC 49134
7.81
S. epidermidis ATCC 35984
62.5
S. epidermidis ATCC 12228
15.6
S. pneumoniae ATCC 6003
> 1000
S. pyogenes ATCC 19615
> 1000
L. monocytogenes ATCC 7644
> 1000
L. monocytogenes ATCC 1392
> 1000
E. hirae ATCC 10541
>1000
M. catarrhalis ATCC 8176
>1000
Values are expressed as g ml-1 and represent the mean of three determinations.
3,4-DHPEA-EA: (methyl-4-(2-(3,4-dihydroxyphenethoxy)-2-oxoethyl)-3-formyl-2methyl-3,4-dihydro-2H-pyran-5-carboxylate).
all the strains tested (data not shown). Analogue values
of MICs were obtained with the broth microdilution
method and in the Bioscreen C. No differences in the
MIC values were recorded with the two solvents utilized
(methanol or DMSO). Amongst the Gram-positive bacteria tested, 3,4-DHPEA-EA was active against staphylococci, the most sensitive strains being S. epidermidis
ATCC 49134 and S. epidermidis ATCC 12228, followed
by S. aureus spp. The effect was bacteriostatic rather than
bactericidal.
Table 2 reports the MICs of 3,4-DHPEA-EA against
the clinical and food isolates tested. MIC values of 7.8
and 15.6 g ml-1 3,4-DHPEA-EA, respectively, inhibited
the growth of 50% and 90% of the S. epidermidis strains
tested, whereas 125 and 250 g ml-1 3,4-DHPEA-EA,
respectively, inhibited the growth of 50% and 90% of
the S. aureus strains tested. All the other isolates were
Table 2 Minimum inhibitory concentration (MIC) of
3,4-DHPEA-EA against food and clinical isolates
Strain
Page 3 of 4
MIC 50
MIC 90
S. aureus
125
250
S. epidermidis
7.8
15.6
S. pyogenes
> 1000
> 1000
S. pneumoniae
> 1000
> 1000
M. catarrhalis
> 1000
> 1000
L. monocytogenes
> 1000
> 1000
E. faecalis
> 1000
> 1000
E. fecium
> 1000
> 1000
Values are expressed as g ml-1 and represent the mean of three determinations.
3,4-DHPEA-EA: (methyl-4-(2-(3,4-dihydroxyphenethoxy)-2-oxoethyl)-3-formyl-2methyl-3,4-dihydro-2H-pyran-5-carboxylate).
Discussion
The present study has evaluated the antimicrobial effect
of a metabolite from oleuropein, 3,4-DHPEA-EA, against
a range of Gram-positive bacteria, which included ATCC
strains, food and clinical isolates. We have recently demonstrated that polyphenols from pistachios had a bactericidal effect against S. aureus and L. monocytogenes
[21], whereas almond skin extracts rich in polyphenols
were active against a range of Gram-positive bacteria
[22]. The effect on S. aureus could be used to find potential applications as a topical treatment for S. aureus.
Other authors have reported on the antioxidant and
antimicrobial activities of individual and combined phenolics in Olea europea leaf extract: oleuropein and caffeic
acid were active against Salmonella enteridis, Bacillus
cereus and Escherichia coli and the antimicrobial effect of
the combined phenolics was significantly higher than
those of the individual compounds [23]. Using agar dilution and broth microdilution techniques, Sudjana et al.
[24] found that a commercial olive leaf extract was active against Campylobacter jejuni, Helicobacter pylori
and Staphylococcus aureus with low MIC concentrations (0.310.78% v/v). Another investigation on the
antimicrobial effect of an olive leaf extract showed that
Bacillus subtilis was less susceptible than E. coli, Pseudomonas aeruginosa, Klebsiella pneumoniae and S. aureus
[25]. A commercial olive powder and 4-hydroxytyrosol
were able to inactivate S. aureus and its enterotoxin A,
secreted by the bacteria in 78% of the outbreaks [26]. Although no reports have identified the possible mechanisms of action of the phenolic compounds present in
olive leaf, some authors report the activity of phenolics on
Gram-positive bacteria may be due to the cell wall or cell
membrane disruption together with cell enlargement,
which is more susceptible compared to Gram-negative
strains [27]. Fabiani et al. [28] demonstrated that hydrogen
peroxidase production is responsible for the induction of
apoptosis by hydroxytyrosol on HL60 cells.
In our previous study we demonstrated that oleuropein and hydroxytyrosol were active against ATCC
strains and clinical isolates: the MIC values of hydroxytyrosol ranged between 0.24 and 7.85 g ml-1 for ATCC
strains and between 0.97 and 31.25 g ml-1 for clinically
isolated strains, whereas the MIC values of oleuropein
ranged between 62 and 500 g ml-1 for ATCC strains
and between 31.25 and 250 g ml-1 for clinically isolated
strains [3]. Although oleuropein was found effective
against a range of Gram-positive strains, in the present
work we demonstrated that 3,4-DHPEA-EA, a metabolite obtained by hydrolysis of oleuropein, was only active
against ATCC and clinical isolates of S. aureus and
Bisignano et al. Annals of Clinical Microbiology and Antimicrobials 2014, 13:24
http://www.ann-clinmicrob.com/content/13/1/24
S. epidermidis. The use of Olea metabolites could therefore be tested in combination with traditional antibiotics
in order to identify new mechanisms of synergism and
antibiotic-resistant modulating properties for the development of novel drugs. On the basis of previous investigations on the absorption and metabolism of olive oil
secoiridoids in the small intestine [29], further studies
need to be performed to elucidate the formation of 3,4DHPEA-EA in the stomach.
Conclusions
In summary, the results of the present study showed that a
metabolite from oleuropein was effective against staphylococci and could therefore be a potential source of natural
antimicrobials for the treatment of skin infections. However, further studies are needed to understand the mechanisms responsible for these activities and the obtained
in vitro results need to be translated both in food and
in vivo.
9.
10.
11.
12.
13.
14.
15.
16.
17.
Competing interest
The authors declare no conflict of interest.
Authors contributions
GM designed research, CB, AF, GG, SVG carried out research, GM, MN, RR
analysed results, GM wrote the manuscript. All authors read and approved
the final manuscript.
Acknowledgements
This research was funded by the University of Messina and INBB (Consorzio
interuniversitario Istituto Nazionale di Biostrutture e Biosistemi).
Author details
1
Dipartimento di Scienze Biologiche ed Ambientali, University of Messina,
Salita Sperone 31, 98100 Messina, Italy. 2Dipartimento di Scienze del Farmaco
e Prodotti per la Salute, University of Messina, Viale Annunziata, 98100
Messina, Italy.
Received: 4 February 2014 Accepted: 27 May 2014
Published: 1 July 2014
References
1. Martin-Pelaez S, Covas MI, Fito M, Kusar A, Pravst I: Health effects of olive
oil polyphenols: recent advances and possibilities for the use of health
claims. Mol Nutr Food Res 2010, 57:760771.
2. Cicerale S, Conlan XA, Sinclair AJ, Keast RSJ: Chemistry and health of olive
oil phenolics. Crit Rev Food Sci Nutr 2009, 49:218246.
3. Bisignano G, Tomaino A, Lo Cascio R, Crisafi G, Uccella N, Saija A: On the
in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J Pharm
Pharmacol 1999, 51:971974.
4. Omar SH: Oleuropein in olive and its pharmacological effects. Sci Pharm
2010, 78:133154.
5. Martinez I, Wallace G, Zhang CM, Legge R, Benson AK, Carr TP, Moriyama EN,
Walter J: Diet-induced metabolic improvements in a hamster model of
hypercholesterolemia are strongly linked to alterations of the gut
microbiota. Appl Environ Microbiol 2009, 75:41754184.
6. Trautwein EA, Rieckhoff D, Erbersdobler HF: Dietary inulin lowers plasma
cholesterol and triacylglycerol and alters biliary bile acid profile in
hamster. J Nutr 1998, 128:19371943.
7. Alagna F, Mariotti R, Panara F, Caporali S, Urbani S, Veneziani G, Esposto S,
Taticchi A, Rosati A, Rao R, Perrotta G, Servili M, Baldoni L: Olive phenolic
compounds: metabolic and trabscriptional profiling during fruit
development. BMC Plant Biol 2012, 12:162180.
8. Rescifina A, Chiacchio U, Iannazzo D, Piperno A, Romeo G: -cyclodextrin
and caffeine complexes with natural polyphenols from olive and olive
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
Page 4 of 4
oils: NMR, thermodynamic, and molecular modeling studies. J Agric Food
Chem 2010, 58:1187611882.
Carrera-Gonzales MP, Ramirez-Exposito MJ, Mayas MD, Martinez-Martos JM:
Protective role of oleuropein and its metabolite hydroxytyrosol on cancer.
Trends Food Sci Technol 2013, 31:9299.
Daccache A, Lion C, Sibille N, Gerard M, Slomianny C, Lippens G, Cotelle P:
Oleuropein and derivatives from olives as Tau aggregation inhibitors.
Neurochem Int 2011, 58:700707.
Bisignano G, Lagana MG, Trombetta D, Arena S, Nostro A, Uccella N,
Mazzanti G, Saija A: In vitro antibacterial activity of some aliphatic
aldehydes from Olea europaea L. FEMS Microbiol Lett 2001, 198:913.
Furneri PM, Marino A, Saija A, Uccella N, Bisignano G: In vitro
antimycoplasmal activity of oleuropein. Int J Ant Ag 2002, 20:293296.
Medina E, Brenes M, Garcia A, Romero C, de Castro A: Bactericidal activity
of glutaraldehyde-like compounds from olive products. J Food Prot 2009,
72:26112614.
Pereira AP, Ferreira IC, Marcelino F, Valentao P, Andrade PB, Seabra R,
Estevinho L, Bento A, Pereira JA: Phenolic compounds and antimicrobial
activity of olive (Olea europaea L. Cv. Cobrancosa) leaves. Molecules 2007,
12:11531162.
Medina E, Brenes M, Romero C, Garcia A, de Castro A: Main antimicrobial
compounds in table olives. J Agric Food Chem 2007, 55:98179823.
Romero C, Medina E, Vargas J, Brenes M, de Castro A: In vitro activity of
olive oil polyphenols against Helicobacter pylori. J Agric Food Chem 2007,
55:680686.
Ma SC, He ZD, Deng XL, But PPH, Ooi VE, Xu HX, Lee SH, Lee SF: In vitro
evaluation of secoiridoid glucosides from the fruit of Ligustrum lucidum
as antiviral agents. Chem Pharm Bull 2001, 49:14711473.
Bianco AD, Piperno A, Romeo G, Uccella N: NMR experiments of oleuropein
biomimetic hydrolysis. J Agric Food Chem 1999, 47:36653668.
Clinical and Laboratory Standards Institute, M100S18: Performance
Standards for Antimicrobial Susceptibility Testing; Seventeenth Informational
Supplement. Wayne, PA: Clinical Laboratory Standards Institute; 2008.
DArrigo M, Ginestra G, Mandalari G, Furneri PM, Bisignano G: Synergism
and postantibiotic effect of tobramycin and Melaleuca alternifolia (tea
tree) oil against Staphylococcus aureus and Escherichia coli. Phytomedicine
2010, 17:317322.
Bisignano C, Filocamo A, Faulks RM, Mandalari G: In vitro antimicrobial
activity of pistachio (Pistacia vera L.) polyphenols. FEMS Microbiol Lett
2013, 341:6267.
Mandalari G, Bisignano C, DArrigo M, Ginestra G, Arena A, Tomaino A,
Wickham MSJ: Antimicrobial potential of polyphenols extracted from
almond skins. Lett Appl Microbiol 2010, 51:8389.
Lee O-H, Lee B-Y: Antioxidant and antimicrobial activities of individual
and combined phenolics in Olea europaea leaf extract. Bior Technol 2010,
101:37513754.
Sudjana AN, DOrazio C, Ryan V, Rasool N, Ng J, Islam N, Riley TV, Hammer KA:
Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int J
Ant Ag 2009, 33:461463.
Markin D, Duek L, Berdicevsky I: In vitro antimicrobial activity of olive
leaves. Mycoses 2003, 46:132136.
Friedman M, Roasooly R, Do PM, Henika PR: The olive compound
4-hydroxytyrosol inactivates Staphylococcus aureus bacteria and
Staphylococcical enterotoxin A (SEA). J Food Sci 2011, 76:M558M563.
Al-Habib A, Al-Saleh E, Safer A-M, Afzal M: Bactericidal effect of grape seed
extract on methicillin-resistant Staphylococcus aureus (MRSA). J Toxicol Sci
2010, 35:357364.
Fabiani R, Fuccelli R, Pieravanti F, De Bartolomeo A, Morozzi G: Production
of hydrogen peroxidase for the induction of apoptosis by
hydroxytyrosol on HL60 cells. Mol Nutr Food Res 2009, 53:887896.
Pinto J, Paiva-Martins F, Corona G, Debnam ES, Jose Oruna-Concha M,
Vauzour D, Gordon MH, Spencer JP: Absorption and metabolism of olive oil
secoiridoids in the small intestine. Br J Nutr 2011, 105:16071618.
doi:10.1186/1476-0711-13-24
Cite this article as: Bisignano et al.: 3,4-DHPEA-EA from Olea Europaea L.
is effective against standard and clinical isolates of Staphylococcus sp.
Annals of Clinical Microbiology and Antimicrobials 2014 13:24.
Você também pode gostar
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (121)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (400)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (895)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- INFECTIONS IN DIALYSIS PATIENTS Basis & Prevention by Dr.T.V.Rao MDDocumento48 páginasINFECTIONS IN DIALYSIS PATIENTS Basis & Prevention by Dr.T.V.Rao MDtummalapalli venkateswara raoAinda não há avaliações
- Antibiotics in Veterinary UseDocumento83 páginasAntibiotics in Veterinary Usehansmeet100% (1)
- Backup of ANTIMICROBIALS PRINTABLEDocumento4 páginasBackup of ANTIMICROBIALS PRINTABLEinvading_jam7582100% (1)
- Rashes in Children - Paediatric Dermatology Guideline Jan17 PDFDocumento24 páginasRashes in Children - Paediatric Dermatology Guideline Jan17 PDFpatmcgarryAinda não há avaliações
- Bacterial Infection (MRSA Infection)Documento8 páginasBacterial Infection (MRSA Infection)Brijesh Singh Yadav100% (1)
- Oleuropein in Olive Leaf and Extra Virgin OilDocumento22 páginasOleuropein in Olive Leaf and Extra Virgin OilWaqar Ahmad Khan GhoryAinda não há avaliações
- Triptolide - Wikipedia, The Free EncyclopediaDocumento2 páginasTriptolide - Wikipedia, The Free EncyclopediaDomitian PascaAinda não há avaliações
- Single Oral Dose Pharmacokinetics of Decursin, Decursinol Angelate, and Decursinol in RatsDocumento2 páginasSingle Oral Dose Pharmacokinetics of Decursin, Decursinol Angelate, and Decursinol in RatsDomitian PascaAinda não há avaliações
- Rhapontin (155!58!8) Chengdu Biopurify Phytochemicals LTDDocumento1 páginaRhapontin (155!58!8) Chengdu Biopurify Phytochemicals LTDDomitian PascaAinda não há avaliações
- Rhaponticin From Rhubarb Rhizomes Alleviates Liver Steatosis and Improves Blood Glucose and Lipid Profiles in KK - Ay Diabetic MiceDocumento2 páginasRhaponticin From Rhubarb Rhizomes Alleviates Liver Steatosis and Improves Blood Glucose and Lipid Profiles in KK - Ay Diabetic MiceDomitian PascaAinda não há avaliações
- Oleuropein in Olive Leaf and Extra Virgin OilDocumento22 páginasOleuropein in Olive Leaf and Extra Virgin OilWaqar Ahmad Khan GhoryAinda não há avaliações
- Lannaconitine 98%Documento2 páginasLannaconitine 98%Domitian PascaAinda não há avaliações
- Decursinol and Decursin Protect Primary Cultured Rat Cortical Cells From Glutamate-Induced NeurotoxicityDocumento2 páginasDecursinol and Decursin Protect Primary Cultured Rat Cortical Cells From Glutamate-Induced NeurotoxicityDomitian PascaAinda não há avaliações
- Arctigenin, A Natural Compound, Activates AMP-Activated Protein Kinase Via Inhibition of Mitochondria Complex I and Ameliorates Metabolic Disorders..Documento2 páginasArctigenin, A Natural Compound, Activates AMP-Activated Protein Kinase Via Inhibition of Mitochondria Complex I and Ameliorates Metabolic Disorders..Domitian PascaAinda não há avaliações
- Neuroprotective Effect of A New Synthetic Aspirin-Decursinol Adduct in Experimental Animal Models of Ischemic StrokeDocumento12 páginasNeuroprotective Effect of A New Synthetic Aspirin-Decursinol Adduct in Experimental Animal Models of Ischemic StrokeDomitian PascaAinda não há avaliações
- Arctigenin From Fructus Arctii Is A Novel Suppressor of Heat Shock Response in Mammalian CellsDocumento8 páginasArctigenin From Fructus Arctii Is A Novel Suppressor of Heat Shock Response in Mammalian CellsDomitian PascaAinda não há avaliações
- Inhibitory Activities of Cyanidin and Its Glycosides and Synergistic Effect with Acarbose against Intestinal α -Glucosidase and Pancreatic α -AmylaseDocumento10 páginasInhibitory Activities of Cyanidin and Its Glycosides and Synergistic Effect with Acarbose against Intestinal α -Glucosidase and Pancreatic α -AmylaseDomitian PascaAinda não há avaliações
- Effects of The Olive-Derived Polyphenol Oleuropein On Human HealthDocumento17 páginasEffects of The Olive-Derived Polyphenol Oleuropein On Human HealthDomitian PascaAinda não há avaliações
- Cyanidin-3-Glucoside Chloride (7084!24!4) Chengdu Biopurify Phytochemicals LTDDocumento2 páginasCyanidin-3-Glucoside Chloride (7084!24!4) Chengdu Biopurify Phytochemicals LTDDomitian PascaAinda não há avaliações
- Traditional Uses, Phytochemistry, and Pharmacology of Olea Europaea (Olive)Documento29 páginasTraditional Uses, Phytochemistry, and Pharmacology of Olea Europaea (Olive)Domitian PascaAinda não há avaliações
- Comparative Pharmacokinetics of Arctigenin in Normal and Type 2 Diabetic Rats After Oral and Intravenous AdministrationDocumento2 páginasComparative Pharmacokinetics of Arctigenin in Normal and Type 2 Diabetic Rats After Oral and Intravenous AdministrationDomitian PascaAinda não há avaliações
- Contact - IrconDocumento2 páginasContact - IrconDomitian PascaAinda não há avaliações
- Arctiin (20362-31-6) Chengdu Biopurify Phytochemicals LTDDocumento1 páginaArctiin (20362-31-6) Chengdu Biopurify Phytochemicals LTDDomitian PascaAinda não há avaliações
- Arctigenin Increases Hemeoxygenase-1 Gene Expression by Modulating PI3K/AKT Signaling Pathway in Rat Primary AstrocytesDocumento6 páginasArctigenin Increases Hemeoxygenase-1 Gene Expression by Modulating PI3K/AKT Signaling Pathway in Rat Primary AstrocytesDomitian PascaAinda não há avaliações
- Arctigenin (7770!78!7) Chengdu Biopurify Phytochemicals LTDDocumento1 páginaArctigenin (7770!78!7) Chengdu Biopurify Phytochemicals LTDDomitian PascaAinda não há avaliações
- Decursin and Decursinol Angelate Inhibit Estrogen-Stimulated and Estrogen-Independent Growth and Survival of Breast Cancer CellsDocumento12 páginasDecursin and Decursinol Angelate Inhibit Estrogen-Stimulated and Estrogen-Independent Growth and Survival of Breast Cancer CellsDomitian PascaAinda não há avaliações
- Arctigenin Protects Focal Cerebral Ischemia-Reperfusion Rats Through Inhibiting NeuroinflammationDocumento2 páginasArctigenin Protects Focal Cerebral Ischemia-Reperfusion Rats Through Inhibiting NeuroinflammationDomitian PascaAinda não há avaliações
- Arctigenin Efficiently Enhanced Sedentary Mice Treadmill EnduranceDocumento2 páginasArctigenin Efficiently Enhanced Sedentary Mice Treadmill EnduranceDomitian PascaAinda não há avaliações
- 4-Hydroxyisoleucine (781658-23-9) Chengdu Biopurify Phytochemicals LTDDocumento1 página4-Hydroxyisoleucine (781658-23-9) Chengdu Biopurify Phytochemicals LTDDomitian PascaAinda não há avaliações
- 02 Broschuere WitepsolDocumento25 páginas02 Broschuere WitepsolDomitian PascaAinda não há avaliações
- Antithrombotic and Antiallergic Activities of Rhaponticin From Rhei Rhizoma Are Activated by Human Intestinal BacteriaDocumento2 páginasAntithrombotic and Antiallergic Activities of Rhaponticin From Rhei Rhizoma Are Activated by Human Intestinal BacteriaDomitian PascaAinda não há avaliações
- Antifungal Activity of Lavandula Angustifolia Essential Oil Against Candida Albicans Yeast and Mycelial FormDocumento2 páginasAntifungal Activity of Lavandula Angustifolia Essential Oil Against Candida Albicans Yeast and Mycelial FormDomitian PascaAinda não há avaliações
- Absorption, Distribution, Metabolism, and Excretion of Decursin and Decursinol Angelate From Angelica Gigas NakaiDocumento2 páginasAbsorption, Distribution, Metabolism, and Excretion of Decursin and Decursinol Angelate From Angelica Gigas NakaiDomitian PascaAinda não há avaliações
- Antimicrobials - New and Old Molecules in The Fight Against Multi-Resistant BacteriaDocumento363 páginasAntimicrobials - New and Old Molecules in The Fight Against Multi-Resistant BacteriaŽaba Od ŽadaAinda não há avaliações
- Microbiology - Exercise 3e AntibiogramDocumento5 páginasMicrobiology - Exercise 3e Antibiogramapi-253346521Ainda não há avaliações
- International Journal of Pharmaceutical Science Invention (IJPSI)Documento2 páginasInternational Journal of Pharmaceutical Science Invention (IJPSI)inventionjournalsAinda não há avaliações
- Le ExamrevDocumento32 páginasLe Examrevnicolas dionisio ordonez barruetaAinda não há avaliações
- What Is MrsaDocumento17 páginasWhat Is MrsaAnonymous NVBCqKWHAinda não há avaliações
- Cellulitis Definition, Etiology, and Clinical FeaturesDocumento10 páginasCellulitis Definition, Etiology, and Clinical Featuresrosscharles1006869Ainda não há avaliações
- Key Answers Part 2 December 2019 PDFDocumento29 páginasKey Answers Part 2 December 2019 PDFNimer Abdelhadi AliAinda não há avaliações
- Exposure To World Health Organization's AWaRe Antibiotics and Isolation of Multidrug Resistant Bacteria - A Systematic Review and Meta-AnalysisDocumento10 páginasExposure To World Health Organization's AWaRe Antibiotics and Isolation of Multidrug Resistant Bacteria - A Systematic Review and Meta-AnalysisLee Foo WengAinda não há avaliações
- Fournier's Gangrene GuidelinesDocumento2 páginasFournier's Gangrene GuidelinesDavid Morales ZepedaAinda não há avaliações
- Hospital Acquired Infections:: Yesterday, Today & TomorrowDocumento87 páginasHospital Acquired Infections:: Yesterday, Today & TomorrowSharad BhallaAinda não há avaliações
- Multidrug Resistance in BacteriaDocumento31 páginasMultidrug Resistance in BacteriaSudeep GoswamiAinda não há avaliações
- Foundations in Microbiology: TalaroDocumento71 páginasFoundations in Microbiology: Talaromertx013Ainda não há avaliações
- Clinical Treatment of Rabbits Experimentally Infected With Staphylococcus Aureus Using Different AntibioticsDocumento4 páginasClinical Treatment of Rabbits Experimentally Infected With Staphylococcus Aureus Using Different Antibioticsarum pratiwiAinda não há avaliações
- Central Line Infection PathwayDocumento36 páginasCentral Line Infection PathwaycignalAinda não há avaliações
- A-Z On AntibioticDocumento5 páginasA-Z On AntibioticRama JogjaAinda não há avaliações
- 2015 - Staphylococcus Aureus Mandell, Douglas, and Bennett's Principles and Practice of Infectious DiseasesDocumento41 páginas2015 - Staphylococcus Aureus Mandell, Douglas, and Bennett's Principles and Practice of Infectious DiseasesBianca DanielaAinda não há avaliações
- Olitorius) IN SOAP: Martin Lawrence L Asinas John Edison V. Brillo Acel Deane D. CironDocumento17 páginasOlitorius) IN SOAP: Martin Lawrence L Asinas John Edison V. Brillo Acel Deane D. CironEllen Joy Velasco BrilloAinda não há avaliações
- Guideline For Prevention of Surgical Wound InfectionsDocumento14 páginasGuideline For Prevention of Surgical Wound InfectionsosaqerAinda não há avaliações
- Mannitol Salt Phenol-Red Agar Acc. Harm. EP/USP/JP: Ordering Number: 1.05404.0500Documento4 páginasMannitol Salt Phenol-Red Agar Acc. Harm. EP/USP/JP: Ordering Number: 1.05404.0500Andrea PardoAinda não há avaliações
- JurnalDocumento7 páginasJurnalHardiAinda não há avaliações
- Microbiology at A GlanceDocumento126 páginasMicrobiology at A GlanceMuhammad UsmanAinda não há avaliações
- Challenges AbDocumento15 páginasChallenges AboinkAinda não há avaliações
- Gram Positive Bacteria ChartDocumento1 páginaGram Positive Bacteria ChartAngelina IafanoAinda não há avaliações
- Research IIIDocumento14 páginasResearch IIIRS CYAinda não há avaliações
- Reading Guide V 6.0 EUCAST Disk Test 2019Documento26 páginasReading Guide V 6.0 EUCAST Disk Test 2019oinkAinda não há avaliações