Escolar Documentos
Profissional Documentos
Cultura Documentos
Closing in On Alzheimer's Disease - Feature - Pharmaceutical Journal
Enviado por
Matilde ConradTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Closing in On Alzheimer's Disease - Feature - Pharmaceutical Journal
Enviado por
Matilde ConradDireitos autorais:
Formatos disponíveis
17/08/2015
ClosinginonAlzheimersdisease|Feature|PharmaceuticalJournal
Dementia
ClosinginonAlzheimersdisease
ThePharmaceuticalJournal,14AUG2015 ByDawnConnelly
KnowledgeabouttheneuropathologyofAlzheimersdiseasehas
blossomedoverthepastfewdecades,leadingtoabetterunderstandingof
theconditionandnewwaystoattackit.
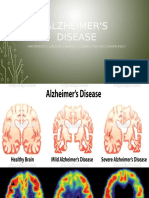
Alzheimersdiseaseisaprogressivedisorder.Aftersymptomsfirstappear,lifeexpectancyisaround810
years.Memoryistypicallythefirstproblem,thendifficultieswithcognitionandplanningbecomemarked,
speechandlanguageareaffected,andpatientsmayexperiencealteredpersonalityandbehaviour.Eventually,
individualsareunabletocareforthemselves(seePDFofgraphic).
Twomainprocessesarethoughttodrivedegeneration:intracellularaccumulationofaproteincalledtauand
extracellulardepositionofaproteincalledbetaamyloid(A),leadingtoformationofneurofibrillatorytangles
andamyloidplaques.Althoughmostpeopledevelopsometanglesandplaquesastheyage,thosewith
symptomsofAlzheimersdiseasetendtodevelopfarmore.
http://www.pharmaceuticaljournal.com/newsandanalysis/features/closinginonalzheimersdisease/20069081.article
1/5
17/08/2015
ClosinginonAlzheimersdisease|Feature|PharmaceuticalJournal
Pathology
Oligomersarethoughttobemoredamagingthanplaquesbecausetheyblockcelltocellsignallingat
synapsesandmayalsoactivateimmunesystemcellstriggeringanattack
Tauandtangles
(1)Insidethehealthyneuron,parallelmicrotubulesprovidethecellstransportsystemfornutritionandkey
materialstobedelivered.Tauproteinactasscaffoldingforthemicrotubules,keepingtheminplace.
(2)Hyperphosphorylationoftauleadstoformationoftauoligomers.Theseoligomersclumptogetherwith
othermoleculesandformtangles.
(3)Bydistortingthespacingofmicrotubules,tanglesimpairaxonaltransportthusaffectingthenutritionof
axonterminalsanddendrites,eventuallyleadingtocelldeath.
BetaAmyloid
(1)APPisatransmembraneprotein,madebyneuronsandotherbraincells.(2)AiscutfromAPPbythe
enzymessecretase(alsocalledBACE1)andsecretasereleasingAmonomersintotheextracellular
environment.(3)Onceclipped,Amonomerscanassembleinatleasttwoways:onepathwayleadsto
solubleoligomersofA42,whicharesmallenoughtoentersynapses.(4)AnotherpathwayforA
monomersistoassembleintoinsolublefibrilsandlargerplaques,whichcanprovokeharmful
inflammation.
Biologicaltargets
Licenseddrugstreatsymptomsbyregulatinglevelsoftheneurotransmittersacetylcholineandglutamate,which
areimportantforneuronalcommunication.Incontrast,drugsindevelopmentaimtoinhibitthemechanismsthat
leadtothebuildupofplaquesandtanglescharacteristicoftheconditionbytargetingA,tauorthe
inflammationcausedbyplaquesandtangles.
http://www.pharmaceuticaljournal.com/newsandanalysis/features/closinginonalzheimersdisease/20069081.article
2/5
17/08/2015
ClosinginonAlzheimersdisease|Feature|PharmaceuticalJournal
Licensedtreatments
ThreecholinesteraseinhibitorsarecurrentlylicensedtotreatAlzheimersdiseasesymptoms,aswellas
oneNmethylDaspartate(NMDA)blocker.
Pipelinedrugs
Monoclonalantibodies(blue)aimtocleartoxicAeitherdirectlyorthroughmicrogliaorastrocyte
activation.However,antibodieshavelimitedbioavailabilityinthebrainandcaninduceimmunogenicity.
Smallmolecules(yellow)aimtoreduceAproduction,preventtauaggregationortarget
neuroinflammation.Theyhavebetterbioavailabilityinthebrainthantheantibodies.Seetablebelowfor
breakdownofdrugs
Monoclonalantibodies
1
ChugaiPharmaceutical/HoffmannLaRochesgantenerumabisafullyhumanIgG1monoclonalantibodythatrecognises
primarilyfibrilsofA.Itstimulatesmicrogliatoclearamyloidplaques.
Biogensaducanumabisahighaffinity,fullyhumanIgG1monoclonalantibodythatbindsaggregatedformsofA,not
monomers.Itstimulatesmicrogliatoclearamyloidplaques.
EliLillyssolanezumabisahumanisedmonoclonalIgG1antibody,whichrecognisessolublemonomersof
3 A,notfibrils.Itstimulatesmicrogliatocleartoxicamyloidmonomers.
GenentechscrenezumabisafullyhumanIgG4monoclonalantibody,whichrecognisesmultipleformsofaggregatedA,
4 includingoligomersandfibrilsandamyloidplaqueswithhighaffinity,andmonomerswithlowaffinity.Itstimulatesmicroglia
enoughtoclearA,butnotenoughtoinduceaninflammatoryresponse.
Biogen/EisaisBAN2401isahumanisedIgG1monoclonalantibodythatselectivelybindstolarge,solubleAprotofibrilsand
stimulatestheirclearancebymicroglialcells.
Smallmolecules
6
Gammasecretasemodulators,suchasMercksGSM1andEisaisE2012,reducethecleavageofAPPthatproducesthemost
toxicpeptide,A42,whilestillallowingtheenzymetocleaveitsothersubstrates.
MercksMK8931andAstraZenecasAZD3293bothinhibitBACE1,sointerferewithproductionofA.
http://www.pharmaceuticaljournal.com/newsandanalysis/features/closinginonalzheimersdisease/20069081.article
3/5
17/08/2015
ClosinginonAlzheimersdisease|Feature|PharmaceuticalJournal
TauRxTherapeuticssLTMXisatauaggregationinhibitorthatreduceslevelsofaggregatedormisfoldedtauproteins.
Twolicenseddrugs(namesnotreleased)havebeenfoundtoinhibittheenzymePERK,whichisthoughttoswitchoff
productionofallnewproteinsinthebraininresponsetoaccumulationofamyloidplaques.Newproteinsarenecessaryfor
neuronalplasticityandmemoryconsolidation.
10
T3DTherapeuticssT3D959isadualnuclearreceptoragonist,whichhasthepotentialtoinhibitboththeAandtau
pathways.
11
Pfizer/TransTechsazeliragonisanantagonistofthecellsurfacereceptorRAGE,whichcanleadtoinflammationand
oxidativedamagewhenoccupied.RAGEmayalsomediatethetoxiceffectsofAoligomers.
Drugdevelopment
Azheimersdisease(AD)wasfirstidentifiedbyGermandoctorAloisAlzheimerin1906butitwasnotuntil
1993thatthefirstdrugtotargetthesymptomsofAlzheimerswasapprovedbytheUSFoodandDrug
Administration(FDA).Despitea99.6%attritionrateforADdrugsbetween2002and2012,thereishopefor
currentpipelinedrugsbeingtestedinprodromalandmildAD,withtheaimofstoppingdiseasedevelopment
ratherthantreatingsymptoms.
1993:WarnerLambertstacrine(Cognex)receivesFDAapprovalformildtomoderateADbutislater
discontinued.
1996:Eisaisdonepezil(Aricept)receivesFDAapprovalformildtomoderateAD.Thiswasextendedto
includesevereADin2006.
2000:Novartissrivastigmine(Exelon)receivesFDAapprovalformildtomoderateAD.
2000:Shiresgalantamine(Reminyl)receivesEuropeanMedicinesAgency(EMA)approvalformildto
moderateAD.
2002:ForestLaboratoriessmemantine(Ebixa)receivesEMAapprovalformoderatetosevereAD.
2012:TwophaseIIItrialsofEliLillysmonoclonalantibodysolanezumabinpatientswithmildto
moderateADdonotreachtheirendpoints.
2012:PhaseIItrialofBiogen/EisaismonoclonalantibodyBAN2401beginsrecruiting800peoplewith
earlystageAD.EstimatedcompletiondateisJuly2018.
2012:TwophaseIIItrialsofTauRxTherapeuticssLMTX,asecondgenerationtauaggregationinhibitor,
begininpatientswithmildandmildtomoderateAD.Studyresultsareexpectedin2016.
2013:PhaseIItrialofGenentechsmonoclonalantibodycrenezumabstartsinpatientswithpreclinicalAD,
withresultsduein2020.Twoadditionalphase2trialsinmildtomoderateADareongoing.
2013:PhaseIIItrialbeginstoassesssafetyandefficacyofMSDsBACEinhibitorMK8931compared
withplaceboinpatientswithprodromalAD.Thetrialissettorununtil2018.
2014:Actavis/Adamassdonepezil/memantinecombinationproduct(Namzaric)receivesFDAapprovalfor
moderatetosevereAD.
2015:Biogenreportsadosedependentreductioninamyloidplaqueandslowingofcognitivedeclinefor
monoclonalantibodyaducanumabinaninterimanalysisofphase1bdataforprodromalormildAD
patients.
2015:NewdelayedstartanalysisofnegativephaseIIIsolanezumabtrialssuggestsitmayslow
progressionofmildAD.
Citation:ThePharmaceuticalJournal,22/29August2015,Vol295,No7876/7,online|URI:20069081
http://www.pharmaceuticaljournal.com/newsandanalysis/features/closinginonalzheimersdisease/20069081.article
4/5
17/08/2015
ClosinginonAlzheimersdisease|Feature|PharmaceuticalJournal
http://www.pharmaceuticaljournal.com/newsandanalysis/features/closinginonalzheimersdisease/20069081.article
5/5
Você também pode gostar
- Emergency Care Algorithms 2019 PDFDocumento75 páginasEmergency Care Algorithms 2019 PDFsiraj ammed75% (4)
- Dimentia DiseaseDocumento10 páginasDimentia Diseaseernie16estreraAinda não há avaliações
- 2021-05-15 New ScientistDocumento62 páginas2021-05-15 New ScientistAgenor Phillips100% (1)
- Alzhymas DiseaseDocumento12 páginasAlzhymas Diseasekids dance BAinda não há avaliações
- Demensia Vs AlzheimerDocumento16 páginasDemensia Vs AlzheimerUmmu ZaFaAinda não há avaliações
- How To Slow Cognitive Decline - Based On The Teachings Of Dr. Andrew Huberman: Unlocking The Secrets To Preserving Cognitive VitalityNo EverandHow To Slow Cognitive Decline - Based On The Teachings Of Dr. Andrew Huberman: Unlocking The Secrets To Preserving Cognitive VitalityAinda não há avaliações
- Step by Step Interventional Ultrasound in Obstetrics and GynaecologyDocumento116 páginasStep by Step Interventional Ultrasound in Obstetrics and GynaecologySahal Beli100% (1)
- ALZHEIMERSDocumento16 páginasALZHEIMERSKathleen DiangoAinda não há avaliações
- AlzheimerDocumento11 páginasAlzheimerSoniya G08Ainda não há avaliações
- Alzheimer's Disease Research PaperDocumento24 páginasAlzheimer's Disease Research Papershayne86% (22)
- Clinical Chemistry Notes With BlanksDocumento34 páginasClinical Chemistry Notes With Blanksepson printerAinda não há avaliações
- Counselling Plab2Aspired19Documento137 páginasCounselling Plab2Aspired19osamaeAinda não há avaliações
- Alzheimer's Disease ResearchDocumento61 páginasAlzheimer's Disease ResearchshayneAinda não há avaliações
- Alzheimer's DiseaseDocumento6 páginasAlzheimer's DiseaseDelia Lingcong100% (1)
- Renal MCQ 4Documento10 páginasRenal MCQ 4AzizAinda não há avaliações
- Alzheimer Disease: Petra Nowotny, Jennifer M Kwon, Alison M GoateDocumento6 páginasAlzheimer Disease: Petra Nowotny, Jennifer M Kwon, Alison M GoatedineshhissarAinda não há avaliações
- Boy Scout First Aid Merit Badge TrainingDocumento109 páginasBoy Scout First Aid Merit Badge TrainingMac100% (2)
- The Basics of Alzheimer's Disease: Dipil PatelDocumento15 páginasThe Basics of Alzheimer's Disease: Dipil Patelgore-patelAinda não há avaliações
- Enf Alzheimer RevisionDocumento8 páginasEnf Alzheimer RevisionLuis Collar ViñuelasAinda não há avaliações
- Alzheimer'S Disease: Miss Sana HanifDocumento27 páginasAlzheimer'S Disease: Miss Sana HanifDr-umar AliAinda não há avaliações
- A Public Health Approach TO Alzheimer'S and Other DementiasDocumento47 páginasA Public Health Approach TO Alzheimer'S and Other DementiasAlaka KondaAinda não há avaliações
- Alzheimer's Disease Fact Sheet: Symptoms DementiaDocumento16 páginasAlzheimer's Disease Fact Sheet: Symptoms DementiaJovelyn Morales JosolAinda não há avaliações
- Alzheimers Case StudyDocumento4 páginasAlzheimers Case Studygamecockusc1992Ainda não há avaliações
- Understanding Alzheimer's and DementiaDocumento16 páginasUnderstanding Alzheimer's and DementiaWaltWritmanAinda não há avaliações
- Alzheimer'S Disease: Is A Progressive and Fatal Brain Disease. More Than 5 Million Americans Now HaveDocumento7 páginasAlzheimer'S Disease: Is A Progressive and Fatal Brain Disease. More Than 5 Million Americans Now Havebored_stiffAinda não há avaliações
- Alzheimer'S Research PapeDocumento11 páginasAlzheimer'S Research PapeAnonymous yQQKF5qLiRAinda não há avaliações
- Alzheimer's Disease: by Franci Papić and Erik PrceDocumento23 páginasAlzheimer's Disease: by Franci Papić and Erik PrceFranciAinda não há avaliações
- Dementia & AlzhiemersDocumento8 páginasDementia & AlzhiemersEileen BarrettAinda não há avaliações
- Alzheimer'S Disease: Amoranto - Azugue - Bamba - Caser - Nhiyam - RaymundoDocumento34 páginasAlzheimer'S Disease: Amoranto - Azugue - Bamba - Caser - Nhiyam - RaymundoRia り あ HitsugayaAinda não há avaliações
- Chap 2 AlzeihmerDocumento4 páginasChap 2 AlzeihmerSabina MoolyeAinda não há avaliações
- Alzheimer's and Dementia Basics: What We Know Today Understanding DementiaDocumento9 páginasAlzheimer's and Dementia Basics: What We Know Today Understanding DementiaVal Lalicon100% (1)
- Thesis Statement On Alzheimers DiseaseDocumento7 páginasThesis Statement On Alzheimers Diseasegbwygt8n100% (1)
- Whatis DementiaDocumento3 páginasWhatis DementiaCamara Cu ZambeteAinda não há avaliações
- Alzheimer Cover Page (Hard Cover)Documento21 páginasAlzheimer Cover Page (Hard Cover)seanfaria100% (1)
- Introduction - Alzheimer's DiseaseDocumento9 páginasIntroduction - Alzheimer's Diseasepriya swathiAinda não há avaliações
- Diseases & Disorders Dementia, Alzheimer's DiseaseDocumento11 páginasDiseases & Disorders Dementia, Alzheimer's DiseaseAngela DepedroAinda não há avaliações
- What Is Alzheimer's - Alzheimer's AssociationDocumento6 páginasWhat Is Alzheimer's - Alzheimer's AssociationRatnaPrasadNalamAinda não há avaliações
- Alzheimer's and DementiaDocumento31 páginasAlzheimer's and DementiaDheepa SrinivasanAinda não há avaliações
- AlzheimersDocumento9 páginasAlzheimersNader Smadi100% (1)
- Alzheimer and SocietyDocumento10 páginasAlzheimer and SocietyAdriana PalomaresAinda não há avaliações
- Bio InvestigatoryDocumento12 páginasBio InvestigatorySonakshi BadlaniAinda não há avaliações
- Products & Services: Book: Mayo Clinic Guide To Stress-Free LivingDocumento10 páginasProducts & Services: Book: Mayo Clinic Guide To Stress-Free LivingoliviaAinda não há avaliações
- Alzheimer's Disease: Causes, Symptoms and TreatmentsDocumento5 páginasAlzheimer's Disease: Causes, Symptoms and Treatmentsfaradilla ramadaniAinda não há avaliações
- Proiect EnglezaDocumento4 páginasProiect EnglezaRebecaAinda não há avaliações
- Levi Santana, R.N. Bulacan State University College of NursingDocumento66 páginasLevi Santana, R.N. Bulacan State University College of NursingMary Grace MasAinda não há avaliações
- Alzheimer'sDocumento11 páginasAlzheimer'sdianaAinda não há avaliações
- What Is DementiaDocumento5 páginasWhat Is DementiaIuly ManacAinda não há avaliações
- Alzheimers - Association - Topics - 12 - 10 - 2019 2 PDFDocumento34 páginasAlzheimers - Association - Topics - 12 - 10 - 2019 2 PDFRonnie MalletAinda não há avaliações
- What Is DementiaDocumento24 páginasWhat Is DementiaJorey QuietaAinda não há avaliações
- Alzheimers Disease,,syringoyeloma by VineethaDocumento42 páginasAlzheimers Disease,,syringoyeloma by VineethaDR. KUMARASWAMI HEALTH CENTRE COLLEGE OF NURSING KANYAKUMARIAinda não há avaliações
- AlzheimersDocumento37 páginasAlzheimerskhizerboi75Ainda não há avaliações
- Alzheimer's BrochureDocumento2 páginasAlzheimer's Brochurebluebeary22Ainda não há avaliações
- The Causes and Effects of Alzheimer's Disease: The Silent DiseaseDocumento35 páginasThe Causes and Effects of Alzheimer's Disease: The Silent DiseaseNina Canares100% (2)
- Alzheimer FolioDocumento15 páginasAlzheimer FolioNurul AshikinAinda não há avaliações
- Alzeimer'S Disease: Lontoc, Aina Gail B. BSED-Science 211 Mrs. Bessielyn Aledo December 15, 2019Documento11 páginasAlzeimer'S Disease: Lontoc, Aina Gail B. BSED-Science 211 Mrs. Bessielyn Aledo December 15, 2019guia macatangayAinda não há avaliações
- Alzimer's Dse P1Documento5 páginasAlzimer's Dse P1Sam AraAinda não há avaliações
- AlzheimerDocumento8 páginasAlzheimerKrizhia MacayaonAinda não há avaliações
- Definition DementiaDocumento8 páginasDefinition DementiaShuhada Abu HassanAinda não há avaliações
- Biology Project File Alzheimer: Akshita AgrawalDocumento15 páginasBiology Project File Alzheimer: Akshita AgrawalAkshita100% (1)
- "The Alarming Impact of Alzheimer's Disease on Society"No Everand"The Alarming Impact of Alzheimer's Disease on Society"Ainda não há avaliações
- Alzheimer'S Disease: By: Ibadat Qayyum Mrs. Cameron Human Anatomy&Physiology Period 4Documento19 páginasAlzheimer'S Disease: By: Ibadat Qayyum Mrs. Cameron Human Anatomy&Physiology Period 4qwertyuiop2012Ainda não há avaliações
- Dissertation On Alzheimers DiseaseDocumento7 páginasDissertation On Alzheimers DiseaseDoMyCollegePaperSingapore100% (1)
- Alzheimer's and Dementia BasicsDocumento2 páginasAlzheimer's and Dementia BasicsVictor Alejandro Larios SalazarAinda não há avaliações
- The Role of Plaques and TanglesDocumento2 páginasThe Role of Plaques and TanglesOrlando BernardoAinda não há avaliações
- AlzheimerDocumento6 páginasAlzheimerCharlie CansteinAinda não há avaliações
- Alziehmer SeminarDocumento15 páginasAlziehmer Seminarsantosh kumarAinda não há avaliações
- DiphtheriaDocumento10 páginasDiphtheriaAgustin IskandarAinda não há avaliações
- Pathophysiology and Management of Diabetes Mellitus GppqeDocumento60 páginasPathophysiology and Management of Diabetes Mellitus GppqeOlivia OliverAinda não há avaliações
- Breast Cancer Astrological Indicators BW PDFDocumento29 páginasBreast Cancer Astrological Indicators BW PDFGargaAinda não há avaliações
- NCM 3114 Acid Base Imbalance-2Documento13 páginasNCM 3114 Acid Base Imbalance-2Fayeh Harah PadrillanAinda não há avaliações
- Acta Pediatrica Enero 2021 - Topicos NeonatalesDocumento113 páginasActa Pediatrica Enero 2021 - Topicos NeonatalesalbertoAinda não há avaliações
- Bpharm Winter 2014Documento1 páginaBpharm Winter 2014babaf79912Ainda não há avaliações
- Question Paper 1 2018 PDFDocumento38 páginasQuestion Paper 1 2018 PDFAyeshaAinda não há avaliações
- IndianJPsychiatry618254-624277 172027Documento16 páginasIndianJPsychiatry618254-624277 172027Fatma BakrAinda não há avaliações
- Contents of Wetland Medicinal Plants in Taiwan: Antioxidant Properties and Total PhenolicDocumento12 páginasContents of Wetland Medicinal Plants in Taiwan: Antioxidant Properties and Total Phenolicvaishali shuklaAinda não há avaliações
- Potts Spine Case StudyDocumento9 páginasPotts Spine Case StudyK. KAinda não há avaliações
- Country Courier - 08/09/2013Documento10 páginasCountry Courier - 08/09/2013bladepublishingAinda não há avaliações
- Ho Lester OlllDocumento6 páginasHo Lester OlllVlad VladAinda não há avaliações
- Animalogy: Cats and Other FelinesDocumento11 páginasAnimalogy: Cats and Other FelinesShubham Bobby BhattacharyaAinda não há avaliações
- Remodelling of Nola PenderDocumento5 páginasRemodelling of Nola PenderDon Chiaw ManongdoAinda não há avaliações
- Pyle Et Al 1995 - Rapid Direct Count E ColiDocumento7 páginasPyle Et Al 1995 - Rapid Direct Count E ColiFabio SenaAinda não há avaliações
- Q.Paper Science Class IX (Moderated)Documento8 páginasQ.Paper Science Class IX (Moderated)ShivangiAinda não há avaliações
- Atlas of Nerve Conduction Studies and Electromyography (2 Ed.)Documento21 páginasAtlas of Nerve Conduction Studies and Electromyography (2 Ed.)rodrigocorcino899959Ainda não há avaliações
- Hyperhidrosis: Management Options: Hyperhidrosis Is Excessive SweatingDocumento6 páginasHyperhidrosis: Management Options: Hyperhidrosis Is Excessive SweatingHagane TetsuAinda não há avaliações
- Airway ObstructionDocumento32 páginasAirway ObstructionAmirrah LaurenteAinda não há avaliações
- Test Bank For College Algebra 12Th Edition by Lial Hornsby Schneider and Daniels Isbn 0134217454 978013421745 Full Chapter PDFDocumento36 páginasTest Bank For College Algebra 12Th Edition by Lial Hornsby Schneider and Daniels Isbn 0134217454 978013421745 Full Chapter PDFdavid.weldon592100% (12)
- Pengetahuan Mengenai Faktor Risiko Dan Perilaku Pasien Sindrom Koroner Akut Jeki RefialdinataDocumento10 páginasPengetahuan Mengenai Faktor Risiko Dan Perilaku Pasien Sindrom Koroner Akut Jeki RefialdinataFATIMAH WANDAAinda não há avaliações
- Medical Language 3rd Edition Test Bank Susan M TurleyDocumento37 páginasMedical Language 3rd Edition Test Bank Susan M Turleykatieterryrlrb100% (12)
- Drink Water On Empty StomachDocumento4 páginasDrink Water On Empty StomachbabuAinda não há avaliações