Escolar Documentos
Profissional Documentos
Cultura Documentos
9 Flow Cytometric Strategies To Study CNS Development
Enviado por
taroDescrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
9 Flow Cytometric Strategies To Study CNS Development
Enviado por
taroDireitos autorais:
Formatos disponíveis
Flow Cytometric
Strategies
to Study CNS Development
Dragan
Marie,
I, Introduction
lrina Marie,
and leffery
1. Barker
to Flow Cytometry
The technique of flow cytometry was initially developed to
count and size particles. However, it has progressively evolved
into a sophisticated analytic tool for rapidly quantifying multiple
properties of individual cells or cellular constituents in suspended
nonhomogeneous populations. All flow cytometry instruments
share a common feature: single cells or particles are pressured to
flow through a sensing region in which their electrical resistance
or optical properties are recorded. Most commonly, these properties are visualized with fluorescent molecules that bind specifically to the biological constituent(s) to be measured. Typically,
these fluorescent molecules are excited by laser beam(s) tuned at
specific wavelenghtts) and their emission(s) collected with an array
of appropriate filters that convey the signals to photomultiplier
tubes and ultimately to a computer.
Flow cytometry complements other optical and electrical
recording strategies that have recently evolved and offers clear
advantages, including the acquisition of multiple parameters at
very high rates (1000-3000 events/s), objectivity, and powerful
sorting capabilities. Over the last 25 yr, it has become widely used
in the fields of hematology, immunology, oncology. and microbiology. Cell counting, identification and classification, cell cycle
studies, measurements of DNA content and cell proliferation, chromosomal karyotyping, and studies of cellular physiology are
among the most widespread research and clinical applications of
flow cytometry (Melamed et al., 1990).
From
Neuromethods,
vol
Eds A A Boulton,
C B Baker,
33
Cell
Neurohology
and A N. Bateson
287
Techques
0 Humana
Press Inc
288
Marie,
Marie,
and Barker
In the field of developmental
neurobiology,
however, flow
cytometry has not been extensively used so far. In this chapter,
we demonstrate several possible applications of flow cytometry
in the studies of CNS development: rapid identification of specific cell populations in the developing CNS using multiple surface
and cytoplasmic markers putatively specific for neuroepithelial,
neuronal, and glial cell lineages; analysis of cells in specific stages
of cell cycle and apoptosis; physiological recordings of membrane
potential and cytosolic calcium and pharmacological discovery
of functional receptors and ion channels; and precise isolation and
sorting of distinct cell populations, based on a specific epitope
expression or a functional response.
2. Cell Preparation
One of the most crucial steps in using flow cytometry to investigate physiological and pharmacological properties of developing CNS at a single-cell level is cell preparation. Cells composing
the CNS during embryonic (E) and early postnatal (P) periods can
be most completely dissociated into single cell suspensions by
enzymatic digestion with papain (Huettner and Baughman, 1986,
Marie et al., 1997). Other commonly used dissociation protocols,
including mechanical (Mandler et al., 1988), trypsin (Schaffner and
Daniels, 1982), and collagenase (Johnson and Argiro, 1983) can
lead to highly variable cell recoveries, which are associated with
up to 50% reduction in cell yield, together with a markedly
decreased cell viability (Marie et al., 1997).
In our study, the papain dissociation protocol was as follows
Embryonic (Eli-22) and early postnatal (PO-7) CNS tissues were
quickly dissected into telencephalic (Eli-14) and neocortical
(E15-P7), olfactory bulb, hippocampal, thalamic, hypothalamic,
mesencephalic, rhombencephalic, and spinal cord regions (Hebel
and Stromberg, 1986; Altman and Bayer, 1995) and immediately
placed in ice-cold saline to retard further developmental changes.
Tissues were cleaned, minced with forceps, and then completely
dissociated into single-cell suspensions by the enzymatic action
of papain (20 U/mL) for 30-45 min at 37C, and gentle trituration
as described (Huettner and Baughman, 1986). In some experiments, 350~pm thick coronal sections of late embryonic neocortex
were first microdissected along the incipient white matter into
cortical plate/subplate (CP/SP) zone, including layer I cells, and
CNS Development
Studies
289
ventricular/subventricular
(VZ/SVZ) zone, including lower intermediate zone (IZ) cells, and then dissociated as described. This
protocol routinely yielded single-cell suspensions with greater
than 95% vitality as determined by trypan blue exclusion on the
microscope stage and confirmed using vital (acridine orange) and
nonvital (propidium
iodide) dye staining of cell suspensions
analyzed by flow cytometry.
After isolation, the cells were labeled with fluorescent antibodies and/or indicator dyes, and passed through the laser-based fluorescence activated cell sorter (FACS), where up to five different
parameters of each single cell (including cell size and complexity,
and immunocytochemical, membrane potential and calcium fluorescence signals) were measured simultaneously, at the rate of
several thousand cells per second. In some experiments, precise
sorting of different cell subpopulations then followed, based on
any one or a combination of these different cell parameters. A schematic outline of the method and some of the cell properties that
can be quantified with different indicators by flow cytometry are
depicted in Fig. 1.
All recordings were carried out with a FACSTAR flow cytometer (Becton Dickinson, Mountain View, CA). Cells were excited
using an argon ion laser (Spectra Physics, Model 2016, Mountain
View, CA) operated at 500 mW and tuned to 488 nm. Forward
angle light scatter (FALS), a property related to cell size, and different fluorescence emissions of individual
elements were
randomly recorded at 1000-2000 events/s. This rate of data
acquisition allowed profiling the properties of approx 10,000 cells
in 5-10 s. FALS data were collected in a linear mode using a combination of 488 + 10 nm bandpass and neutral density filters,
whereas fluorescence emissions were logarithmically
amplified
and filtered at appropriate wavelengths. In multiple labeling
experiments, fluorescence emissions were corrected for color crossover by using electronic compensation. FALS properties and fluorescence intensities were each resolved into 1024 channels. The data
were analyzed using Cell Quest Analysis software operating on a
FACStation Macintosh-based computer platform (Becton Dickinson).
3. lmmunocytochemistry
One of the major difficulties encountered when studying the
development of the CNS is the inability to readily identify specific
290
CM
Development
Stud/es
291
cell lineages at distinct phases of proliferation and differentiation.
One of the reasons IS the lack of availability of uniquely specific
cell markers. There is at present a rapidly growing number of commercially available polyclonal and monoclonal antibodies that can
be used to detect specific cell surface, cytoplasmic, or nuclear
epitopes in CNS cells. However, many of these epitopes are shared
among neuroepithelial,
neuronal, and glial cell types at some
stages of their development. Therefore, there is an increasing need
for using double- and triple-immunostaining
procedures, in order
to obtain a more precise identification of specific cell populations
under mvestigation. A flow cytometer equipped with dual and
triple emission filter sets is ideally suited to access this complexity and diversity of specific CNS populations in a very rapid and
precise manner. In the following sections, we describe the identification of putative neuroepithelial, nerve- and glia-specific markers on several populations of acutely dissociated embryonic and
early postnatal CNS cells using flow cytometry and double- or
triple-immunolabeling
protocols with specific antibodies against
cytoplasmic and plasma membrane epitopes.
3.1. lmmunocharacterization
by Cytoplasmic
Markers
Different cytoplasmic markers tagged with fluorochrome-conjugated antibodies can be identified by flow cytometry, but prior
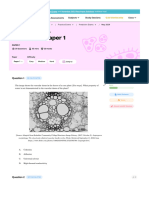
cell fixation and membrane permeabilization are necessary. Laserbased flow cytometry is more sensitive in detection of immunoFig. 1. ~~WZVOUS
page)Accessing CNS development by flow cytometry
(A) In order to study the biological properties of developing neuroeplthehal, neuronal, and glial cell lineages during CNS development, the cells
first have to be dissociated into uniform single-cell suspensions.
(B) The
cells are then immunoreacted,
stained or loaded with reagents that target
their distinct phenotyprc or physiological
properties
The labeled cells are
passed through a nozzle tip (with an aperture of 70 pm> and Illuminated
one at a time with a laser set at a desired excitation wavelength.
Then
light-scattering
and fluorescence
emission properties
are collected with
an array of specific filters connected to their respective photomultiplier
tubes, which convey the signals to the computer. (C) By vibrating the
nozzle tip at high frequencies
(typically
24,000 Hz) and electronically
charging the mdivrdual droplets of salme in which each cell is suspended,
It is possible to sort specific populations of cells based on a distmct combrnation of then light scattering and fluorescence emission properties.
292
Mar/c,
Marie,
and Barker
fluorescence signals than conventional lamp-based fluorescence
microscopy and offers the advantage of precise and objective electronic quantification of different fluorescence intensities in tens
of thousands of cells virtually all at the same time. This is particularly important
in studies on the developmental
appearance
and
disappearance
of different cell markers, since it is often difficult
to distinguish
precisely
between
background
and very low
immunopositive
signals with conventional
methods. Figure 2 represents the results obtained
after immunostaining
acutely disso-
ciated and ethanol-fixed
El9 neocortical cells with a rabbit
polyclonal class IgG anti-nestin antibody, an intermediate filament
protein associated with neuroepithelium-derived
progenitor cells
(Hockfield
Bethesda,
and McKay,
1985) (a gift from R. McKay,
NIH,
MD), and a mouse monoclonal
class IgG anti-MAP2
antlbody, a neuronal cytoskeletal marker (Sigma, St. Louis, MO).
For flow cytometry,
these immunoreactions
were respectively
visualized with a phycoerythrin (PE)-conjugated goat anti-rabbit
IgG and biotinylated
goat anti-mouse
IgG (Jackson ImmunoResearch
Laboratories
Inc.,
West
Grove,
PA),
followed
by
Fig. 2. (opposzte page) Double immunolabeling
of cytoskeletal markers
in fixed cell preparations.
(A) Flow cytometric assessment of antmestm
and anti-MAP2
immunostaming
of El9 neocortlcal cells reveals four
distinct subpopulatlons.
Nestin- /MAP2-,
Nestm+/MAP2-,
Nestin-/
MAP2+, and Nestin+/MAl??
Whereas most of the Nestin+/MAP2and
Nestm/MAP2+
cells are located m the VZ/SVZ and CP/SP, respectively,
both regions contain Nestin+/MAlY
subpopulations.
However, there
are marked region-specific
fluorescence
intensity
differences
in both
cytoskeletal
markers
between these two subpopulations
Nestmhgh/
MAP2 expressors
are located in the VZ/SVZ and Nestinow/MAP2t~h
expressors
appear m the CP/SP (B) Immunostainmg
of acutely plated
CP/SP and VZ/SVZ
cells with the same antibodies
clearly reveals
MAl2hgh immunopositive
cells in the CP/SP and Nestinhgh cells m the
VZ/SVZ,
whereas the quantification
of Nestn+
and MAP2
subpopu-
lations IS somewhat
ambiguous using the light microscope
(C) Immunostaining
of the El9 coronal sections of the cortex under identical
conditions used for flow cytometry confirms that nestin-lmmunoposltlve
cell bodies are for the most part located in the VZ/SVZ, whereas MAP2-
lmmunopositlve
cells are present mainly
sections can not resolve the intensity
marker m individual
cells.
m the CP/SP
differences
However,
tissue
of either cytoskeletal
Anti-Nestin-PE
Anti-Nestin
Anti-Nestin
Anti-MAP2h..
Anti-MAP2
I
CP
SP
IZ
I.
svz
vz
Fig. 2(A-C)
293
i,,
I
!
i
294
Max,
Mar/c,
and Barker
streptavidin-Red670
(Life Technologies Inc., Gaithersburg, MD).
Cell immunofluorescence
characteristics were acquired using a
488 nm laser excitation and fluorescence filters set at 575 rt 25 and
670 _+ 20 nm to detect PE and Red670 emissions, respectively.
Reactions in acutely plated cells were visualized with appropriate blotinylated secondary antibodies, followed by streptavidinperoxidase (Jackson ImmunoResearch
Laboratories Inc., West
Grove, PA) and the development of peroxidase reaction product
in 3-amino-9-ethylcarbazole
(AEC) containing 0 001% HzO,.
Quantitative flow cytometric assessment of logarithimlcallyamplified anti-nestin and anti-MAP2 immunofluorescence intensities revealed different levels of nestin and MAP2 expression in
neocortlcal cells, as some transformed from progenitor stages in
the VZ/SVZ to more differentiated neuronal stages in the CP/SP
(Fig 2A). We akl y expressing nestin- and MAP2-immunoposltive
cells comprised distinct subpopulations in the flow cytometric
recordings, although they could not be easily accounted for under
the microscope (Fig. 2B), despite the fact that the percent of highexpressing immunopositive cells obtained with both methods was
quite similar. For example, it was very difficult to precisely quantify the large population (approx 50%) of nesti@ positive cells in
the CP/SP dissociates without a flow cytometer, even when the
antibody reaction in acutely plated cells was visualized with a
much more sensitive enzymatic endpoint, instead of a fluorescent
endpoint. Because of this objective, extremely sensitive and rapid
data acquisition, the results obtained with flow cytometry are often
more complete compared to the results obtained with conventional
microscopy techniques.
3.2. lmmunocharacterization
by Cell Surface Markers
Living CNS cells in different stages of neuronal and glial lineage progression can be identified using antibodies against distinct cell-surface markers. A variety of monoclonal antibodies are
now available that recognize specific ganghosides and other
epitopes on the plasma membranes of developing CNS cells. In
our studies, we have used a mixture of tetanus toxin fragment C
(TnTx) and a mouse monoclonal class IgG anti-TnTx antibody, a
marker of terminally postmitotic developing neurons (Koulakoff
et al., 19831, a mouse monoclonal class IgM anti-A2B5 antibody, a
neuronal and O-2A progenitor marker (Abney et al., 1983), a mouse
monoclonal class IgM anti-04, and a mouse monoclonal class IgG
CNS Development
295
Studies
anti-galacto-cerebroside
(GalC) antibodies (Boehringer Mannheim
Biochemicals, Indianapolis, IN), two markers of early and late
stages of oligodendrocyte lineage development (Raff et al., 1978,
Schachner et al., 1981). Acutely dissociated cells were double
labeled with different combinations of these antibodies and primary immunoreactions were then visualized by immunostaininmg
with PE-conjugated goat anti-mouse IgM antibody and a biotinylated goat anti-mouse IgG (Fey fragment specific) antibody
(Jackson ImmunoResearch
Laboratories, West Grove, PA) followed by streptavidin-Red670
(Life Technologies, Gaithersburg,
MD). Results of TnTx/A2B5
and GalC/04
double-immunostaining reactions at several ages and regions during CNS development revealing qualitative and quantitative
differences in
expressions and coexpressions of these surface epitopes are presented in Fig 3.
4. Assay of Proliferative
and Apoptotic
of Neocortical
Subpopulations
Potentials
It is well accepted that development of the CNS system involves
both cell proliferation
and naturally occurring cell death, or
apoptosis (Naruse and Keino, 1995). Here we show that these processes can be expeditiously
detected and quantified by flow
cytometry using fluorescently labeled antibodies against thymidine analog bromodeoxyuridine
(BrdU), a marker of S-phase cells
(Gratzner, 19821, annexin V, an anticoagulant protein that preferentially binds to phosphatidyl serine phospholipids exposed on
the outer leaflet of the cytoplasmic membrane early in apoptosis
(Koopman et al., 1994; Martin et al., 1995), and propidium iodide
(PI), a fluorescent dye that binds to all double-stranded
nucleic
acids and can be used to measure total DNA content (Dolbeare
et al., 1983).
4.1. Detection of BrdU Incorporation
by DNA -replicating
Cells
Timed pregnant dams at embryonic day 16 were given a single
intraperitoneal injection of BrdU (50 ug/g body weight) (Sigma)
and sacrificed 60 min later. The pups were removed and several
regions of the developing CNS acutely dissociated as previously
described. Detection of BrdU incorporation
was conducted by
permeabilizing the ethanol-fixed cells with 2 N HC1/0.5% Triton
296
Marie,
Neocortex K!P/SP)
Marie,
and Barker
Neocortex NZISVZI
I 1
32.3
Spinal Cords
PI
Anti-A2BS-PE
Neocortex
Fluorescence
Olfactory
Cerebellum
Bulb
Hippocampus
Rhombencephalon
1.7
hl
I7
Anti-040PE
Fluorescence
Fig. 3(A,B)
CNS Development
297
Studies
X-100 and immunoreacting the exposed DNA with FITC-conjugated
mouse anti-BrdU monoclonal antibody (Becton Dickinson). Finally,
the immunoreacted nuclei were counter-stained for total DNA content by resuspending the cells in PBS containing 5 ug/mL PI.
Bivariate distributions of BrdU incorporation and total DNA content were then assessed on a single-cell level by flow cytometry
(Fig. 4). Upon excitation at 488 nm, the green (FITC-conjugated
anti-BrdU) and red (PI) fluorescence intensities emitted by each
cell were acquired using bandpass filters set at 530 + 30 and
575 + 20 nm, respectively. Electronic gating was used to exclude
any residual cellular aggregates, which consistently accounted
for ~5% of the total number of events. The percentages of BrdU+
(S-phase cells) and BrdU- subpopulations with diploid or tetraploid DNA content (reflecting cells in GJG, and GJM stages of
the cell cycle, respectively) were quantified using a Cell Quest
data analysis system.
4.2. Detection
and Nuclear
of Plasma Membrane
Markers
of Apoptotic
Cells
Apoptotic cells can be first detected at the level of plasma membrane using annexin V (Koopman et al., 1994; Martin et al., 1995).
Fig. 3 fprevzous page) Double immunolabeling
of surface markers on
viable cell preparations
quantified by flow cytometry. (A) Anti-A2B5
and anti-TnTx immunostaining
of El9 neocortical cells reveals four distinct subpopulations.
A2B5-/TnTx-,
A2B5+/TnTx;
A2B5-/TnTx+,
and
A2B5+/TnTx+. Whereas all four subpopulations
can be found in the proliferative and early differentiating
VZ/SVZ regions of the El9 neocortex, the differentiating
CP/SP region is for the most part composed of
A2B5-/TnTx+ cells, which we independently
identified as a vrrtually pure
neuronal population using cytoskeletal markers and expressed morphological characterrstics m short-term cultures (see Fig. 2). Other mvestigated CNS regions at El9 reveal a variable presence of all of the above
populatrons with the exception of the hippocampus, which notably lacks
A2B5-immunopositive
cells. (8) Anti-04
and anti-GalC
immunoreactions of P6 neocortical cells also reveal 4 distinct subpopulations.
04-/GalC-, 04+/GalC-, 04-/GalC+, and 04*/GalC+. Whereas 04+/GalCsubpopulation
1s detected in all CNS regions tested at P6, the rhombencephalic and spinal cord regions exhibit the greatest abundance of
04+/GalC+
cells, with the former also showing the greatest percentage of 04-/GalC+
cells.
298
Marie,
Marie,
and Barker
HYPOTHALAMUS
CORTEX
Km
II n-. _<nLlm
RHOMBENCEPHALON
W-0
$4
e!
GdGv91.8%
SPINAL CORD
lumbosacral (SC 1)
bl:
9.4%
ag
;
43
\ +*,
SPINAL CORD
thoracic (SC t )
Gn/G1:86.0%
Fig. 4. Three-dimensronal
histograms of bivariate DNA data acquired
by flow cytometry illustrate an anatomical gradient of cellular prohferatron and differentiation
throughout
the neuroaxis at Elk Relatively
few cells are actively syntheslzmg new DNA in the cervical spinal cord
and rhombencephalon,
whereas cerebral cortrcal and lumbosacral
spinal cord populations
exhibit the greatest percentages of cells m S-phase
CNS Development
299
Studies
In our studies we have triple-stained acutely dissociated El9 neocortical cells with anti-A2B5-PE, anti-TnTx-Red670, and annexin
V-FITC and analyzed them by flow cytometry (Figs. 5A and B).
Green (FITC), orange (PE), and red (Red6701 fluorescence emissions were simultaneously
measured with bandpass filters at
530 _+30,575 A 20, and 670 + 20 nm, respectively. Percentages of
single-, double- and triple-positive and negative cells were quantified with the Cell Quest Analysis software using a logical gating
strategy.
Late stages of apoptosis were assessed by measuring total DNA
content of fixed cells stained with PI (Fig. 50, Cells doubleimmunoreacted
with A2B5-PE and TnTx-Red670 were sorted
based on their surface epitopes into A2B5-/TnTx-, A285+/TnTx-,
A2B5-/TnTx+, and A2B5+/TnTx+ subpopulations (seeSection 7.1.)
and fixed in 70% ethanol. The A2B5 and TnTx immunoreactions
were then stripped off the cell membranes in Triton/HCl
solution and the cells further processed for detection of their total DNA
content (seeSection 4.1.). Late apoptotic or A,, cells were identified as cells with hypodiploid DNA content with respect to that
of G,/G, cells, which have diploid DNA. This cytometric property, also referred to as sub-G,/G, peaks, depicting cells undergoing DNA fragmentation, has been shown to be a reliable marker
of cell death by apoptosis (Telford et al., 1991; Darzynkiewicz et
al., 1992; McCloskey et al., 1994).
5. Potentiometric
of Dissociated
Signals
Embryonic
Neocortical
Cells
One of the most crucial processes in CNS cytogenesis is the
development of membrane excitability. Although classical electrophysiological techniques have been extensively used to characterize membrane receptor/channel
and ion properties of cells,
technical difficulties can be encountered in recording small, proliferating, and immature cells, which constitute the majority of
cytoarchitecture during the earliest stages of CNS development
(reviewed by Barry and Lynch, 1991). In addition, microelectrode
techniques can be invasive to the cell membrane and only a limited number of cells can be recorded at any one time under the
same experimental conditions. One way of overcoming these difficulties is to use noninvasive techniques with fluorescent voltage-sensitive indicator dyes. Several studies have already reported
300
Marie,
Marie,
and Barker
A2BS+iTnTx+
A2B5PE
Immunofluorescence
Annexin V-FITC
Fluorescence
A2BS+fhTf
Propidium
Iodide Fluorescence
Fig. 5. Flow cytometric assessment of early and late apoptotic neocortical cells at E19: (Al Live, unfixed cells were triple stained with antiA2B5 and anti-TnTx antibodies
and annexin V-FITC. We have used
electronic gates (depicted by the cross-hairs) on A2B5 and TnTx doubleimmunoreacted
cells to reveal their expression of annexin V. (B) Annexin
V-FITC binding to the plasma membranes reveals a differential
presence of annexin V and annexin Vhigh-positive cells in the four immuno-
CNS Development
301
Stud/es
the utility of voltage-sensitive dyes in investigations of potentiometric signals of developing CNS tissues using digital video
microscopy (Walton et al., 1993) and flow cytometry (Mandler et
al., 1988; Krieger et al., 1991; di Porzio et al., 1993; Fiszman et al.,
1993). The advantage of these methods is that they allow experimental access to the physiological properties of entire populations
of intact cells regardless of their size or morphology, and can provide a statistically complete account of potentiometric signals randomly acquired from hundreds to thousands of individual cells
within a matter of seconds.
In our studies, we have used flow cytometry and oxonol, an
anionic voltage-sensitive indicator dye that partitions into the cells
according to membrane potential (Petit et al., 1993), to investigate
the development of membrane excitability throughout the embryonic CNS. After papain dissociation, cells were resuspended in a
physiological saline (in n-&I): 145 NaCl, 5 KCl, 1.8 CaCl,, 0.8 MgCl,,
10 HEPES,
10 glucose,
and 1 mg/mL
fatty-acid-free
bovine
serum
albumin (Sigma), the pH and osmolarity of which was adjusted
to 7.3 and 290 mOsm, respectively. Cells were then stained with
bis(l,3-dibutyl
barbituric acid) trimethine oxonol (Molecular
Probes, Eugene, OR), a potentiometric
dye that is negatively
charged at physiological pH. Since virtually all living cells exhibit
negative potentials, the dyes negative charge opposes its cellular
accumulation at resting potentials, whereas depolarized cells stain
lo-fold
or more
relative
to cells at rest. All of the potentiometric
results were recorded using 200 nM oxonol, since this concentration effectively stains cells well above autofluorescence levels.
Staining with oxonol requires approx 2 min to equilibrate at room
temperature, after which the mode and distribution of fluorescence signals remain stable for at least the duration of the typical
(Frg. 5, contznuedfvom prevtous page) identified subpopulatlons
Separate
experiments
using unfixed cells stained with annexm V-FITC and PI
revealed that only a few of annexin V and the majority of annexin
Vh~hcells were PI positive, demonstrating
that membrane permeability
of most annexm Vi cells is not significantly
compromised,
thus mdicatmg the early phase of apoptosis. (Cl PI staming of sorted and fixed
A2B5-ITnTx-,
A2B5+/TnTx-,
A2B5-/TnTx+,
and A2B5+/TnTx+ subpopulations reveals a percentage of hypodiploid
cells that positively
correlates with the percentage
of annexin V-positive
cells m the same
subpopulations
302
Marie,
Marie,
and Barker
recording period (5-15 min). Oxonol-stained cells were passed
through a flow cytometer at a rate of 2000 cells/s, excited one at a
time by 488 nm and the resulting emissions detected with a single
filter set at 530 + 30 nm. A typical recording involved the acquisition of oxonol fluorescence intensities of 10,000 randomly sampled
cells recorded over 5 s, the distribution of which was then plotted
as a single-parameter frequency histogram (Fig 6).
5.1. Calibration
of Membrane
Potential
The relationship between oxonol fluorescence intensity and cell
membrane potential can be calibrated by recording the oxonol fluorescence intensity profile under resting conditions and again after
exposing cells in the same test tube to gramicidin, a monovalent
cationophore previously used to relate oxonol fluorescence to theoretical membrane potential in spectrophotometric (Breuer et al.,
1988; MacDougall et al., 1988; Cruciani et al., 1991; Brent et al.,
1993) and flow cytometric studies (di Porzio et al., 1993). In electrophysiological recordings, 1 PM gramicidin depolarizes cells to
0 mV in physiological saline. In our recordings, we have empuitally determined that saturating concentrations (21 PM) of gramicidin increase the fluorescence modes of oxonol-stained cortical
cells in a [Na+lO-dependent manner (Fig. 6). These results allowed
us to use a Goldman-Hodgkin-Katz
formulation to relate oxonol
fluorescence to membrane potential and to calibrate the signals
Assuming that after gramicidin permeabilization total intracellular concentration of permeant Na and K cations remains constant at approx 150 mM during the 10-s recording period, then
oxonol fluorescence modes of the signals in altered Na+O salines
can be related to membrane potential using a simplified GoldmanHodgkin-Katz equation in which the membrane potential is E, E0 = RT/ZF log [Na + K+ll/[Na+ + K+lO,where R, T, Z, and F have
their usual meanings. Modes of oxonol fluorescence can be calibrated in terms of membrane potential over much of the physiological range, i.e., -90 mV-0 mV (Fig. 6B).
5.2. Survey of Membrane
in Developing
Neurons
Excitability
We have investigated the chemosensitivity of acutely dissociated El9 CP/SP neurons to saturating concentrations of various
neuroactive agents including acetylcholine, y-aminobutyric acid
CNS Development
303
Studies
Oxonohained
pmicidin-treated
[Na+l,bM)
0
145
+I0
V,,,= 0307FL,,-228
R = 0.99
0
gB
145
-10
7257
'i;j -20
P
$ -30
2
207
-40
if! -50
aa
69
P -60
0
3 -70
RMP
-80
/
-loll~-~
400
Oxonol
Fluorescence
Intensity
(Channels)
450
Oxonol
500
550
Fluorescence
600
650
Intensity
700
750
4
800
(Channels)
Fig 6. Calibration of oxonol fluorescence signals in terms of estrmated
membrane potential values. (A) Oxonol-stained
neurons isolated from
the El9 CP/SP were resuspended in salines containing varying iNa+&
(145, 72.5, 20.7,6.9, and 0 n-M), which was replaced by equimolar concentrations of membrane impermeable
N-methyl-D-glucamine.
The cells
were then treated with 1 uM gramicidin
and their fluorescence levels
recorded (B) The modal fluorescence values of the oxonol fluorescence
distributions
(FL,,) m different [Na+10 are plotted against theoretical
membrane
potentials
(V,,,) as calculated
by a simplified
GoldmanHodgkin-Katz
equation, The slope of this relationship
reveals a relatively constant conversion factor, which defined that a change in approx
3 fluorescence channel units in oxonol intensity is equivalent to 1 mV
change in membrane potential
By substituting
the modal oxonol fluorescence value of El9 CP/SP neurons under our control resting conditions for FL,,, we estimated the modal resting membrane potential of
these cells at -85 mV.
(GABA), glycine, kainic acid (agonist of a subtype of glutamate
receptors), and veratridine (agonist of voltage-dependent
Na+
channels). Potentiometric responses were quantified in terms of
percentage of responsive cells and the amplitude of the responses,
which were, after calibration, converted into mV. After recording
a control profile, cells were exposed to different ligands and change
in oxonol fluorescence recorded after 2 min. All recordings were
performed at room temperature. The heterogeneity of excitatory
responses obtained in recordings of these cells (Fig. 7) indicate
304
Mar/c,
f;
a
1
:9
B 60
z
Mar/c,
and Barker
40
c2
20
80
60
I
loo
40
20
.
(;*:*?,
\:j::
.J
:
Membrane
Potential
(mV)
Fig. 7. Excitatory
membrane
potential
responses
of El9 CP/SP
neurons.
Approx
90-95% of cells depolarize
to either 10 PM GABA
or 100 pM veratridine,
whereas less than 5% depolarize
to 10 FM acetylcholme.
Veratrldme
depolarizes
cells to +20 mV, whereas
GABA
CM
Development
30.5
Studies
that FACS potentrometry
can serve as a powerful
tool for the
investigation
of the cellular distribution
of functional
receptor/ion
channels in different subpopulations
of developing
CNS tissues.
6. Intracellular
Calcium Signals
of Dissociated Embryonic Neocortical
Cells
Measurement
of cytoplasmic
calcium
([Ca],)
concentrations
in living cells is of great interest to many investigators,
since Ca*+
is a ubiquitous
second messenger throughout
the CNS. Cazcc signals have been implicated
in the development
of many neuronal
and glial cell functions.
Cell survival
(Spitzer, 1994) and death
(Franklin
and Johnson,
1994), neurotransmitter
release (Hille,
1992), growth cone motility
and neurite elongation
(Mattson and
Kater, 1987), cell migration
(Komuro
and Rakic, 1992), synaptic
plasticity
(Bear and Malenka,
1994), and regulation
of gene
expression
(Gallin and Greenberg,
1995) are only some of the Ca2+related processes that have been described.
Investigation
of the
mechanisms
that regulate intracellular
Ca2+ during histogenesis
of the CNS is therefore of crucial importance
in understanding
the physiology
of cell proliferation,
migration,
differentiation,
death, and cell-to-cell
communication.
Initially,
electrophysiological
methods were used to study Ca2+
homeostasis
and plasma membrane
expression
of Ca2+-selective
channels. However, recent development
of a growing family of Ca*+sensitive fluorescence
indicator
probes has led to alternative
optical and confocal microscopic
strategies of recording
Ca*+signals at
cellular and subcellular
levels. One such probe is l-[2-amino-5-(2,7dichlor-6-hydroxy-3-oxy-9-xanthenyl)
phenoxyl-2-(2-amino-5methyl
phenoxy)
ethanel-N,N,N,N-tetra-acetic
acid or Fluo-3.
Flu03 is a fluorescein-derived
Ca*+-sensitive
dye that produces a
40-fold increase in fluorescence
intensity
upon bmding
with free
(Fzg 7, confinuedfvom previous page) depolarizes virtually all cells to -40
mV. Only 50% of cells depolarize to -40 mV after exposure to 100 PM
glycme, whereas 100 PM kainic acid depolarizes approx 70% of cells
mainly to approx 0 mV. Gramicidin,
which chemically clamps all cells
at 0 mV by permeabilrzing
their plasma membranes with monovalent
cation-selective
channels, is always used as a control at the end of each
experiment
to reveal the fluorescence mtensrty and distribution
of
potentrometrlc
srgnals corresponding
to 0 mV
306
Mar/c,
Marie,
and Barker
cytosolic calcium (Minta et al., 1989). Although other types of Ca*+sensitive dyes have been described, e.g., quin-2, fura- and indo-l
(reviewed by Tsien, 1980; Grynkiewicz et al., 19851, the major advantage of Fluo-3 lies in its high signal-to-noise resolution, low cytotoxic
and mitogenic properties as well as in optimal excitation properties,
at wavelengths in the visible as opposed to UV range (Tsien, 1989).
In our studies, we have used Flu03 in conjunction with flow
cytometry in order to reveal and characterize, on qualitative and
quantitative levels, the presence of functional intracellular Ca*+stores,
Na+-Ca*+ exchange mechanisms, and several voltage- and ligandstimulated Ca2 channels in acutely isolated El9 CP/SP neurons. The
cells were loaded with 1 PM Flu03 for 45 mm at room temperature,
then washed and resuspended in physiological saline, passed through
a flow cytometer, excited one at a time by 488 nm and the resulting
emissions detected with a single filter set at 530 + 30 nm.
6.1. Calibration
of [Ca2+lc
The fluorescence of Fluo-3-loaded cells is measured in arbitrary
intensity units, i.e., fluorescence channels, which can be converted
into estimated [Ca], values after the calibration procedure is performed (Kao et al., 1989) at the end of each experiment (Fig. 8). Fluo3-loaded cells were acutely treated with lo-20 PM ionomycin, a Ca*+
ionophore, and the resulting saturated levels of Flu03 fluorescence
then maximally quenched by the addition of 2 mM MnCl,. [Ca2+lclevels under resting and experimental conditions were calculated
according to the following equation: [Ca*+], = Kd x [F - F,,,] / [FmaX- F].
Kd is defined as the dissociation constant for Ca2+-bound Flu03 and
is 400 nM at room temperature (Minta et al., 1989). Fmaxrepresents
the maximum Flu03 fluorescence value, whereas F represents the
fluorescence value of cells under resting or experimental conditions.
FmInis defined as the minimum Flu03 fluorescence in the presence
of saturating concentrations of MnCl,. Since Mn*+ ions readily displace Ca*+ ions from Flu03 (Hesketh et al., 1983) and the Mn*+/Fluo3 complex is only one fifth as fluorescent as Ca*+ /Flu03 complex
(Kao et al., 1989; Minta et al., 19891, F,,, is then calculated as follows
(Vandenberghe and Ceuppens, 1990): F,,, = F,,, - (FmuX- F,,,,,,) x
1.25. In our flow cytometric experiments, the fluorescence values of
F, Lx~ and h4na2 were defined as the arbitrary channel number of
the mode of Flu03 fluorescence distributions recorded from 10,000
randomly sampled cells under appropriate resting and experimental conditions (Fig. 8).
CNS Development
Studies
9000
8000
F,,= 814
Fbfna2= 451
Fmln= 360
7000
6000
ct-"
5000
cl
4000
ICa2+lc = 400
3000
Flue-3Fluorescence
Intensity
Khannels)
2000
1000
0
400
440
400
520
560
Flue-3 Fluorescence
600
640
Intensity
l""I"T'~l~~~~l600
720
760
(Channels)
Fig. 8 Calibration
of Fluo-3 signals. Flu03 loaded El9 CP/SP neurons were treated wrth 10 pM ionomycin
for 2 min followed by 2 mM
MnCl, for an additional
1 min. F,,, and FMvlnCIz
were measured from the
modal values of the resulting Fluo-3 fluorescence distributions.
By substituting the modal Fluo-3 fluorescence value of El9 CP/SP neurons
under our control resting conditions for F, we estimated the modal resting lCaz+lc levels of these cells at approx 140 nM
6.2. Survey
of the Contributors
to Calcium
Homeostasis
Regulation
of Ca 2+ levels in most cells is achieved
through
interactions
of Ca2+ &ansport
mechanisms
in the plasma
and
endo(sarco)plasmic
reticulum membranes
and Ca*+ buffering mechanisms in the cytoplasm (Kostyuk and Verkhratsky,
1994). Ca2+ transport in transmembranes
is regulated by different Ca*+ channels, Ca*+
pumps, and Ca*+ exchangers, whereas intracellular
Ca2+ stores and
Ca*+-binding
molecules serve as a Ca2+-buffering
system.
In our studies, we have surveyed the developmental
expression of several mechanisms
involved
in Ca2+ homeostasis
of El9
CP/SP neurons. intracellular
calcium stores, Na+-Ca*+-exchange,
and voltage-sensitive
calcium channels (VSCC) (Fig 9). Intracel-
rl
1100
308
Marie,
Max,
and Barker
Voltaee-Sensitive
Cytosolic Calcium Concentration
control
Calcium Channels
(CLM)
1
.=I1
I *sa
Fig. 9. Survey of several mecharusms
involved in Ca2+ homeostasis
in El9 CP/SP neurons
(A) All cells exhibit
micromolar
levels of calcium mobilization
after treatment
with 10 uA4 ionomycin,
a Ca2+ ionophore.
Whereas
thapsigargin
elevates [Ca2+lc to submicromolar
levels m virtually
every cell recorded,
only 64% and 35% of cells
respond to caffeine and ryanodine stimulation,
respectively.
(Bl Resuspension
in Na+O-free saline produces approx
100 nM increase of [Ca2+lc in the majority of cells, and a micromolar
increase of [Ca2+Jc in the remaining
5% of the
cells. Subsequent exposure of the same cells to 10 mM caffeine produces nucromolar
levels of [Ca], in many cells,
which are sustamed even after 5 min of continuous
recording
(right-most
panel) This response is in sharp contrast
to the response of the cells suspended
in regular physiological
saline (145 n-&I Na+,), where caffeine produces
a
transient increase in [Ca*+lc that recovers to resting levels wlthm 5 min of stimulation
in most cells These results
suggest that Na+-Ca*+-exchange
may play an important
role in regulatron
of resting levels of [Ca2+lE. (Cl Stimulation of El9 CP/SP neurons with 40 mM K+O produces a significant calcium entry in 65% of the population,
which is
totally abolished by repeating the experiment
in Ca2+0- free saline. A complete block of calcium entry induced with
40 mM K0 is also achieved by pre-exposing
the cells to 100 uM nitrendrpine,
implying that virtually
all cells have
L-type VSCC. Prestimulation
of cells with 100 nM o-conotoxin
GVIA or 100 nM o-agatoxin
VIA reveals that 18% of
the cells lack N-type and 23% lack P-type VSCC, respectively.
loo
310
Marie,
Marie,
and Barker
lular calcium stores were studied by resuspending the cells in Ca2+free saline and then stimulating them separately with 10 mM caffeine, 10 pM ryanodine, 10 pM thapsigargin, or 10 uM ionomycin
(Fig. 9A). Na+-C a*+-exchange mechanisms revealed under resting
conditions and after exposure to caffeine were studied by resuspending the cells u-t Na+O-free salines (Fig. 9B). Functional L-type,
N-type, and P-type VSCC were individually
revealed by exposing the cells suspended in physiological salme to 40 mM K*O in
the presence of nitrendipine, o-conotoxin GVIA, or cu-agatoxin
VIA, respectively (Fig. 90. Due to the transient nature (lo-300 s
range) of the Ca2+cresponses obtained with some of the above conditions, these recordings involved the acquisition of Ca2+csignals
at higher rates (3000 cells/s) than used in other experiments. In
addition, the dead time between application of the stimulus and
the recording was reduced to approx 2 s by using a Time Zero
module equipped with an injector system (Cytek Development,
Fremont, CA).
6.3. Caztc responses to neurotransmitter
ligands
We tested Ca2+cresponses in El9 CP/SP neurons to asymptotic
concentrations of several neuroactive agents, including acetylcholine, GABA, glycine, kainic acid, and veratridine. The responses
were recorded using a Time Zero module as described above. After
recording a control profile, cells were exposed to different ligands
and changes in Fluo-3 fluorescence recorded after approx 2 s. Typical peak Ca2+cresponses, recorded at room temperature, are illustrated in Fig. 10.
7. Flow Cytometric
Sorting
of Embryonic Neocortical
Subpopulations
The studies of specific populations of the CNS in vitro are complicated by our limited abilities to unequivocally
identify and
expeditiously isolate pure cell types. Investigators commonly use
Fig. 10. (upposzte page) Survey of Ca2+c responses of El9 CP/SP neurons to several neuroactrve
ligands After the addrtion of 10 pM acetyl-
choline, approx 70% of the cells exhibit an immediate submicromolar
rise in [Ca2+Jc that recovers to resting levels within 2 min of stimulation
(kinetics data not shown). At peak response, GABA and kainic acid mduce a Ca2+c rise in approx 60% of neurons to submrcromolar
and micro-
CNS Development
Studies
Rcs(fllg
,f J
SO-
SO
40-
100
0 OS 3
1 ZSIO
I
60-
;!
,
40-
00512
Cytosolic
Calcium
Concentration
(PM)
molar levels, respectively,
whereas glycine affects 30% of the cells,
elevating their Ca 2+cby 400 nM. Veratridine affects approx 90% of cells,
increasing [Ca+] levels above 1 PM. Ionomycin typically mduces a maxImum rise in [Ca5+lc in all cells recorded.
312
Max,
Marie,
and Barker
selective culture conditions to isolate neurons, astrocytes, oligodendrocytes, and other cell populations. However, these methods usually require several days to weeks of culturing, during which
time cell properties may change and no longer reflect those
expressed in vivo. A variety of methods now exist that permit
enrichment of specific subpopulations based on surface epitopes (i.e.,
panning and complement lysis). However, these are complicated by
the fact that many antigenic epitopes are shared among different cell
types during development and hence a combination of markers is
required for the identification and isolation of specific cell subpopulations. Using a flow cytometer equipped for sorting, it is possible to
isolate very pure specific cell subpopulations based on the presence
of multiple phenotypic or functional cell markers.
7.1. Sorting Based on Surface Epitope Expression
El9 neocortical cells were double immunostained with anti-A2B5
and TnTx antibodies, as described previously (seeSection 3.2.) and
categorized into four populations (TnTx+/A2B5-, TnTx+/A2B5+,
TnTx-/A2B5+, and TnTx-/A2B5-) based on their fluorescence signatures determined by FACS electronic gates (Fig. 11, left most
panel). The four populations were sorted by means of electrically
charged saline droplets, which were deflected by charged plates
directly into appropriate test tubes (see Fig. 1). Sorted cells were
then washed twice in physiological saline and re-analyzed to test
for sorting purity, which was greater than 96% in all cases (Fig.
11, four right panels). After sorting, the viability of the cells
remained unchanged, with less than 5% trypan blue or PI-positive (dead or dying) cells in every sorted subpopulation.
7.2. Sorting Based on functional
Response
Flow cytometers equipped for sorting also have a unique capability of isolating purified responding and nonresponding
cell
populations based on sustained or transient functional responses
in different cells. El9 CP/SP neurons stained with oxonol or loaded
with Fluo-3 were stimulated with 100 pM kainic acid or 10 PM acetylcholine, respectively. Responding and nonresponding subpopulations were sorted based on a sustained membrane depolarization
induced by kainic acid or a transient calcium increase induced by
acetylcholine using electronic gates as shown in Fig. 12 (shaded
areas>. To test for the purity of kainic acid responding
and
CNS Development
Studies
I2
Y4
a,- - - - - - -, 2
I
I
314
Maw,
Maw,
and Barker
Before Sorting
loo .
Kainlc Acid
(59%)
Sorted-Responders
loo .
100
Acetylcholine
Kainic Acid
,I!
80-
t
, I
1 ,
60-
.
;
40-
Sorted-Non-Responders
l-
Membrane
Potential
(mV)
Fig. 12
[Cytosolic
Calcium]
(PM)
CNS Development
315
Studies
nonresponding populations after sorting, the cells were rinsed
twice in physiological saline, restained with oxonol and restimulated with 100 IAM kainic acid. Virtually all sorted responders
depolarized again after restimulation, confirming the purity and
functional viability of the sort. By contrast, none of sorted nonresponder cells depolarized to kainic acid after restimulation.
Similarly, acetylcholine restimulation of sorted acetylcholineresponding and nonresponbding subpopulations revealed >95%
purity of each sort. The results confirm that functional sorting
according to both membrane potential and Ca2+cresponses is very
effective, and provides the opportunity for further study of very
specific cell subpopulations in developing CNS.
8. Conclusion
In this chapter, we have described several strategies
fying
and studying
and physiological
cytometry.
different
phenotypic,
proliferative,
properties of developing
The sort capability
for identiapoptotic,
CNS cells using flow
of flow cytometers
further
allows
isolation and purification of subpopulations of CNS cells expressing specific epitopes or functional receptors for more detailed cellular and molecular
analyses in culture. With
have begun to map the biological
properties
these strategies, we
of CNS cells in the
context of lineage progression. In sum, the versatility,
objectivity
and sort capability
of flow cytometry
may be ideally suited for
confronting
the complexity
of CNS development,
providing
an
unparalleled
perspective
on the distribution
of physiologically
relevant properties
as the cells transform
from proliferative
to a
more differentiated
state.
Fig. 12. (previous page) Functional sorting of responding and nonresponding cells according to membrane potential and calcium signals.
El9 cells were loaded with either oxonol, a voltage-sensitive
dye (panel
A), or Fluo-3, a calcium-sensitive
dye (panel B) and sorted into responding and nonrespondmg
populations
after the addition of 100 ~JM kainic
acid to oxonol-loaded
cells or 10 PM acetylcholine to Fluo-3-loaded
cells
(sorting gates are shown as shaded areas). Reanalyses of sorted and
restimulated
subpopulations
revealed > 95% purity of functionally
responsive and nonresponsive cells.
316
Marie,
Marie,
and Barker
Bibliography
Abney, E R , Wllhams, B P , and Raff, M C. (1983) Tracmg the development of
olrgodendrocytes
from precursor cells using monoclonal antrbodies, fluorescence-activated cell sorting, and cell culture Dev Bzol 100, 166-171
Altman, J and Bayer, S A (1995) Atlas of Prenatal Rat Brazn Development, CRC
Press,Boca Raton, FL
Barry, P H and Lynch, J. W (1991) Liquid Junctron potentials and small cell
effects m patch-clamp analysis [published erratum appearsm ] Membr Blol
1992Feb,125(3)2861 ] Membr Brol 121,101-117
Bear, M F and Malenka, R C (1994)Synaptic plastlclty LTP and LTD Curr
Open Neurobtol
4,389-399
Brent, L. H , Gong, Q , Ross,J M , and Wreland, SJ (1993) Mrtogen-activated
Ca++channelsm human B lymphocytes J Cell Physrol 155,520-529
Breuer, W V , Mack, E , and Rothstem, A (1988)Actlvatron of K and Cl- channels by Ca2+and cyclrc AMP m dlssocrated kidney eprthehal (MDCK) cells
Pflugers Arch 411,450-455
Cruclam, R A , Barker, J L , Zasloff, M., Chen, H C , and Colamomcr, 0 (1991)
Antrbrotrc magamins exert cytolytrc actrvrty against transformed cell lines
through channel formatron. Proc Nat1 Acad Sci USA 88,3792-3796
Darzynkrewlcz, Z , Bruno, S , Del Bmo, G , Gorczyca, W , Hotz, M A, Lassota,
P , and Traganos, F (1992) Features of apoptotrc cells measured by flow
cytometry Cytometry 13, 795-808
dr Porzro, U , Smith, S V , Novotny, E A , Morelh, F , and Barker, J L (1993)
Two functronally different glutamate receptors of the kamate subtype m embryonic rat mesencephahccells Exp Neural 120,202-213
Dolbeare, F , Gratzner, H , Pallavrcml, M G , and Gray, J W (1983) Flow
cytometric measurement of total DNA content and mcorporated bromodeoxyurrdme Proc Nat1 Acad Scz USA 80,5573-5577
Flszman, M L , Behar, T , Lange, G D , Smith, S V , Novotny, E A, and Barker,
J L (1993) GABAerglc cells and signals appear together in the early postmrtotic period of telencephalrcand strratal development Brazn Res Dev Brain
Res 73,243-251
Franklin, J L and Johnson, E M (1994) Block of neuronal apoptosts by a sustamed increase of steady-state free Ca2+concentration Phllos Trans Royal
Sot London B Brol Scl 345,251-256
Gallm, W J and Greenberg, M E (1995)Calcium regulation of gene expression
m neurons the mode of entry matters Curr Opm Neurobrol
5,367-374
Gratzner, H G (1982) Monoclonal antibody to 5-bromo- and 5-rododeoxyurrdme a new reagent for detection of DNA repllcatlon Scrence 218,
474-475
Grynkrewrcz, G , Poeme, M , and Tslen, R Y (1985) A new generation of Ca2+
mdrcators with greatly improved fluorescence properties ] Blol Chem 260,
3440-3450
Hebel, R and Stromberg, M W (1986)Anatomy and Embryology @the Laboratory
Rat, BloMed Verlag , Worthsee
Hesketh, T R, Smith, G A, Moore, J P , Taylor, M V, and Metcalfe, J C
(1983) Free cytoplasmrc calcium concentratron and the mltogemc strmulatron of lymphocytes J Blol Chem 258,4876-4882
CNS Development
Studies
317
Hille, B (1992) lonlc Channels of Excttable Membranes, Smauer Assocrates,
Sunderland, MA
Hockheld, S and McKay, R D. (1985) Identification of major cell classes m the
developing mammalian nervous system J Neuroscr 5,3310-3328
Huettner, J E and Baughman, R W (1986) Primary culture of identified neurons from the visual cortex of postnatal rats. I Neurosct 6,3044-3060.
Johnson, M I and Argiro, V. (1983) Techniques m the tissue culture of rat sympathetic neurons Mefhods Enzymol 103,334-347
Kao, J P , Harootuman, A T , and Tsien, R. Y. (1989) Photochemically generated cytosollc calcium pulses and therr detection by flue-3 I Brol Chem 264,
8179-8184
Komuro, H and Rakrc, P (1992) Selective role of N-type calcium channels m
neuronal migration. Scrence 257,806-809.
Koopman, G., Reutelmgsperger, C P , Kuilten, G A, Keehnen, R M , Pals, S.
T , and van Oers, M H (1994) Annexm V for flow cytometric detection of
phosphatidylserme
expression on B cells undergoing apoptosls Blood 84,
1415-1420
Kostyuk, P and Verkhratsky,
A (1994) Calcium stores m neurons and gha
Neuroscience 63,381-404
Koulakoff, A, Bizzmi, B , and Berwald-Netter,
Y (1983) Neuronal acqursrtion
of tetanus toxin bmdmg sites relatronship with the last mrtotic cycle Dev
Bzol 100,350-357
Krueger, C., Pull, E , and Kim, S U. (1991) Development of resting membrane
potentials of embryonic murme spinal cord cells evaluated by flow cytometric
analysis Dev Neuroscl 13,11-19
MacDougall, S L , Grmstein, S , and Gelfand, E W (1988) Activation of Ca2+dependent K channels m human B lymphocytes by anti-immunoglobulin
J
Clan lnvest 81,449-454
Mandler, R N., Schaffner, A E , Novotny, E A., Lange, G D , and Barker, J L
(1988) Flow cytometric analysis of membrane potential in embryomc rat sprnal cord cells. J Neurosct Meth 22203-213.
Marie, D , Marie, I, Ma, W., Lahjoulr, F , Somogyi, R , Wen, X , Sieghart, W ,
Fritschy, J-M, and Barker, J L (1997) Anatomical gradients m proliferation
and differentiation of embryonic rat CNS accessed by buoyant density fractionation a3, 83 and y2 GABA, receptor subunit co-expression by post-mrtotrc neocortical neurons correlates directly with cell buoyancy
Eur J
Neuroscl 9,101-116
Martin, S J , Reutelmgsperger, C P , McGahon, A J , Rader, J A , van Schie, R
C , LaFace, D M , and Green, D R (1995) Early redistribution
of plasma
membrane phosphatidylserme
IS a general feature of apoptosls regardless
of the imtratmg stimulus mhrbitron by overexpression of Bcl-2 and Abl. J
Exp Med 182,1545-1556
Mattson, M I and Kater, S B (1987) Calcium regulation of neurite elongation
and growth cone motility J Neuroscz 7,4034-4043
McCloskey, T W , Oyaizu, N , Coronesl, M., and Pahwa, S (1994) Use of a flow
cytometric assay to quantitate apoptosis in human lymphocytes
Clan
Immunol lmmunopathol 71,14-18
Melamed, M. R , Lmdmo, T., and Mendelsohn, M. L. (1990) Flow Cytometry and
Sortrng, Wiley-Liss, NY
318
Mar/c,
Marie,
and Barker
Minta, A , Kao, J I-, and Tsien, R Y. (1989) Fluorescent mdlcators for cytosolic
calcium based on rhodamme and fluorescem chromophores
J Blol Chem
264,8171-8178
Naruse, I. and Keino, H (1995) Apoptosis m the developmg CNS Prog Neurobrol
47,135-155
Petit, J M , Derus-Gay, M , and Ratinaud, M. H (1993) Assessment of fluorochromes for cellular structure and function studies by flow cytometry Blol
Cell 78, 1-13
Raff, M C , Mirsky, R, Frelds, K L , Lisak, R I, Dorfman, S H , Srlberberg, D
H , Gregson, N A, Leibowitz, S , and Kennedy, M. C. (1978) Galactocerebroslde is a specific cell-surface antigemc marker for ohgodendrocytes
in culture Nature 274,813-816
Schachner, M , Kim, S K , and Zehnle, R (1981) Developmental expression m
central and peripheral nervous system of olrgodendrocyte
cell surface
antrgens (0 antigens) recognized by monoclonal antibodies Dev Brol 83,
328-338
Schaffner, A E and Daniels, M P (1982) Conditioned medium from cultures
of embryonic neurons contains a high molecular weight factor which
induces acetylcholme
receptor aggregation on cultured myotubes
1
Neurosci 2,623-632
Spitzer, N. C. (1994) Spontaneous Ca*+ spikes and waves in embryonic neurons
slgnalmg systems for differentiation. Trends Neuroscr 17, 115-118
Telford, W. G., King, L. E , and Fraker, P J (1991) Evaluation of glucocorticoidinduced DNA fragmentation in mouse thymocytes by flow cytometry Cell
Pro14 24,447-459
Tsien, R Y (1980) New calcium indicators and buffers with high selectivity
against magnesium and protons. design, synthesis, and properties of prototype structures. Bzochemistry 19,2396-2404
Tsien, R Y. (1989) Fluorescent probes of cell signaling Ann Rev Neurosct 12,
227-253.
Vandenberghe, P. A. and Ceuppens, J. L (1990) Flow cytometric measurement
of cytoplasmic free calcium m human peripheral blood T lymphocytes with
fluo-3, a new fluorescent calcurm indicator J Immunol Meth 127,197-205
Walton, M K., Schaffner, A E , and Barker, J L. (1993) Sodium channels, GABA,
receptors, and glutamate receptors develop sequentrally on embryomc rat
spinal cord cells J. Neuroscl 13,2068-2084
Você também pode gostar
- Human Chromosomes: An Illustrated Introduction to Human CytogeneticsNo EverandHuman Chromosomes: An Illustrated Introduction to Human CytogeneticsNota: 5 de 5 estrelas5/5 (1)
- Principles, Applications and InterpretationsDocumento64 páginasPrinciples, Applications and InterpretationsAbbi Yanto ArtAinda não há avaliações
- Tissue Engineering and Regeneration in Dentistry: Current StrategiesNo EverandTissue Engineering and Regeneration in Dentistry: Current StrategiesRachel J. WaddingtonAinda não há avaliações
- Flow CytometryDocumento7 páginasFlow CytometryWAinda não há avaliações
- Chapter 2- Jnu Techniques in Cell and Molecular Biology - 複本Documento40 páginasChapter 2- Jnu Techniques in Cell and Molecular Biology - 複本Wai Kwong ChiuAinda não há avaliações
- Flourscence Activated Cell SortingDocumento6 páginasFlourscence Activated Cell SortingSalman KhanAinda não há avaliações
- 1 Repiska 2010Documento6 páginas1 Repiska 2010Thamyres BrancoAinda não há avaliações
- Flow Cytometry: Meroj A. JasemDocumento58 páginasFlow Cytometry: Meroj A. Jasemahmad100% (1)
- 824-Texto Del Manuscrito Completo (Cuadros y Figuras Insertos) - 4381-1!10!20120828Documento6 páginas824-Texto Del Manuscrito Completo (Cuadros y Figuras Insertos) - 4381-1!10!20120828Tallie ZeidlerAinda não há avaliações
- Photonics: Identification and Differentiation of Single Cells From Peripheral Blood by Raman Spectroscopic ImagingDocumento9 páginasPhotonics: Identification and Differentiation of Single Cells From Peripheral Blood by Raman Spectroscopic ImagingmpmileticAinda não há avaliações
- ImunoDocumento11 páginasImunoAulia Azizah KosmanAinda não há avaliações
- Flow CytometryDocumento6 páginasFlow Cytometrytanweer_elevenAinda não há avaliações
- Experiment and Mechanism Research of SKOV3 Cancer Cell Apoptosis Induced by Nanosecond Pulsed Electric FieldDocumento4 páginasExperiment and Mechanism Research of SKOV3 Cancer Cell Apoptosis Induced by Nanosecond Pulsed Electric Fieldjuliogomez008Ainda não há avaliações
- ATC: Lecture 4: Cell Separation Cell Quantification Cell CharacterizationDocumento42 páginasATC: Lecture 4: Cell Separation Cell Quantification Cell Characterizationchan yi hanAinda não há avaliações
- Neurochemistry International: Oliver Maier, Julia Böhm, Michael Dahm, Stefan Brück, Cordian Beyer, Sonja JohannDocumento10 páginasNeurochemistry International: Oliver Maier, Julia Böhm, Michael Dahm, Stefan Brück, Cordian Beyer, Sonja JohannPili CárdenasAinda não há avaliações
- Prinsip Sysmex Hematology AnalyzerDocumento5 páginasPrinsip Sysmex Hematology AnalyzerLince Wijoyo100% (1)
- Ajpcell 00166 2015Documento22 páginasAjpcell 00166 2015JasonAinda não há avaliações
- Flow CytometryDocumento7 páginasFlow CytometryMayHnin KhaingAinda não há avaliações
- In-Vitro Growth Study of Cell - Scaffold Implants For Cartilage Tissue EngineeringDocumento85 páginasIn-Vitro Growth Study of Cell - Scaffold Implants For Cartilage Tissue EngineeringcmbbsrrklAinda não há avaliações
- Electrical Characters On Surface of Apoptotic Cell in Maize Roots Induced by CytotoxinsDocumento10 páginasElectrical Characters On Surface of Apoptotic Cell in Maize Roots Induced by CytotoxinsUmiatin RamdhaniAinda não há avaliações
- Histo - Course HeroDocumento16 páginasHisto - Course HeroNatalie EnriquezAinda não há avaliações
- Distinct RNA Profiles in Subpopulations of Extracellular Vesicles: Apoptotic Bodies, Microvesicles and ExosomesDocumento10 páginasDistinct RNA Profiles in Subpopulations of Extracellular Vesicles: Apoptotic Bodies, Microvesicles and ExosomesOlavo Amorim SantosAinda não há avaliações
- FACSDocumento24 páginasFACSMudit MisraAinda não há avaliações
- Bendall Et Al ScienceDocumento11 páginasBendall Et Al SciencePratip ChattopadhyayAinda não há avaliações
- Biochemical and Biophysical Research CommunicationsDocumento6 páginasBiochemical and Biophysical Research CommunicationsSebastián GallegosAinda não há avaliações
- Collagen Types Analysis and Differentiation by FTIR SpectrosDocumento9 páginasCollagen Types Analysis and Differentiation by FTIR SpectrosGuilherme de OliveiraAinda não há avaliações
- Comparison of The Leukocyte Differentiation PDFDocumento8 páginasComparison of The Leukocyte Differentiation PDFDyah LaksmiAinda não há avaliações
- Complete Basic Flow Cytometry Principles ManualDocumento37 páginasComplete Basic Flow Cytometry Principles ManualcandiddreamsAinda não há avaliações
- LAPORAN PRAKTIKUM Apoptosis Moh Jaml 226070103141001Documento11 páginasLAPORAN PRAKTIKUM Apoptosis Moh Jaml 226070103141001jimi jamalAinda não há avaliações
- Campeanu Et Al 2014 PDFDocumento8 páginasCampeanu Et Al 2014 PDFBeatriceMihaelaRaduAinda não há avaliações
- Pluripotent Stem Cell BiologyDocumento227 páginasPluripotent Stem Cell BiologyhrbasuAinda não há avaliações
- Micromachines 08 00015Documento26 páginasMicromachines 08 00015yashbhardwajAinda não há avaliações
- Journal of Medical Genetics Volume 32 Issue 9 1995 (Doi 10.1136/jmg.32.9.759) Harris, A. - Cell Biology - A Laboratory HandbookDocumento2 páginasJournal of Medical Genetics Volume 32 Issue 9 1995 (Doi 10.1136/jmg.32.9.759) Harris, A. - Cell Biology - A Laboratory HandbooksnailmonstahAinda não há avaliações
- Chromosone AnalysisDocumento234 páginasChromosone AnalysisSpataru VasileAinda não há avaliações
- Celllll Term PaperrrDocumento12 páginasCelllll Term PaperrrNarveer SinghAinda não há avaliações
- Distribution of Melatonin MT1 Receptor Immunoreactivity in Human RetinaDocumento7 páginasDistribution of Melatonin MT1 Receptor Immunoreactivity in Human RetinaANAAinda não há avaliações
- Fluorescence Activated Cell SortingDocumento6 páginasFluorescence Activated Cell SortingAjit YadavAinda não há avaliações
- Cell-Type-Specific Profiling of Brain MitochondriaDocumento22 páginasCell-Type-Specific Profiling of Brain MitochondriaFrancini MontemarAinda não há avaliações
- Fluorescence Activated Cell Sorter: Sudhanshu Shekhar M.Tech (Biotech) III Sem A7110709009Documento24 páginasFluorescence Activated Cell Sorter: Sudhanshu Shekhar M.Tech (Biotech) III Sem A7110709009Sudhanshu ShekharAinda não há avaliações
- CytometryDocumento11 páginasCytometrysachinpardeshiAinda não há avaliações
- Immune Cell PhenotypingDocumento24 páginasImmune Cell PhenotypingSavina SabahAinda não há avaliações
- Thomson 2007 Functional Maps of Neocortical CircuitryDocumento24 páginasThomson 2007 Functional Maps of Neocortical CircuitrySebastián GallegosAinda não há avaliações
- Characterization of Circulating Tumor Cells by Fluorescence in Situ HybridizationDocumento8 páginasCharacterization of Circulating Tumor Cells by Fluorescence in Situ HybridizationNidhi JaisAinda não há avaliações
- Flow CytometryDocumento35 páginasFlow CytometryAdwika DeoAinda não há avaliações
- Explain About Flourocytometry.Documento4 páginasExplain About Flourocytometry.mandolinjobAinda não há avaliações
- Paper Flow CytometryDocumento21 páginasPaper Flow CytometryReena SharmaAinda não há avaliações
- Flow CytometryDocumento16 páginasFlow Cytometrywildana PATKLINAinda não há avaliações
- AcidoDocumento6 páginasAcidoDark WolfAinda não há avaliações
- Flow Cytometry Facs ServicesDocumento3 páginasFlow Cytometry Facs ServicesSteven4654Ainda não há avaliações
- Flowcytometry: Dr. Noune Ahs CihsrDocumento30 páginasFlowcytometry: Dr. Noune Ahs CihsrAniapthingAinda não há avaliações
- 2012 MC Proteomics - Proteomics and Transcriptomics Approach To Identify Leukemic Stem Cell MarkersDocumento34 páginas2012 MC Proteomics - Proteomics and Transcriptomics Approach To Identify Leukemic Stem Cell MarkersJosé Nivaldo Ifatunmibi Aworeni OdusolaAinda não há avaliações
- Complementarity of Flow Cytometry and Fluorescence MicrosDocumento2 páginasComplementarity of Flow Cytometry and Fluorescence MicrosAni IoanaAinda não há avaliações
- Cell Signalling Biology - M 1 - IntroductionDocumento63 páginasCell Signalling Biology - M 1 - IntroductionIoana BodescuAinda não há avaliações
- Electrorelease of Escherichia Coli NucleoidsDocumento6 páginasElectrorelease of Escherichia Coli Nucleoidserhan6936Ainda não há avaliações
- Telepathology and Virtual MicrosDocumento14 páginasTelepathology and Virtual MicrosCristinaAinda não há avaliações
- 2020 The Application of Cell Surface Markers To Demarcate Distinct Human Pluripotent States (Experimental Cell Research)Documento9 páginas2020 The Application of Cell Surface Markers To Demarcate Distinct Human Pluripotent States (Experimental Cell Research)Thakoon TtsAinda não há avaliações
- tmpD776 TMPDocumento6 páginastmpD776 TMPFrontiersAinda não há avaliações
- Article 2 - Infrared SpectrosDocumento8 páginasArticle 2 - Infrared SpectrosSharmaine L. LozanoAinda não há avaliações
- Brouzes 2009Documento6 páginasBrouzes 2009Salman AhmadAinda não há avaliações
- Tmp7a25 TMPDocumento5 páginasTmp7a25 TMPFrontiersAinda não há avaliações
- Genetics of RetinoblastomaDocumento5 páginasGenetics of RetinoblastomadesmawitaAinda não há avaliações
- Rat DissectionDocumento15 páginasRat Dissectionapi-233187566Ainda não há avaliações
- PRE-BOARD EXAMINATION, January 2015 Class XII Biology (044) : G.G.N. Public School, Rose Garden, LudhianaDocumento5 páginasPRE-BOARD EXAMINATION, January 2015 Class XII Biology (044) : G.G.N. Public School, Rose Garden, LudhianaSamita BhallaAinda não há avaliações
- Electrophysiological Recording Techniques PDFDocumento7 páginasElectrophysiological Recording Techniques PDFAndrei TatomirAinda não há avaliações
- Lipids BiochemistryDocumento7 páginasLipids BiochemistryPojangAinda não há avaliações
- TFSB 3 (SI2) 54-65oDocumento12 páginasTFSB 3 (SI2) 54-65oJohn ShipAinda não há avaliações
- Marker For Wheat Stem Rust ResistanceDocumento9 páginasMarker For Wheat Stem Rust Resistancesaurav100% (1)
- Genome Chapter SummariesDocumento8 páginasGenome Chapter SummariesAshley YaoAinda não há avaliações
- Fall 2015 Schedule of CoursesDocumento15 páginasFall 2015 Schedule of CoursesThiago Antonio ZogbiAinda não há avaliações
- Molecules: The New Challenge of Green Cosmetics: Natural Food Ingredients For Cosmetic FormulationsDocumento28 páginasMolecules: The New Challenge of Green Cosmetics: Natural Food Ingredients For Cosmetic FormulationsalbertoAinda não há avaliações
- CumulativetestDocumento14 páginasCumulativetestapi-254428474Ainda não há avaliações
- Heberprot PDocumento5 páginasHeberprot PManzoor A. ShaikhAinda não há avaliações
- CIE Alevel Biology Mock Papers Paper 2 As Structured Questions Sample PagesDocumento96 páginasCIE Alevel Biology Mock Papers Paper 2 As Structured Questions Sample PagesSalman Farsi TaharatAinda não há avaliações
- Mammalian Oocyte Regulation Methoda and ProtocolesDocumento316 páginasMammalian Oocyte Regulation Methoda and ProtocolesTlad AljazeraAinda não há avaliações
- Microbial DiversityDocumento6 páginasMicrobial DiversityDev AnandhAinda não há avaliações
- Fertilization and MaturationDocumento21 páginasFertilization and MaturationRenz L. SalumbreAinda não há avaliações
- IchthyosisDocumento154 páginasIchthyosisprajnamitaAinda não há avaliações
- Nisa Kartal TezDocumento64 páginasNisa Kartal TezalpAinda não há avaliações
- Biotechnology Patenting in India and Related IssuesDocumento22 páginasBiotechnology Patenting in India and Related IssuesShruti KaushikAinda não há avaliações
- Heredity UnitDocumento60 páginasHeredity Unitapi-224842598Ainda não há avaliações
- Flowchart GaDocumento1 páginaFlowchart GaSidney Bruce ShikiAinda não há avaliações
- Haemin Crystals LabDocumento6 páginasHaemin Crystals LabNaiomiAinda não há avaliações
- Jam 14704Documento17 páginasJam 14704Raja ChandraAinda não há avaliações
- AQA Immunity BookletDocumento6 páginasAQA Immunity Bookletnchauhan212Ainda não há avaliações
- Course Catalogue 21 22 UpdatedDocumento286 páginasCourse Catalogue 21 22 UpdatedKumarAinda não há avaliações
- PATHOPHYSIOLOGY OF OSTEOSARCOMaDocumento1 páginaPATHOPHYSIOLOGY OF OSTEOSARCOMakyawAinda não há avaliações
- BIOLOGY Form 5 Chapter 5 InheritanceDocumento52 páginasBIOLOGY Form 5 Chapter 5 InheritanceCabdicasiis Maxamuud Guuleed100% (1)
- Bio2 Set BDocumento22 páginasBio2 Set BAdrienaAinda não há avaliações
- IB Biology SL - 2024 Prediction Exam - May 2024 Paper 1Documento16 páginasIB Biology SL - 2024 Prediction Exam - May 2024 Paper 1Christy HuynhAinda não há avaliações
- Histology and Cell Biology An Introduction To Pathology 3rd Edition Kierszenbaum Test BankDocumento12 páginasHistology and Cell Biology An Introduction To Pathology 3rd Edition Kierszenbaum Test Bankapostolicembetterxrymjn100% (28)
- 10% Human: How Your Body's Microbes Hold the Key to Health and HappinessNo Everand10% Human: How Your Body's Microbes Hold the Key to Health and HappinessNota: 4 de 5 estrelas4/5 (33)
- Masterminds: Genius, DNA, and the Quest to Rewrite LifeNo EverandMasterminds: Genius, DNA, and the Quest to Rewrite LifeAinda não há avaliações
- Why We Die: The New Science of Aging and the Quest for ImmortalityNo EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityNota: 4.5 de 5 estrelas4.5/5 (6)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisNo EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisNota: 3.5 de 5 estrelas3.5/5 (2)
- Tales from Both Sides of the Brain: A Life in NeuroscienceNo EverandTales from Both Sides of the Brain: A Life in NeuroscienceNota: 3 de 5 estrelas3/5 (18)
- The Rise and Fall of the Dinosaurs: A New History of a Lost WorldNo EverandThe Rise and Fall of the Dinosaurs: A New History of a Lost WorldNota: 4 de 5 estrelas4/5 (597)
- Buddha's Brain: The Practical Neuroscience of Happiness, Love & WisdomNo EverandBuddha's Brain: The Practical Neuroscience of Happiness, Love & WisdomNota: 4 de 5 estrelas4/5 (216)
- Return of the God Hypothesis: Three Scientific Discoveries That Reveal the Mind Behind the UniverseNo EverandReturn of the God Hypothesis: Three Scientific Discoveries That Reveal the Mind Behind the UniverseNota: 4.5 de 5 estrelas4.5/5 (52)
- A Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsNo EverandA Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsNota: 4.5 de 5 estrelas4.5/5 (6)
- The Molecule of More: How a Single Chemical in Your Brain Drives Love, Sex, and Creativity--and Will Determine the Fate of the Human RaceNo EverandThe Molecule of More: How a Single Chemical in Your Brain Drives Love, Sex, and Creativity--and Will Determine the Fate of the Human RaceNota: 4.5 de 5 estrelas4.5/5 (517)
- Gut: the new and revised Sunday Times bestsellerNo EverandGut: the new and revised Sunday Times bestsellerNota: 4 de 5 estrelas4/5 (393)
- Seven and a Half Lessons About the BrainNo EverandSeven and a Half Lessons About the BrainNota: 4 de 5 estrelas4/5 (111)
- Undeniable: How Biology Confirms Our Intuition That Life Is DesignedNo EverandUndeniable: How Biology Confirms Our Intuition That Life Is DesignedNota: 4 de 5 estrelas4/5 (11)
- All That Remains: A Renowned Forensic Scientist on Death, Mortality, and Solving CrimesNo EverandAll That Remains: A Renowned Forensic Scientist on Death, Mortality, and Solving CrimesNota: 4.5 de 5 estrelas4.5/5 (397)
- The Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionNo EverandThe Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionNota: 4 de 5 estrelas4/5 (812)
- Who's in Charge?: Free Will and the Science of the BrainNo EverandWho's in Charge?: Free Will and the Science of the BrainNota: 4 de 5 estrelas4/5 (65)
- Good Without God: What a Billion Nonreligious People Do BelieveNo EverandGood Without God: What a Billion Nonreligious People Do BelieveNota: 4 de 5 estrelas4/5 (66)
- Moral Tribes: Emotion, Reason, and the Gap Between Us and ThemNo EverandMoral Tribes: Emotion, Reason, and the Gap Between Us and ThemNota: 4.5 de 5 estrelas4.5/5 (116)
- Remnants of Ancient Life: The New Science of Old FossilsNo EverandRemnants of Ancient Life: The New Science of Old FossilsNota: 3 de 5 estrelas3/5 (3)
- The Lives of Bees: The Untold Story of the Honey Bee in the WildNo EverandThe Lives of Bees: The Untold Story of the Honey Bee in the WildNota: 4.5 de 5 estrelas4.5/5 (44)
- Darwin's Doubt: The Explosive Origin of Animal Life and the Case for Intelligent DesignNo EverandDarwin's Doubt: The Explosive Origin of Animal Life and the Case for Intelligent DesignNota: 4 de 5 estrelas4/5 (19)
- The Consciousness Instinct: Unraveling the Mystery of How the Brain Makes the MindNo EverandThe Consciousness Instinct: Unraveling the Mystery of How the Brain Makes the MindNota: 4.5 de 5 estrelas4.5/5 (93)
- Human: The Science Behind What Makes Your Brain UniqueNo EverandHuman: The Science Behind What Makes Your Brain UniqueNota: 3.5 de 5 estrelas3.5/5 (38)
- The Other Side of Normal: How Biology Is Providing the Clues to Unlock the Secrets of Normal and Abnormal BehaviorNo EverandThe Other Side of Normal: How Biology Is Providing the Clues to Unlock the Secrets of Normal and Abnormal BehaviorAinda não há avaliações