Escolar Documentos
Profissional Documentos
Cultura Documentos
Elements and Compounds
Enviado por
PaulDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Elements and Compounds
Enviado por
PaulDireitos autorais:
Formatos disponíveis
KISS Resources for the Australian Curriculum - Science
KEEP IT SIMPLE SCIENCE
keep it simple science
KISS topic
number
Elements & Compounds
Year level
designation in
Nat.Curriculum
Topic 10.8C
Science Understanding Strand
B = Biological Sciences
C = Chemical Sciences
E = Earth & Space Sciences
P = Physical Sciences
Year 8 Chemical Sciences
WORKSHEETS
Attention Teachers
INSPECTION COPY
for schools only
1.
KISS Worksheets are designed to consolidate students knowledge &
understanding and/or develop or practice a skill, such as graphing,
calculating, reporting prac.work, etc. Some are suitable to issue as
homework assignments. Some can be used as a quick quiz.
2.
In both the PhotoMaster and OnScreen resources,
an information box (as shown) indicates the appropriate
point for each worksheet to be completed.
Please complete
Worksheets 1 & 2
before going on.
3.
KISS Worksheets are formatted for photocopying so that they may be used
as in-class paper exercises, quiz tests or homework assignments.
They can also be converted for use as Microsoft WordTM documents, or
with software allowing annotations, (eg Microsoft OneNoteTM) or apps such
as NotabilityTM and iAnnotate PDFTM in tablets & iPads. This allows
KISS Worksheets to be completed by students in their computer, then
submitted by email, for example.
Software titles underlined above are registered trademarks of Microsoft Corp., GingerLabs, Branchfire Inc.
Answer Section begins on p8
Suggested answers to the Discussion / Activity pages
(OnScreen resources) are in a separate file in the folder for this topic.
Topic 10.8C Elements & Compounds Worksheets
copyright 2013 KEEP IT SIMPLE SCIENCE
page 1
www.keepitsimplescience.com.au
INSPECTION COPY for
schools only
KISS Resources for the Australian Curriculum - Science
Make your own Mind-Map TITLE PAGE.
Cut out the boxes. Sort them into an appropriate
lay-out on a page of your workbook, then glue them down.
Add connecting arrows and colour in.
keep it simple science
Elements
&
Compounds
Particles
& Atoms
Chemical
Reactions
Chemical
Compounds
Metals &
Non-Metals
Physical Changes
&
Chemical Changes
The
Elements
Compounds
v. Mixtures
Introduction
to the
Periodic Table
INSPECTION COPY
for schools 2014
Make your own Mind-Map TITLE PAGE.
Cut out the boxes. Sort them into an appropriate lay-out on a page of your
workbook, then glue them down. Add connecting arrows and colour in.
Elements
&
Compounds
Particles
& Atoms
Physical Changes
&
Chemical Changes
The
Elements
Compounds
v. Mixtures
Chemical
Compounds
Metals &
Non-Metals
Topic 10.8C Elements & Compounds Worksheets
copyright 2013 KEEP IT SIMPLE SCIENCE
page 2
www.keepitsimplescience.com.au
Chemical
Reactions
Introduction
to the
Periodic Table
INSPECTION COPY for
schools only
KISS Resources for the Australian Curriculum - Science
Worksheet 1 Student Name......................................
The Elements Fill in the blank spaces.
keep it simple science
The ancient Greek, a)....................................
believed that everything was made of 4
elements; earth, air, b).................... and
.............................
The aim of Alchemy was to turn ordinary
metals into c)............................. and to find a
chemical which could make a person
d)..........................................
While searching for these impossible
chemicals, the alchemists discovered
many new chemicals and invented
equipment and processes such as filtration
and e)..............................
By learning to break chemicals down into
the simplest parts (f)..................................)
the true concept of a chemical element was
finally discovered.
We now know there are about g)................
naturally occurring elements. These are
listed on the h)............................ Table. Each
element has its own unique i)......................
and j).............................. number.
An element can be defined as a substance
composed of atoms which are k).................
........................... It can also be defined as a
substance which cannot be l)........................
into anything simpler.
Each elements atoms have the same
number of m).................................
This number is equal to the n)...................
....................... shown on the Periodic Table.
Worksheet 2
Elements & Periodic Table
Student Name......................................
Search the Periodic Table and find the information to complete the table
Element
Name
Chemical
Symbol
Atomic
Number
Zinc
Number of Electrons
in each atom
INSPECTION COPY
for schools only
Krypton
Ne
Ba
15
74
11
53
Fluorine
79
Am
Topic 10.8C Elements & Compounds Worksheets
copyright 2013 KEEP IT SIMPLE SCIENCE
page 3
www.keepitsimplescience.com.au
INSPECTION COPY for
schools only
KISS Resources for the Australian Curriculum - Science
Worksheet 3 Student Name......................................
INSPECTION COPY
Names of the Elements
for schools only
keep it simple science
1. At least 6 of the elements were named
after countries (or places) of the world.
Search the Periodic Table and find 2.
Name
Atomic No.
......................................
...............
3.
Some minerals have been named because
they contain a lot of certain elements, or
the element was named after being
discovered in that mineral.
Can you find them?
......................................
...............
Mineral
2. About a dozen elements have been
named in honour of famous scientists.
List 2 of these. (hint: very high atomic numbers)
Element
At. No.
Calcite
.............................
............
Fluorite
.............................
............
Atomic No.
Beryl
.............................
............
......................................
...............
Zircon
.............................
............
......................................
...............
Name
Worksheet 4
Student Name......................................
Black Shading = gases
Grey Shading = liquids
White Shading = solids
Classifying the Elements
Solid, Liquid or Gas?
The vast majority of the elements are solid
at room temperature. About a dozen are
gases. Only 2 are liquids.
(In Chemistry, room temperature is defined
to be 25oC)
1.
Use the information on the right, and refer
to a Periodic Table to list all the elements
which are liquids at room temperature.
Atomic Number
Q2 (cont)
Atomic Number
Name
Symbol
...............
...............................
.............
...............
...............................
.............
Name
Symbol
...............
...............................
.............
...............
...............................
.............
...............
...............................
.............
...............
...............................
...............
...............................
.............
...............
...............................
.............
...............
...............................
.............
...............
...............................
.............
...............
...............................
.............
.............
2. List all the elements which are gases at
room temperature.
...............
...............................
...............
...............................
.............
.............
Topic 10.8C Elements & Compounds Worksheets
copyright 2013 KEEP IT SIMPLE SCIENCE
page 4
www.keepitsimplescience.com.au
INSPECTION COPY for
schools only
KISS Resources for the Australian Curriculum - Science
Worksheet 5
Metals & Non-Metals
keep it simple science
Most of the elements are metals.
They typically have these properties:
Student Name......................................
In contrast, the non metals are
generally:
h)........................... in appearance.
They are a).......................... in appearance.
poor i).................................. of electricity.
They are good b)............................. of both
electricity and c)...........................
They are d)................................, which
means they can be flattened into sheets.
They are e).................................., which
means they can be drawn out into wires.
At room temperature, they are all
f)............................., except the liquid metal
g)...................................
j)........................, which means they will
shatter or snap if hammered or
stretched.
Many are solids, but there are also
many k)........................... and 1 liquid.
In the l).................................. Table, the
non-metals are clustered in the
m).............. (top or bottom)
n)......................... (left or right)
Worksheet 6
Useful Elements
1. We use the element copper for electrical
wires. Which 2 typical properties of a
metal make it suitable for this use?
2. Aluminium is familiar to you in the form
of aluminium foil. Which property of
metals allows thin sheets of aluminium to
be made like this?
3. Pure iodine is a solid non-metal, in the
form of shiny, purple crystals. What do
you expect to happen if you were to tap it
with a hammer? Explain.
Student Name......................................
4. Silicon is an element used to make
silicon chips for computer circuits. Silicon
is shiny, brittle and a semi-conductor of
electricity. On balance, should we classify
silicon as metal or non-metal? Explain.
INSPECTION COPY
for schools only
5. Helium is a gas with such low density that
it can make balloons rise into the air.
a) Why do you think it has such a low
density?
b) There is one other element which can also
lift balloons. Name it.
c) Of these 2, helium is preferred.
Find out why.
Topic 10.8C Elements & Compounds Worksheets
copyright 2013 KEEP IT SIMPLE SCIENCE
page 5
www.keepitsimplescience.com.au
INSPECTION COPY for
schools only
KISS Resources for the Australian Curriculum - Science
keep it simple science
Worksheet 7
Compounds & Reactions.
Student Name......................................
Fill in the blank spaces.
A compound is formed when 2 or more
a).............................. combine. The atoms
are not just mixed but are chemically
b)................................ together to form a
new particle called a c)...............................
When a chemical reaction occurs, the
atoms remain the same, but are
j).................................. to form new
substances. The signs of a chemical
change are that:
The elements always combine in a fixed
d)....................... which is described by the
chemical e)............................... for that
compound. For example, H2O means that
there are 2 atoms of f).......................... and
1 atom of g)............................ in each
molecule of h)...........................
original substance(s) k).............................
new substance(s) l)........................... This
may show as a change of m).....................,
or n)......................... of a gas.
the o)............................... changes.
The properties of a compound are usually
i)..................................................................
compared to the properties of the
elements in the compound.
Compounds are p).................... substances
and cannot be separated by any
q)................................. process. They can
be chemically split into r)...........................
by the process of s).....................................
INSPECTION COPY
for schools only
Worksheet 8
Student Name......................................
Chemical Formulas. Physical & Chemical Changes
1. For each compound below, state which
elements are present, and how many
atoms of each are in 1 molecule.
The first one is done for you.
2. For each change described, state if it is a
physical change, or a chemical change.
a) melting ice
.........................
a) Water, H2O contains:
b) burning paper
.........................
2 atoms of hydrogen & 1 atom of oxygen
....................................................................
b) carbon dioxide, CO2 contains:
c) grinding sugar to a powder
....................................................................
c) aluminium chloride. AlCl3 contains
....................................................................
d) ethane, C2H6 contains
....................................................................
e) copper sulfate, CuSO4 contains
....................................................................
.........................
d) collecting clear water
by filtering mud
e) decomposing salt to
sodium and chlorine
f) mixing two solutions
which change colour
and form a sediment
g) water is heated so that
bubbles of steam form
h) water is zapped with
electricity so that bubbles
of hydrogen and oxygen form
Topic 10.8C Elements & Compounds Worksheets
copyright 2013 KEEP IT SIMPLE SCIENCE
page 6
www.keepitsimplescience.com.au
.........................
.........................
.........................
.........................
.........................
INSPECTION COPY for
schools only
KISS Resources for the Australian Curriculum - Science
Topic Test
Student Name......................................
Score
/ 22
Elements & Compounds
keep it simple science
Answer all questions
in the spaces provided.
1. (8 marks)
True or False?
a) Alchemy was mainly concerned
with making gold.
b) There are about 20-30
chemical elements.
c) The atoms of an element are
all the same as each other.
d) An element can be chemically
decomposed into simpler things.
e) Every metal is a solid at
room temperature.
f) Non-metals are found on the
left side of the Periodic Table.
g) A compound contains elements
chemically bonded together.
h) Compounds can be decomposed
into elements.
(T or F?)
........
........
........
........
........
........
........
........
2. (6 marks)
a) The ancient Greek, Aristotle, believed
that everything was composed of just 4
basic substances, or elements.
Name 2 of Aristotles elements.
................................ and ...............................
b) If the atoms of 2 different elements are
represented by these symbols,
use a sketch to show:
i) a mixture of these
elements.
ii) a compound of these
elements.
3. (5 marks)
Give one word for:
a) a substance which cannot be separated
by any physical processes, but can be
decomposed chemically into simpler
substances.
...............................................
b) the general name for a shiny, malleable
element which conducts electricity.
...............................................
c) a substance which can be separated
into parts by physical processes.
...............................................
d) the property of being able to stretch a
substance to form wires.
...............................................
e) a substance which cannot be
decomposed into any simpler substances.
...............................................
4. (3 marks)
Answer each part by clearly marking the
blank Periodic Table as instructed.
a) Write a where you would find an
element which is a gas at room temperature.
b) Rule a straight line to show the
approximate dividing line between the
metals and the non-metals. Indicate on
which side of the dividing line the metals
are located.
c) Write c to show the location of the
element with Atomic Number = 11.
c) List 2 things you might observe or
measure which indicate that a chemical
reaction has occurred.
Topic 10.8C Elements & Compounds Worksheets
copyright 2013 KEEP IT SIMPLE SCIENCE
page 7
www.keepitsimplescience.com.au
INSPECTION COPY for
schools only
KISS Resources for the Australian Curriculum - Science
keep it simple science
Answer Section
Worksheet 1
Worksheet 4
a) Aristotle
b) fire and water
c) gold
d) immortal
e) distillation / crystalisation, etc
f) decomposition
g) 90
h) Periodic
i) symbol
j) Atomic
k) identical
l) decomposed
m) electrons
n) Atomic Number
1.
35
80
Bromine
Mercury
Br
Hg
2.
1
2
7
8
9
10
17
18
36
54
86
Hydrogen
Helium
Nitrogen
Oxygen
Fluorine
Neon
Chlorine
Argon
Krypton
Xenon
Radon
H
He
N
O
F
Ne
Cl
Ar
Kr
Xe
Rn
Worksheet 2
Zinc
Zn
Krypton
Kr
Neon
Ne
Barium
Ba
Phosphorus P
Tungsten
W
Sodium
Na
Iodine
I
Fluorine
F
Gold
Au
Americium Am
30
36
10
56
15
74
11
53
9
79
95
30
36
10
56
15
74
11
53
9
79
95
Worksheet 3
1.
Any 2 of Germanium (32), Francium (87),
Polonium (84), Europium (63), Americium
(95), Californium (98)
INSPECTION COPY
for schools only
Worksheet 5
a) shiny
c) heat
e) ductile
g) mercury
i) conductors
k) gases
m) top
b) conductors
d) malleable
f) solids
h) dull (not shiny)
j) brittle
l) Periodic
n) right
Worksheet 6
1. ductile & electrical conductor
2.
Curium (96) and Einsteinium (99) are best
known, but also elements 100 - 109.
3.
Calcium
Fluorine
Beryllium
Zirconium
2. malleable
3. It would shatter. Being a non-metal it is
brittle, not malleable.
20
9
4
40
4. non-metal. Although it is shiny like a
metal, it is brittle and not a good
conductor.
5.
a) Its atoms are very small & light weight.
b) Hydrogen
c) Hydrogen is explosively inflammable,
so helium is much safer to use.
Topic 10.8C Elements & Compounds Worksheets
copyright 2013 KEEP IT SIMPLE SCIENCE
page 8
www.keepitsimplescience.com.au
INSPECTION COPY for
schools only
KISS Resources for the Australian Curriculum - Science
keep it simple science
Answer Section
Worksheet 7
a) elements
c) molecule
e) formula
g) oxygen
i) totally different
k) disappear
m) colour
o) temperature
q) physical
s) decomposition
(cont.)
Topic Test
b) bonded
d) ratio
f) hydrogen
h) water
j) re-arranged
l) appear
n) bubbles
p) pure
r) elements
1.
a) T
g) T
b) F
h) T
c) T
d) F
e) F
f) F
2.
a) earth, air, fire, water (any 2)
b) i) (separate, different
particles)
1.
ii) (identical molecules,
each one made of different
atoms bonded together)
a) 2 atoms of hydrogen & 1 atom of oxygen
b) 1 atom of carbon & 2 atoms of oxygen
c) 1 atom of aluminium & 3 atoms of chlorine
d) 2 atoms of carbon & 6 atoms of hydrogen
e) 1 atom of copper, 1 atom of sulfur &
4 atoms of oxygen
c) (any 2)
Original substance(s) disappear.
New substance(s) appear.
Temperature changes (as energy is
released or absorbed)
Worksheet 8
2.
a) physical
c) physical
e) chemical
g) physical
b) chemical
d) physical
f) chemical
h) chemical
INSPECTION COPY
for schools only
3.
a) compound
b) metal
c) mixture
d) ductility, or substance is ductile
e) element
4.
a) a at any one of the positions shown
b) aprox. as shown. metals to left of line.
c) as shown
a
c
Topic 10.8C Elements & Compounds Worksheets
copyright 2013 KEEP IT SIMPLE SCIENCE
page 9
www.keepitsimplescience.com.au
a
a a a a
a a
a
a
a
INSPECTION COPY for
schools only
Você também pode gostar
- Evolution WebquestDocumento6 páginasEvolution WebquestPaul100% (1)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
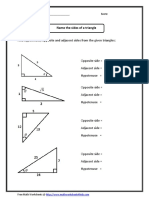
- Name Sides With MeasuresDocumento2 páginasName Sides With MeasuresPaulAinda não há avaliações
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (895)
- Name SidesDocumento2 páginasName SidesPaulAinda não há avaliações
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- Trigonometry Worksheet T1 - Labelling TrianglesDocumento4 páginasTrigonometry Worksheet T1 - Labelling TrianglesPaul50% (2)
- 5024Documento2 páginas5024Luis JesusAinda não há avaliações
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (400)
- Recruitment Process Outsourcing PDFDocumento4 páginasRecruitment Process Outsourcing PDFDevesh NamdeoAinda não há avaliações
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- TheBigBookOfTeamCulture PDFDocumento231 páginasTheBigBookOfTeamCulture PDFavarus100% (1)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- VectorCAST QA Factsheet ENDocumento2 páginasVectorCAST QA Factsheet ENChaos XiaAinda não há avaliações
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2259)
- This Study Resource Was: MCV4U Exam ReviewDocumento9 páginasThis Study Resource Was: MCV4U Exam ReviewNathan WaltonAinda não há avaliações
- Dell Inspiron 5547 15Documento7 páginasDell Inspiron 5547 15Kiti HowaitoAinda não há avaliações
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- Text Descriptive Tentang HewanDocumento15 páginasText Descriptive Tentang HewanHAPPY ARIFIANTOAinda não há avaliações
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Diagnostic Test - Tle8Documento2 páginasDiagnostic Test - Tle8rose mae marambaAinda não há avaliações
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- Arabian Choice General Trading Co. LLCDocumento1 páginaArabian Choice General Trading Co. LLCjaanAinda não há avaliações
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (266)
- Christine Remembered That Today Is The Birthday of Her BossDocumento1 páginaChristine Remembered That Today Is The Birthday of Her BossA.Ainda não há avaliações
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (345)
- KRPL Shahjahanpur Check List For Arc Welding MachineDocumento1 páginaKRPL Shahjahanpur Check List For Arc Welding MachineA S YadavAinda não há avaliações
- Thermal ComfortDocumento50 páginasThermal ComfortSSAinda não há avaliações
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- Valuing Construction Variation by Using PWA, FIDIC, ICWMF and CEDA Fluctuation Formula MechanismDocumento5 páginasValuing Construction Variation by Using PWA, FIDIC, ICWMF and CEDA Fluctuation Formula MechanismAzman YahayaAinda não há avaliações
- Application of SPACE MatrixDocumento11 páginasApplication of SPACE Matrixdecker444975% (4)
- ArticleDocumento9 páginasArticleElly SufriadiAinda não há avaliações
- Calculating Staff Strength:: Find Latest Hospitality Resources atDocumento8 páginasCalculating Staff Strength:: Find Latest Hospitality Resources atPriyanjali SainiAinda não há avaliações
- Pamphlet On Arrangement of Springs in Various Casnub Trolleys Fitted On Air Brake Wagon PDFDocumento9 páginasPamphlet On Arrangement of Springs in Various Casnub Trolleys Fitted On Air Brake Wagon PDFNiKhil GuPtaAinda não há avaliações
- Alternatoer Lvsi804s WDG 12 v9 TdsDocumento8 páginasAlternatoer Lvsi804s WDG 12 v9 TdsCris_eu09Ainda não há avaliações
- Best S and Nocella, III (Eds.) - Igniting A Revolution - Voices in Defense of The Earth PDFDocumento455 páginasBest S and Nocella, III (Eds.) - Igniting A Revolution - Voices in Defense of The Earth PDFRune Skjold LarsenAinda não há avaliações
- Jungbluth Main Catalogue-LanacDocumento60 páginasJungbluth Main Catalogue-LanacMilenkoBogdanovicAinda não há avaliações
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- Kimberly Jimenez Resume 10Documento2 páginasKimberly Jimenez Resume 10kimberlymjAinda não há avaliações
- Transformational Leadership in The UmcDocumento17 páginasTransformational Leadership in The Umcapi-202352366Ainda não há avaliações
- Sketch NotesDocumento32 páginasSketch NotesFilipe Rovarotto100% (8)
- Science: BiologyDocumento22 páginasScience: BiologyMike RollideAinda não há avaliações
- PS410Documento2 páginasPS410Kelly AnggoroAinda não há avaliações
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (121)
- MINDSET 1 EXERCISES TEST 1 Pendientes 1º Bach VOCABULARY AND GRAMMARDocumento7 páginasMINDSET 1 EXERCISES TEST 1 Pendientes 1º Bach VOCABULARY AND GRAMMARanaAinda não há avaliações
- 4040 SERIES: Hinge (Pull Side) (Shown) Top Jamb (Push Side) Parallel Arm (Push Side)Documento11 páginas4040 SERIES: Hinge (Pull Side) (Shown) Top Jamb (Push Side) Parallel Arm (Push Side)Melrose FabianAinda não há avaliações
- RG-RAP6260 (G) Hardware InstallationDocumento26 páginasRG-RAP6260 (G) Hardware InstallationrazuetAinda não há avaliações
- Tom Rockmore - Hegel's Circular EpistemologyDocumento213 páginasTom Rockmore - Hegel's Circular Epistemologyluiz100% (1)