Escolar Documentos
Profissional Documentos
Cultura Documentos
PS1
Enviado por
Elah PalaganasDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
PS1
Enviado por
Elah PalaganasDireitos autorais:
Formatos disponíveis
Chem 28.
1 THW/THUV1
de la Fuente August2015
PROBLEM SET 1
Concentrations and Solution Stoichiometry
CONCENTRATIONS
1. Why is it more accurate to say that the concentration of a solution of acetic acid (CH3COOH) is 0.01 F rather than
0.01 M? (Despite this distinction, we will usually write 0.01 M)
2. How many grams of methanol (CH3OH, MW 32.04) are contained in 0.100 L of 1.71 M aqueous methanol (i.e.
1.71 mol CH3OH/L solution)?
3. The concentration of C20H42 (MW 282.55) in winter rainwater is 0.2 ppb. Assume that the density of rainwater is
close to 1.00 g/mL; find the molar concentration of C20H42.
4. How many grams of perchloric acid, HClO4, are contained in 37.6 g of 70.5 wt % aqueous perchloric acid? How
many grams of water are in the same solution?
5. The concentration of sugar (glucose, C6H12O6) in human blood ranges from about 80 mg/100 mL before meals up
to 120 mg/100 mL after eating. Find the molarity of glucose in blood before and after eating.
6. An aqueous solution of antifreeze contains 6.067 M ethylene glycol (OHCH2CH2OH, MW 62.07) and has a density
of 1.046 g/mL.
a. Find the mass of 1.00 L of this solution and the number of grams of ethylene glycol per liter.
b. Find the molality of ethylene glycol in this solution.
7. What is the molality of 50.00 mL of 5.75 M sulfuric acid solution with a density of 1.45 g/mL?
8. For an acid-base titration experiment, 6.625 grams of the sodium salt of a diprotic acid was weighed, dissolved,
and diluted to 5.000 L. The concentration of the solution was 0.0250 N. Calculate the molar mass of the salt.
9. A 0.2121-g sample of pure Na2C2O4 (134.00 g/mol) was titrated with 43.31 mL of KMnO4. What is the normality
of the KMnO4 solution? What is the titer in terms of g C2O4 per mL of KMnO4?
10. A 0.8040-g sample of an iron ore was dissolve in acid. The iron was then reduced to Fe2+ and titrated with 47.22
mL of 0.1121 N KMnO4 solution. Calculate the results of this analysis in terms of (a) percent Fe (55.847 g/mol)
and (b) percent Fe3O4 (231.54 g/mol). The reaction of the analyte with the reagent is as follows:
MnO4- + 5Fe2+ + 8H+ Mn2+ + 5Fe3+ + 4H2O
PREPARING SOLUTIONS
1. How many grams of boric acid (B(OH)3, MW 61.83) should be used to make 2.00 L of 0.0500 M solution?
2. How many grams of 50.0 wt % NaOH (FW 40.00) should be diluted to 1.00 L to make 0.10 M?
3. A bottle off concentrated aqueous sulfuric acid, labeled 98.0 wt % H2SO4, has a concentration of 18.0M.
a. How many milliliters of reagent should be diluted to 1.00 L to give 1.00 M H2SO4?
b. Calculate the density of 98.0 wt % H2SO4.
c. What is the density of 53.4 wt % aqueous NaOH if 16.7 mL of the solution diluted to 2.00 L gives 0.169 M
NaOH?
SOLUTIONS AND STOICHIOMETRY
1. How many milliliters of 3.00 M H2SO4 are required to react with 4.35 g of solid containing 23.2 wt % Ba(NO3)2 if
the reaction is Ba2+ + SO42- BaSO4(s)
2. How many grams of 0.491 wt % aqueous HF are required to provide a 50% excess to react with 25.0 mL of
0.0236 M Th4+ by the reaction Th4+ + 4F- ThF4(s)?
Você também pode gostar
- HYDROLYSIS OF SALTS AND PH OF BUFFER SOLUTIONSDocumento13 páginasHYDROLYSIS OF SALTS AND PH OF BUFFER SOLUTIONSfadz607100% (2)
- Chem16 LE3 SamplexDocumento3 páginasChem16 LE3 SamplexmariemfranciscoAinda não há avaliações
- Gravimetric Determination of Moisture CoDocumento5 páginasGravimetric Determination of Moisture CoDEFIN BIMA REYNANDAAinda não há avaliações
- Lab 6 Using PH ElectrodeDocumento14 páginasLab 6 Using PH ElectrodeMina VoAinda não há avaliações
- S.3 Chemistry Notes Mole Concept: Molar MassDocumento90 páginasS.3 Chemistry Notes Mole Concept: Molar MassmelohAinda não há avaliações
- UP Academic League of Chemical Engineering Students (UP ALCHEMES)Documento10 páginasUP Academic League of Chemical Engineering Students (UP ALCHEMES)Jerremiah YuAinda não há avaliações
- Calorimetry Chem17Documento6 páginasCalorimetry Chem17Frances Abegail QuezonAinda não há avaliações
- KEM Tutorials Chem 17 Module (3rd Exam)Documento10 páginasKEM Tutorials Chem 17 Module (3rd Exam)Nyka C.Ainda não há avaliações
- UP Academic League of Chemical Engineering Students (UP ALCHEMES)Documento6 páginasUP Academic League of Chemical Engineering Students (UP ALCHEMES)kennethleo69100% (1)
- Chem 17 Expt 8 Fr2 FinalDocumento12 páginasChem 17 Expt 8 Fr2 FinalMarrod CruzAinda não há avaliações
- Chemistry 17 (Second Long Sample Exam)Documento2 páginasChemistry 17 (Second Long Sample Exam)Nyka C.Ainda não há avaliações
- Samples: Experiment 6 - Comparative Investigations of Organic CompoundsDocumento2 páginasSamples: Experiment 6 - Comparative Investigations of Organic CompoundsAlyssa CubillaAinda não há avaliações
- SOURCE: General Chemistry: Principles and Modern Applications 10Documento3 páginasSOURCE: General Chemistry: Principles and Modern Applications 10Jerremiah YuAinda não há avaliações
- Experiment 4 - Quantitative Analysis of Soda Ash by Double-Indicator TitrationDocumento2 páginasExperiment 4 - Quantitative Analysis of Soda Ash by Double-Indicator TitrationMelchiAinda não há avaliações
- Long Quiz 2: Mipmalgapo (Chem 17 X2)Documento3 páginasLong Quiz 2: Mipmalgapo (Chem 17 X2)Paolo QuinteroAinda não há avaliações
- Quantitative Analysis of Soda Ash by Double Indicator Titration Chem 28Documento2 páginasQuantitative Analysis of Soda Ash by Double Indicator Titration Chem 28Frances Abegail QuezonAinda não há avaliações
- S E C H: Olubility Quilibrium of Alcium YdroxideDocumento6 páginasS E C H: Olubility Quilibrium of Alcium YdroxideGiselle ReyesAinda não há avaliações
- Formal Report Experiment 2 and 3Documento5 páginasFormal Report Experiment 2 and 3Zyra Camille Giron HacheroAinda não há avaliações
- Experiment 1: CalorimetryDocumento4 páginasExperiment 1: CalorimetryNeil Mark EnriquezAinda não há avaliações
- FR3 CalculationsDocumento5 páginasFR3 CalculationsJoeco Abay-abayAinda não há avaliações
- Making Molar & Normal SolutionsDocumento10 páginasMaking Molar & Normal SolutionsAbhijit GadheAinda não há avaliações
- Quantitative Analysis of Soda Ash by Double Indicator TitrationDocumento4 páginasQuantitative Analysis of Soda Ash by Double Indicator TitrationYamiyoAinda não há avaliações
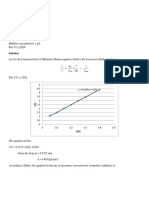
- Chem 28.1 Problem Set Coplex TitrationsDocumento1 páginaChem 28.1 Problem Set Coplex TitrationsIda Anne Cacharel FuentespinaAinda não há avaliações
- Quantitative Determination of Total Hardness in Drinking Water by Complexometric EDTA TitrationDocumento14 páginasQuantitative Determination of Total Hardness in Drinking Water by Complexometric EDTA Titrationabcd efgAinda não há avaliações
- Chem 17 LE 1 Answers1Documento11 páginasChem 17 LE 1 Answers1alyssa100% (1)
- Chem 28.1 Midterm PSDocumento2 páginasChem 28.1 Midterm PSAnonymous ee5dOjAinda não há avaliações
- Chem 31.1 FR1 SantosDocumento5 páginasChem 31.1 FR1 SantosClaire SantosAinda não há avaliações
- Chem 26.1 FR E11Documento7 páginasChem 26.1 FR E11smile fireAinda não há avaliações
- Application of Statistical Concepts in The Determination of Weight Variation in SamplesDocumento2 páginasApplication of Statistical Concepts in The Determination of Weight Variation in SamplesCharlette InaoAinda não há avaliações
- Acid Base TitrationDocumento12 páginasAcid Base TitrationMsfaeza HanafiAinda não há avaliações
- Winkler Titration FRDocumento4 páginasWinkler Titration FRanaperturescienceAinda não há avaliações
- Elimination Reaction of AlkenesDocumento15 páginasElimination Reaction of AlkenesZyra Camille Giron HacheroAinda não há avaliações
- Quantitative Analysis of Soda Ash or Alkali Mixture by Double-Indicator TitrationDocumento2 páginasQuantitative Analysis of Soda Ash or Alkali Mixture by Double-Indicator TitrationZyrle Nikko UchidaAinda não há avaliações
- Chem 28 Problem Set 2Documento1 páginaChem 28 Problem Set 2Anonymous ee5dOjAinda não há avaliações
- FR1 Chem 28.1 Expt 1Documento11 páginasFR1 Chem 28.1 Expt 1Marrod CruzAinda não há avaliações
- FR PDFDocumento4 páginasFR PDFGoku SanAinda não há avaliações
- Determination of Hardness and Calcium in The SampleDocumento8 páginasDetermination of Hardness and Calcium in The SampleGobe JamAinda não há avaliações
- ATQ E5 Chem 28Documento2 páginasATQ E5 Chem 28Zyra Camille Giron HacheroAinda não há avaliações
- Atq E6Documento2 páginasAtq E6BuiHopeAinda não há avaliações
- Chemistry ReportDocumento5 páginasChemistry ReportAngel Trisha Mae DelMundoAinda não há avaliações
- Chemistry 16 Comprehensive Samplex (ANSWER KEY For Non-PSolv)Documento5 páginasChemistry 16 Comprehensive Samplex (ANSWER KEY For Non-PSolv)Laia Valencia100% (1)
- VinegarDocumento14 páginasVinegarLynn HeimatotoAinda não há avaliações
- Quantitative Determination of Soda Ash Composition by Double Indicator TitrationDocumento2 páginasQuantitative Determination of Soda Ash Composition by Double Indicator TitrationAlexander Gordon InesAinda não há avaliações
- 6.4D - Individual Tests - Chemistry LibreTexts PDFDocumento12 páginas6.4D - Individual Tests - Chemistry LibreTexts PDFBožana TomićAinda não há avaliações
- Preparation and Purification of An Alkyl HalideDocumento4 páginasPreparation and Purification of An Alkyl HalideDaphne MercadoAinda não há avaliações
- Quantitative Analysis of Soda Ash or Alkali Mixture by Double-Indicator TitrationDocumento2 páginasQuantitative Analysis of Soda Ash or Alkali Mixture by Double-Indicator TitrationBret Reall LaoAinda não há avaliações
- Acid Base TitrationDocumento57 páginasAcid Base TitrationRichard Obinna100% (1)
- Spectrophotometric Determination of The Acid Dissociation Constant of Methyl RedDocumento3 páginasSpectrophotometric Determination of The Acid Dissociation Constant of Methyl Red7063673nasAinda não há avaliações
- Exp 4 Chem 17 LabDocumento7 páginasExp 4 Chem 17 LabGabrielle CatalanAinda não há avaliações
- Determination of Acid Content of Vinegars and Wines Lab ReportDocumento3 páginasDetermination of Acid Content of Vinegars and Wines Lab ReportAlleia Mae Urbano MazoAinda não há avaliações
- Lab Report 11Documento3 páginasLab Report 11PaulAinda não há avaliações
- Experiment 2 & 3 FR Chem 28.1Documento5 páginasExperiment 2 & 3 FR Chem 28.1Mara Krista CooAinda não há avaliações
- Back TitrationDocumento2 páginasBack TitrationjohnAinda não há avaliações
- Chem 28.1 E10 ATQDocumento5 páginasChem 28.1 E10 ATQSheenly Anne SaavedraAinda não há avaliações
- Chem 31 AtqE1Documento3 páginasChem 31 AtqE1Anonymous GO6JVW9WudAinda não há avaliações
- Atq 4Documento4 páginasAtq 4Martina BlasAinda não há avaliações
- Experiment 1 LabDocumento9 páginasExperiment 1 LabPatrickAinda não há avaliações
- Preparation and Standardization of Potassium Thiocyanate Solution Lab ActDocumento5 páginasPreparation and Standardization of Potassium Thiocyanate Solution Lab ActALYSSA MAE BURACAinda não há avaliações
- Assessment 6 (Concentration of Solution)Documento2 páginasAssessment 6 (Concentration of Solution)shaneeeeAinda não há avaliações
- Angeles Mark P. Assignment No. 2Documento2 páginasAngeles Mark P. Assignment No. 2MARK ANGELESAinda não há avaliações
- Calculation in ACDocumento4 páginasCalculation in AC23005852Ainda não há avaliações
- Motion Notes: Speed, Velocity, and Acceleration!Documento18 páginasMotion Notes: Speed, Velocity, and Acceleration!Elah PalaganasAinda não há avaliações
- Lesson 1Documento77 páginasLesson 1Elah PalaganasAinda não há avaliações
- Newtonian Revolution: Mechanics Is The Branch of Physics That Focuses On The MotionDocumento12 páginasNewtonian Revolution: Mechanics Is The Branch of Physics That Focuses On The MotionElah PalaganasAinda não há avaliações
- Honors Chemistry - Course OutlineDocumento2 páginasHonors Chemistry - Course OutlineElah PalaganasAinda não há avaliações
- Trends & The Periodic TableDocumento34 páginasTrends & The Periodic TableElah PalaganasAinda não há avaliações
- Line Sizing Vapor LiquidDocumento25 páginasLine Sizing Vapor LiquidElah PalaganasAinda não há avaliações
- Palaganas Hw2 Che 197Documento5 páginasPalaganas Hw2 Che 197Elah Palaganas100% (1)
- Project Proposal PalaganasDocumento1 páginaProject Proposal PalaganasElah PalaganasAinda não há avaliações
- Three Quatrains: 2 PoemDocumento1 páginaThree Quatrains: 2 PoemElah PalaganasAinda não há avaliações
- Created by Unlicensed Version: OCTOBER 9, 2018Documento1 páginaCreated by Unlicensed Version: OCTOBER 9, 2018Elah PalaganasAinda não há avaliações
- Zoology - Genetics-Botany - EcologyDocumento1 páginaZoology - Genetics-Botany - EcologyElah PalaganasAinda não há avaliações
- ChE 133 WKL-FXY - Final StandingDocumento35 páginasChE 133 WKL-FXY - Final StandingElah PalaganasAinda não há avaliações
- Non Verbal CommunicationDocumento40 páginasNon Verbal CommunicationElah PalaganasAinda não há avaliações
- Effect of Different Parameters To % Oil RemainingDocumento3 páginasEffect of Different Parameters To % Oil RemainingElah PalaganasAinda não há avaliações
- Current Issues in The Palm Oil Industry Related To Environmental Sustainability: 1. 2. 3. 4. 5Documento1 páginaCurrent Issues in The Palm Oil Industry Related To Environmental Sustainability: 1. 2. 3. 4. 5Elah PalaganasAinda não há avaliações
- Cost Saving Idea: Do Changeover From PKO To PO in CLSBE Units Only When The PO Demand IsDocumento2 páginasCost Saving Idea: Do Changeover From PKO To PO in CLSBE Units Only When The PO Demand IsElah PalaganasAinda não há avaliações
- "Electric Circuit": Project in ScienceDocumento2 páginas"Electric Circuit": Project in ScienceElah PalaganasAinda não há avaliações
- 1st LE - Seta Item AnalyzedDocumento10 páginas1st LE - Seta Item AnalyzedElah PalaganasAinda não há avaliações
- Profile Profile: Writing Style Educational BackgroundDocumento5 páginasProfile Profile: Writing Style Educational BackgroundElah PalaganasAinda não há avaliações
- HSEDocumento7 páginasHSEElah PalaganasAinda não há avaliações
- Physics 71 EquationsDocumento3 páginasPhysics 71 EquationsElah PalaganasAinda não há avaliações
- Q15-22 And28-41 Page: 64: 1. Solve The NCERT Questions From Chapter 1Documento1 páginaQ15-22 And28-41 Page: 64: 1. Solve The NCERT Questions From Chapter 1jamunAinda não há avaliações
- Titration Lab Instruction SheetDocumento2 páginasTitration Lab Instruction Sheetapi-205419744Ainda não há avaliações
- Experiment 3 Analytical ChemistryDocumento6 páginasExperiment 3 Analytical ChemistryNabila HusnaAinda não há avaliações
- AP Chem 1999Documento21 páginasAP Chem 1999Zenzo LeeAinda não há avaliações
- CRE Lecture NotesDocumento10 páginasCRE Lecture Notesmunding21Ainda não há avaliações
- Analytical Chemistry 7aDocumento9 páginasAnalytical Chemistry 7aGarfield SmithAinda não há avaliações
- Chem ProjectDocumento37 páginasChem ProjectabinashAinda não há avaliações
- Application of Beer's Law To Nickel Solution LabDocumento4 páginasApplication of Beer's Law To Nickel Solution LabAnonymous 7srIZqjAinda não há avaliações
- A2AS CHEM REVISED Support 20842 PDFDocumento8 páginasA2AS CHEM REVISED Support 20842 PDFDanesha MccallumAinda não há avaliações
- Chemistry Form 4 Chapter 7Documento5 páginasChemistry Form 4 Chapter 7Azsyerrah Jahini67% (3)
- Lab ManualDocumento35 páginasLab ManualNaresh Manickam0% (1)
- Analytical Chemistry CH 342 20132Documento1 páginaAnalytical Chemistry CH 342 20132KaizerAinda não há avaliações
- WS21.C11.03 - Acids, Bases and Salts - 03-07-2021 - 1625298900049 - XVGVXDocumento2 páginasWS21.C11.03 - Acids, Bases and Salts - 03-07-2021 - 1625298900049 - XVGVXRAVI ANANTHAKRISHNANAinda não há avaliações
- Preparing Acid Base TitrationsDocumento4 páginasPreparing Acid Base TitrationsRebecca ZgheibAinda não há avaliações
- Unsana, Marifer Rose A. - Gen Chem ChemistryDocumento4 páginasUnsana, Marifer Rose A. - Gen Chem ChemistryZeny NaranjoAinda não há avaliações
- Clinical Chemistry 1 (MKEB2404)Documento5 páginasClinical Chemistry 1 (MKEB2404)kiedd_04100% (2)
- 499347059chemistry Question Bank (2013-14)Documento94 páginas499347059chemistry Question Bank (2013-14)amanverma60% (1)
- Instructors Manual For Experiments in Biochemistry A Hands On Approach 2nd Edition Shawn o Farrell Colorado Lynn e TaylorDocumento36 páginasInstructors Manual For Experiments in Biochemistry A Hands On Approach 2nd Edition Shawn o Farrell Colorado Lynn e Taylorogreish.pontilagnqgi100% (42)
- Expressing ConcentrationDocumento10 páginasExpressing ConcentrationChristopher PierceAinda não há avaliações
- CHEM 111 E-Learning Material-1Documento163 páginasCHEM 111 E-Learning Material-1nattydreadfathelahAinda não há avaliações
- A Volumetric AnalysisDocumento10 páginasA Volumetric AnalysisTDUY059109Ainda não há avaliações
- Molality Practice QuestionsDocumento7 páginasMolality Practice QuestionsMuhammad AhmedAinda não há avaliações
- 61 1 0 MMJ 5 PDFDocumento5 páginas61 1 0 MMJ 5 PDFLipsi MerchánAinda não há avaliações
- MolarityDocumento3 páginasMolarityAaron SamudioAinda não há avaliações
- Solution Colligative Properites - EDocumento29 páginasSolution Colligative Properites - EthinkiitAinda não há avaliações
- CTAB CMCDocumento2 páginasCTAB CMCAnand aashishAinda não há avaliações
- StoichiometryDocumento26 páginasStoichiometryShawn Michael GonzalesAinda não há avaliações
- Chemistry IJSO Stage-1Documento8 páginasChemistry IJSO Stage-1Sonal Gupta100% (4)
- KSP AssignmentDocumento3 páginasKSP AssignmentFahri HusainiAinda não há avaliações