Escolar Documentos
Profissional Documentos
Cultura Documentos
Aortic Isthmus Flow in Fetus
Enviado por
shreyaDescrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Aortic Isthmus Flow in Fetus
Enviado por
shreyaDireitos autorais:
Formatos disponíveis
Ultrasound Obstet Gynecol 2009; 33: 628633
Published online in Wiley InterScience (www.interscience.wiley.com). DOI: 10.1002/uog.6406
Opinion
Technical aspects of aortic isthmus Doppler velocimetry in human fetuses
The aortic isthmus is the segment of aorta located between
the origin of the left subclavian artery and the connection
of the ductus arteriosus to the descending aorta. In the
postnatal period following the closure of the ductus arteriosus, it serves only as a vascular conduit transporting
blood from the aortic arch down to the descending aorta.
However, during prenatal life, it plays an important role
in maintaining an adequate balance between the brachiocephalic circulation supplying the upper body (including
the brain) and the subdiaphragmatic circulation supplying
the lower body and placenta1 . The parallel arrangement
of the fetal circulation allows for unequal right and left
ventricular outputs as the placental, pulmonary and lower
body vascular resistances act mainly on the right ventricle, whereas the upper body resistance acts mainly on the
left ventricle2 . Under physiological conditions and in the
absence of structural cardiovascular malformations, such
as hypoplastic left heart syndrome, critical aortic stenosis
and interrupted aortic arch, the flow in the aortic isthmus
is forward during the whole cardiac cycle. The volume
and direction of aortic isthmus blood flow are determined
by the systolic performance of the individual ventricles
and the peripheral vascular resistances. During systole,
the left ventricular ejection facilitates forward flow while
the right ventricular ejection has the opposite effect. During diastole, when the ventricles are not ejecting blood and
both semilunar valves are closed, the direction of blood
flow in the aortic isthmus depends mainly on the relative difference between the upper body (including brain)
and lower body (including placenta) vascular resistances1 .
Therefore, conditions that lead to an increased right ventricular afterload (e.g. intrauterine fetal growth restriction
due to placental insufficiency) or a reduced left ventricular afterload (e.g. hypoxemia, cerebrovascular aneurysms,
vascular tumors of the neck) may cause a reversal of
aortic isthmus blood flow during diastole. Several experimental studies on sheep fetuses3 6 have established the
pathophysiological basis for using aortic isthmus Doppler
velocimetry in the evaluation of fetal cardiovascular
dynamics. Furthermore, clinical studies have shown the
feasibility of recording aortic isthmus blood flow velocity
waveform in the human fetus7 10 , and abnormal blood
flow pattern has been shown to be associated with fetal
circulatory redistribution or compromise11 15 . Recently,
aortic isthmus Doppler velocimetry has been shown to
predict perinatal16 and long-term neurodevelopmental17
outcomes in placental insufficiency and the routine use
of aortic isthmus Doppler velocimetry has been suggested in the evaluation of fetuses with intrauterine
Copyright 2009 ISUOG. Published by John Wiley & Sons, Ltd.
growth restriction18 . Obviously, some centers with expertise make use of aortic isthmus Doppler velocimetry in
addition to other arterial and venous Doppler parameters
in the investigation of fetal hemodynamics, but the perceived technical difficulties have led to some skepticism
regarding its potential for wider clinical application. A
multicenter study on the feasibility and reliability of aortic isthmus Doppler velocimetry published in this issue of
Ultrasound in Obstetrics and Gynecology19 showed that,
despite adequate visualization and accurate identification
of this vascular segment, appropriate cursor placement
for pulsed-wave Doppler interrogation of aortic isthmus
blood flow velocity waveforms remains challenging. The
purpose of this article is to give some practical advice
to clinicians on how to perform aortic isthmus Doppler
blood flow velocimetry in the human fetus.
Today, with improved ultrasound imaging technology,
appropriately trained obstetricians and fetal/perinatal cardiologists obtain standard views of the fetal heart and
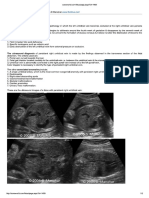
great vessels without much difficulty. The aortic isthmus can be identified easily in both the longitudinal
(Figure 1ac) and cross-sectional (Figure 1d) views that
are used routinely during fetal echocardiography. Once
this vascular segment is identified, Doppler velocimetry
can be performed in any of the views shown in Figure 1
by placing the Doppler gate (cursor) at the appropriate
location, keeping the angle of insonation as low as possible. Although Doppler flow velocity waveforms can be
obtained using B-mode imaging and pulsed-wave Doppler
(Figure 1c), color-directed pulsed-wave Doppler interrogation is recommended, as it helps in the identification
of the vessels and shows the direction of the blood flow,
allowing optimal positioning of the cursor. Pulsed-wave
gate size (sample volume) should be adjusted according to
the size of the aortic isthmus, which depends on the fetal
gestational age, to avoid recording signals from the adjacent vessels. Blood flow velocity waveforms are recorded
during fetal quiescence.
The aortic isthmus flow velocity waveforms obtained
from either of the sonographic planes (longitudinal aortic
arch view or three vessels and trachea view) are quite
similar (Figure 2) and reproducible9,20 . Accurate cursor
positioning may be simpler in the longitudinal view, as
the origin of the left subclavian artery is relatively easier
to visualize in this plane and there is less possibility of
obtaining blood flow velocity waveforms from the transverse aortic arch rather than the isthmus. On the other
hand, it may be simpler, easier and less time-consuming
OPINION
Opinion
629
Figure 1 Longitudinal (ac) and cross-sectional (d) imaging planes demonstrating the aortic isthmus with correct cursor placement for
pulsed-wave Doppler interrogation. The arrow indicates the left subclavian artery. DA, ductus arteriosus; DAo, descending aorta.
to obtain the three vessels and trachea view rather than
the longitudinal aortic arch view.
The aortic isthmus flow velocity waveform has a typical
shape and is easily recognizable in most instances. It
has a quick systolic upstroke (short acceleration time),
with mean peak systolic velocities ranging between
approximately 30 and 100 cm/s from 11 weeks to
term10,21 . This is followed by a more gradual deceleration
of the velocity and a narrow incisura (in most cases) at the
end of systole. A small flow reversal for a very short period
of time is usually seen at end-systole (Figure 3a) in the
third trimester of pregnancy7,8 . However, this brief endsystolic flow reversal is absent before 20 weeks7,21 and
is recorded less commonly when the aortic isthmus flow
velocity waveforms are obtained in the cross-sectional
imaging plane (three vessels and trachea view)10 . Reversal
of blood flow during diastole or net blood flow reversal
(i.e. total retrograde flow > total antegrade flow during
the cardiac cycle) in the aortic isthmus (Figure 3b) is
always abnormal.
As the ductus arteriosus is in close proximity to
the aortic isthmus (Figure 1a and d), even very small
movements may lead to the waveforms being obtained
from one or the other vessel during pulsed-wave Doppler
interrogation (Figure 4). Frequently updating the B-mode
Copyright 2009 ISUOG. Published by John Wiley & Sons, Ltd.
or the color flow images may be necessary to ensure
that the pulsed-wave gate remains in the correct position
during acquisition of Doppler flow velocity waveforms.
Generally speaking, ductus arteriosus Doppler waveforms
differ slightly from those of the aortic isthmus (Figure 5).
Gestational-age specific mean peak systolic velocities
of the ductus arteriosus are much higher than are
those of the aortic isthmus, ranging between 40 and
120 cm/s from 11 weeks to term22 24 . In fact, the ductus
arteriosus has the highest blood flow velocities in the
fetal circulation25 . In contrast to the aortic isthmus, the
brief reversal of flow at end-systole is usually not seen in
the ductus arteriosus and positive diastolic velocities are
almost always present under physiological conditions.
The forward flow during systole starts and peaks in
the aortic isthmus earlier than it does in the ductus
arteriosus (Figures 3 and 6). Experimental studies in sheep
fetuses have shown that the pre-ejection period of the
right ventricle is longer than is that of the left (57 vs.
48 ms)26 , and the ductal flow begins approximately
48 ms later than does the isthmic flow and has a
longer acceleration time (52 vs. 18 ms)27 . Occasionally,
especially while interrogating using a larger sample
volume, one may obtain simultaneously aortic isthmus
and ductus arteriosus blood flow velocity waveforms,
Ultrasound Obstet Gynecol 2009; 33: 628633.
630
Acharya
Figure 2 Doppler flow velocity waveforms obtained from the aortic isthmus using longitudinal (a,b) and cross-sectional (c,d) imaging planes
with and without color Doppler.
Figure 3 Typical normal (a) and abnormal (b) aortic isthmus
Doppler flow velocity waveforms in the third trimester. In (a), the
arrow points to the incisura and the arrowhead points to the brief
retrograde flow at end-systole. In (b), small arrowheads point to the
ductus arteriosus blood flow velocity waveforms in the
background. Note that the aortic isthmus flow is reversed in late
systole and the whole of diastole (net flow is retrograde).
Copyright 2009 ISUOG. Published by John Wiley & Sons, Ltd.
Figure 4 Blood flow velocity recordings from the ductus arteriosus.
(a) is a normal waveform; (b) shows that very small drift can lead
to recording blood flow velocity waveforms from the aortic isthmus
(arrows) and vice versa.
Ultrasound Obstet Gynecol 2009; 33: 628633.
Opinion
631
Figure 6 Doppler flow velocity waveforms obtained at the level of
the aortic isthmus in the longitudinal aortic arch view (a) and three
vessels and trachea view (b) with relatively large sample volumes,
demonstrating the isthmic waveforms (traced in red) superimposed
on the ductal waveforms.
Figure 5 Longitudinal views of the aortic arch (a) with asterisk ( )
indicating the isthmus, and the pulmonary-ductal arch (b) with
asterisk ( ) indicating the ductus arteriosus. Their corresponding
blood flow velocity waveforms are shown in the lower panels.
superimposed on each other (Figure 6), making direct
comparison during the cardiac cycle possible in human
fetuses.
It is possible to estimate the aortic isthmus volume
blood flow (Qai ) non-invasively21 by measuring its
diameter and blood flow velocities: Qai (in mL/min) =
time-averaged maximum velocity (TAMXV, in cm/s)
(diameter/2, in cm)2 60. However, due to the
limitations and complexity of volume flow measurements,
several other indices have been proposed for clinical
Copyright 2009 ISUOG. Published by John Wiley & Sons, Ltd.
use. Basically, these indices differ in one principle:
some use absolute velocities in their calculation whereas
others use the velocity-time integral (VTI). Fouron
et al.7 initially proposed a so-called balance index, i.e.
(peak systolic velocity end-diastolic velocity/(antegrade
VTI retrograde VTI), which is equivalent to pulsatility
index (PI) when absolute velocities are used, as the sum
of systolic and diastolic VTI multiplied by the fetal heart
rate gives TAMXV and PI = (peak systolic velocity
end-diastolic velocity)/TAMXV. An isthmic flow index
(IFI), i.e. (systolic VTI + diastolic VTI)/systolic VTI, was
proposed later8 , but has not gained much popularity
among clinicians. The argument for using VTI-related
indices rather than velocity-related indices has been the
assumption that they provide better information on the
volume and direction of blood flow. However, this
assumption is not entirely true. For example, TAMXV is
likely to reflect the volume blood flow, as it does in other
vessels28,29 , and a positive TAMXV (or PI) signifies that
the net blood flow during the cardiac cycle is antegrade,
while a negative value means that the net flow is retrograde
in the same way as does a VTI of antegrade flow/VTI of
retrograde flow ratio of > 1 or < 1. Similarly, PI is also
likely to reflect the size of the reverse flow component
when present, as TAMXV (which is equivalent to VTI
Ultrasound Obstet Gynecol 2009; 33: 628633.
Acharya
632
over one cardiac cycle heart rate) is the denominator in
the formula used for its calculation.
The IFI for aortic isthmus blood flow can be divided in
five different types8 as follows: Type I: IFI > 1, when the
flow is antegrade throughout the cardiac cycle; Type II:
IFI = 1, when diastolic flow is absent; Type III: IFI = 01,
when the diastolic flow is reversed but the net flow is
still antegrade; Type IV: IFI = 0, when the antegrade
and retrograde flows are equal; and Type V: IFI < 0,
when the net flow is retrograde. However, what really
matters is whether the diastolic blood flow is reversed
and whether the net blood flow is retrograde, as these
are the signs of compromised fetal hemodynamics. In
this context, normal and abnormal waveforms can be
identified by simple qualitative (visual) assessment as
those showing antegrade diastolic flow (IFI values 1)
and those showing retrograde diastolic flow (IFI < 1),
respectively. Further classification of waveform patterns
using IFI into more types does not seem to improve the
predictive value for adverse perinatal outcome8 .
Aortic isthmus retrograde diastolic blood flow signifies
redistribution of fetal circulation, indicating lower upper
body (cerebral) resistance compared with lower body
(placental) resistance, whereas the reversal of net blood
flow indicates that the fetus has problems maintaining
cerebral oxygenation3 . Semiquantitative indices like PI
and the resistance index (RI) are known to reflect
downstream impedance and are easy to calculate
using software packages available with most ultrasound
machines. The aortic isthmus PI is increased and
absolute velocities (especially the TAMXV) are reduced
in intrauterine growth-restricted fetuses, regardless of IFI
type16 . Furthermore, absolute velocities are quantitative
variables that are used in the calculation of PI and RI as
well as of volume blood flow. Therefore, using absolute
blood velocities and PI rather than IFI may be simpler
and more appropriate in clinical practice. However, it is
important to be aware of the fact that the brief reversal
of flow during end-systole, which is a normal finding
in the third trimester, can give falsely high PI values if
one calculates the PI as (maximum velocity minimum
velocity)/TAMXV rather than as (peak systolic velocity
end-diastolic velocity)/TAMXV.
Changes in the aortic isthmus blood flow velocity waveform are evident earlier than are those in the descending
aorta11 , umbilical artery12 and ductus venosus15,30 . An
increase in placental resistance causing a 50% reduction
in umbilical blood flow has been shown to be associated
with reversed aortic isthmus diastolic blood flow in sheep
fetuses, even though the umbilical artery diastolic blood
flow remained forward4 . Fetuses with absent/reversed
end-diastolic flow in the umbilical artery12,16,17 or in the
ductus venosus15,16 consistently appear to have retrograde
diastolic flow in the aortic isthmus. Aortic isthmus blood
flow velocimetry provides important information on fetal
cardiovascular function, i.e. individual performance of
ventricles, relative changes in upper (including brain) and
lower (including placenta) body resistances and fetal oxygenation, and has the potential to become a valuable
Copyright 2009 ISUOG. Published by John Wiley & Sons, Ltd.
clinical tool. However, as this segment of the fetal aorta is
relatively short and has several other blood vessels in its
close proximity, obtaining blood flow velocity waveforms
can be technically challenging. For accurate measurement
and interpretation of the aortic isthmus blood flow using
Doppler ultrasonography, it is important that operators
receive adequate training in obtaining the standard longitudinal aortic arch view and three vessels and trachea
view, visualizing and recognizing the aortic isthmus in
B-mode and the direction of blood flow in color flow
mode, appropriate cursor placement, recognition of the
waveform patterns of the aortic isthmus as well as other
adjacent vessels, and that they have an understanding of
factors that may influence the blood flow in this segment
of fetal circulation.
G. Acharya
Department of Obstetrics and Gynecology, Institute of
Clinical Medicine, University of Troms and University
Hospital of Northern Norway, Troms, Norway
Correspondence.
University Hospital of Northern Norway, Post Box 24,
N-9038 Troms, Norway
(e-mail: ganesh.acharya@uit.no)
REFERENCES
1. Fouron JC. The unrecognized physiological and clinical significance of the fetal aortic isthmus. Ultrasound Obstet Gynecol
2003; 22: 441447.
anen
2. Acharya G, Ras
J, Kiserud T, Huhta JC. The fetal cardiac
function. Curr Cardiol Rev 2006; 2: 4153.
3. Fouron JC, Skoll A, Sonesson SE, Pfizenmaier M, Jaeggi E,
Lessard M. Relationship between flow through the fetal aortic
isthmus and cerebral oxygenation during acute placental
circulatory insufficiency in ovine fetuses. Am J Obstet Gynecol
1999; 181: 11021107.
4. Bonnin P, Fouron JC, Teyssier G, Sonesson SE, Skoll A. Quantitative assessment of circulatory changes in the fetal aortic
isthmus during progressive increase of resistance to umbilical
blood flow. Circulation 1993; 88: 216222.
5. Fouron JC, Teyssier G, Maroto E, Lessard M, Marquette G.
Diastolic circulatory dynamics in the presence of elevated
placental resistance and retrograde diastolic flow in the
umbilical artery: a Doppler echographic study in lambs. Am
J Obstet Gynecol 1991; 164: 195203.
6. Makikallio K, Erkinaro T, Niemi N, Kavasmaa T, Acharya G,
Pakkila M, Rasanen J. Fetal oxygenation and Doppler ultrasonography of cardiovascular hemodynamics in a chronic
near-term sheep model. Am J Obstet Gynecol 2006; 194:
542550.
7. Fouron JC, Zarelli M, Drblik P, Lessard M. Flow velocity
profile of the fetal aortic isthmus through normal gestation.
Am J Cardiol 1994; 74: 483486.
8. Ruskamp J, Fouron JC, Gosselin J, Raboisson MJ, InfanteRivard C, Proulx F. Reference values for an index of fetal
aortic isthmus blood flow during the second half of pregnancy.
Ultrasound Obstet Gynecol 2003; 21: 441444.
9. Del Rio M, Martinez JM, Figueras F, Bennasar M, Palacio M,
Gomez O, Coll O, Puerto B, Cararach V. Doppler assessment
of fetal aortic isthmus blood flow in two different sonographic
planes during the second half of gestation. Ultrasound Obstet
Gynecol 2005; 26: 170174.
10. Del Rio M, Martinez JM, Figueras F, Lopez M, Palacio M,
Gomez O, Coll O, Puerto B. Reference ranges for Doppler
Ultrasound Obstet Gynecol 2009; 33: 628633.
Opinion
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
parameters of the fetal aortic isthmus during the second half of
pregnancy. Ultrasound Obstet Gynecol 2006; 28: 7176.
Fouron JC, Teyssier G, Shalaby L, Lessard M, van Doesburg
NH. Fetal central blood flow alterations in human fetuses with
umbilical artery reverse diastolic flow. Am J Perinatol 1993; 10:
197207.
Sonesson SE, Fouron JC. Doppler velocimetry of the aortic
isthmus in human fetuses with abnormal velocity waveforms
in the umbilical artery. Ultrasound Obstet Gynecol 1997; 10:
107111.
Makikallio K, Jouppila P, Rasanen J. Retrograde aortic isthmus
net blood flow and human fetal cardiac function in placental
insufficiency. Ultrasound Obstet Gynecol 2003; 22: 351357.
Makikallio K, Jouppila P, Rasanen J. Retrograde net blood
flow in the aortic isthmus in relation to human fetal arterial
and venous circulations. Ultrasound Obstet Gynecol 2002; 19:
147152.
Rizzo G, Capponi A, Vendola M, Pietrolucci ME, Arduini D.
Relationship between aortic isthmus and ductus venosus velocity
waveforms in severe growth restricted fetuses. Prenat Diagn
2008; 28: 10421047.
Del Rio M, Martinez JM, Figueras F, Bennasar M, Olivella A,
Palacio M, Coll O, Puerto B, Gratacos E. Doppler assessment
of the aortic isthmus and perinatal outcome in preterm fetuses
with severe intrauterine growth restriction. Ultrasound Obstet
Gynecol 2008; 31: 4147.
Fouron JC, Gosselin J, Raboisson MJ, Lamoureux J, Tison CA,
Fouron C, Hudon L. The relationship between an aortic isthmus
blood flow velocity index and the postnatal neurodevelopmental
status of fetuses with placental circulatory insufficiency. Am J
Obstet Gynecol 2005; 192: 497503.
Makikallio K. Is it time to add aortic isthmus evaluation to the
repertoire of Doppler investigations for placental insufficiency?
Ultrasound Obstet Gynecol 2008; 31: 69.
Fouron JC, Siles A, Montanari L, Morin L, Ville Y. Feasibility
and reliability of Doppler flow recordings in the fetal aortic
isthmus: a multicenter evaluation. Ultrasound Obstet Gynecol
2009; 33: 690693.
Rizzo G, Capponi A, Vendola M, Pietrolucci ME, Arduini D.
Use of the 3-vessel view to record Doppler velocity waveforms
from the aortic isthmus in normally grown and growthrestricted fetuses: comparison with the long aortic arch view.
J Ultrasound Med 2008; 27: 16171622.
Copyright 2009 ISUOG. Published by John Wiley & Sons, Ltd.
633
21. Vimpeli T, Huhtala H, Wilsgaard T, Acharya G. Fetal aortic
isthmus blood flow and the fraction of cardiac output distributed
to the upper body and brain at 1120 weeks of gestation.
Ultrasound Obstet Gynecol 2009; 33: 538544.
22. van der Mooren K, Barendregt LG, Wladimiroff JW. Flow
velocity wave forms in the human fetal ductus arteriosus during
the normal second half of pregnancy. Pediatr Res 1991; 30:
487490.
23. Brezinka C, Huisman TW, Stijnen T, Wladimiroff JW. Normal
Doppler flow velocity waveforms in the fetal ductus arteriosus
in the first half of pregnancy. Ultrasound Obstet Gynecol 1992;
2: 397401.
24. Mielke G, Benda N. Blood flow velocity waveforms of the fetal
pulmonary artery and the ductus arteriosus: reference ranges
from 13 weeks to term. Ultrasound Obstet Gynecol 2000; 15:
21318.
25. Huhta JC, Moise KJ, Fisher DJ, Sharif DS, Wasserstrum N,
Martin C. Detection and quantitation of constriction of the fetal
ductus arteriosus by Doppler echocardiography. Circulation
1987; 75: 406412.
26. De Muylder X, Fouron JC, Bard H, Riopel L, Urfer F. The
difference between the systolic time intervals of the left and
right ventricles during fetal life. Am J Obstet Gynecol 1984;
149: 737740.
27. Schmidt KG, Silverman NH, Rudolph AM. Phasic flow events
at the aortic isthmus-ductus arteriosus junction and branch
pulmonary artery evaluated by multimodal ultrasonography in fetal lambs. Am J Obstet Gynecol 1998; 179:
13381347.
28. Acharya G, Wilsgaard T, Berntsen GK, Maltau JM, Kiserud T.
Doppler-derived umbilical artery absolute velocities and their
relationship to fetoplacental volume blood flow: a longitudinal
study. Ultrasound Obstet Gynecol 2005; 25: 444453.
29. Acharya G,
Erkinaro T,
Makikallio K,
Lappalainen T,
Rasanen J. Relationships among Doppler-derived umbilical
artery absolute velocities, cardiac function, and placental volume blood flow and resistance in fetal sheep. Am J Physiol
Heart Circ Physiol 2004; 286: H1266H1272.
30. Figueras F, Benavides A, Del Rio M, Crispi F, Eixarch E,
E. Monitoring
Martinez JM, Hernandez-Andrade E, Gratacos
of fetuses with intrauterine growth restriction: longitudinal
changes in ductus venosus and aortic isthmus flow. Ultrasound
Obstet Gynecol 2009; 33: 3943.
Ultrasound Obstet Gynecol 2009; 33: 628633.
Você também pode gostar
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- Fetal Cardiac Dimensions Echocardiography Schneider 2005Documento7 páginasFetal Cardiac Dimensions Echocardiography Schneider 2005Heart of the Valley, Pediatric CardiologyAinda não há avaliações
- Proposed Clinical Management of Pregnancies After Combined Screening For Pre-Eclampsia at 35-37 Weeks' GestationDocumento5 páginasProposed Clinical Management of Pregnancies After Combined Screening For Pre-Eclampsia at 35-37 Weeks' GestationshreyaAinda não há avaliações
- Intracranial TranslucencyDocumento8 páginasIntracranial TranslucencyshreyaAinda não há avaliações
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- PRUVDocumento2 páginasPRUVshreyaAinda não há avaliações
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (895)
- Embryology & Sonoappearance of KidneysDocumento17 páginasEmbryology & Sonoappearance of KidneysshreyaAinda não há avaliações
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- Chest WallDocumento26 páginasChest WallDeepti KakkarAinda não há avaliações
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (399)
- Blood VesselsDocumento43 páginasBlood VesselsJessica AcostaAinda não há avaliações
- Heart Dissection Lab Report Guide (GALO)Documento6 páginasHeart Dissection Lab Report Guide (GALO)galo09landivar0902Ainda não há avaliações
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- Anatomy of KidneyDocumento19 páginasAnatomy of KidneydrsooryasridharAinda não há avaliações
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (266)
- Chapter 12Documento9 páginasChapter 12Charlie AbagonAinda não há avaliações
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- Renal Artery Doppler NsDocumento15 páginasRenal Artery Doppler Nss0800841739Ainda não há avaliações
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- CardiomegalyDocumento91 páginasCardiomegalyMariquita Buenafe100% (1)
- Med 1 Block 2 - Wet Lab NotesDocumento36 páginasMed 1 Block 2 - Wet Lab NotesluckyAinda não há avaliações
- REVISED Group4 Congestive Heart FailureDocumento64 páginasREVISED Group4 Congestive Heart FailureNicole Villanueva, BSN - Level 3AAinda não há avaliações
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- Carolina Rabbit Dissection Guide PDFDocumento26 páginasCarolina Rabbit Dissection Guide PDFclairosusAinda não há avaliações
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- The Most CommonsDocumento80 páginasThe Most CommonsJmee8Ainda não há avaliações
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2259)
- Cross Sectional Imaging Made Easy® PDFDocumento207 páginasCross Sectional Imaging Made Easy® PDFsalah subbah100% (2)
- Ab Icms 2018 SofiaDocumento142 páginasAb Icms 2018 SofiaDario PotkonjakAinda não há avaliações
- Pig DissectionDocumento8 páginasPig DissectiondvinalAinda não há avaliações
- Chapter 11 - Cardiovascular SystemDocumento10 páginasChapter 11 - Cardiovascular SystemrishellemaepilonesAinda não há avaliações
- Human Anatomy and Physiology Laboratory Manual Fetal Pig Version Update 10Th Edition Marieb Test Bank Full Chapter PDFDocumento33 páginasHuman Anatomy and Physiology Laboratory Manual Fetal Pig Version Update 10Th Edition Marieb Test Bank Full Chapter PDFAlexandraPerrymzyd100% (10)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- Alvin B. Vibar, M.D.Documento55 páginasAlvin B. Vibar, M.D.Ditas ChuAinda não há avaliações
- List of Eponymously Named Medical SignsDocumento28 páginasList of Eponymously Named Medical SignsEshanth BalaAinda não há avaliações
- Cardiovascular SystemDocumento8 páginasCardiovascular SystemHannah Grace CorveraAinda não há avaliações
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- Lab 2: Anatomy of The Heart: General ConsiderationsDocumento19 páginasLab 2: Anatomy of The Heart: General ConsiderationsDisshiAinda não há avaliações
- Cardiac Surgery MCQDocumento43 páginasCardiac Surgery MCQprofarmah100% (7)
- Pericardium:: AnatomyDocumento4 páginasPericardium:: AnatomyAlya Putri KhairaniAinda não há avaliações
- Schedule D: Drug Regulatory Authority of PakistanDocumento4 páginasSchedule D: Drug Regulatory Authority of Pakistanمحمد عبداللہ چوہدریAinda não há avaliações
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (120)
- ICD9CM-Procedure Index-A5Documento274 páginasICD9CM-Procedure Index-A5Rosdiana OceAinda não há avaliações
- Unit II (B) Intra-Aortic Balloon Pump Counter PulsationDocumento24 páginasUnit II (B) Intra-Aortic Balloon Pump Counter PulsationUmme HabibaAinda não há avaliações
- Anatomy Final Exam Que LF1Documento5 páginasAnatomy Final Exam Que LF1Sheba Dan de WiseAinda não há avaliações
- (Oxford Handbooks in Nursing. - Oxford Medical Publications) Kisiel, Maria - Radford, Mark - Smith, Alison - Oxford Handbook of Surgical Nursing (2016, Oxford University Press)Documento787 páginas(Oxford Handbooks in Nursing. - Oxford Medical Publications) Kisiel, Maria - Radford, Mark - Smith, Alison - Oxford Handbook of Surgical Nursing (2016, Oxford University Press)Nganu AjaAinda não há avaliações
- Curs Engleza Partea 2 Corectat - LariDocumento205 páginasCurs Engleza Partea 2 Corectat - LariVili Robert VoichițoiuAinda não há avaliações
- Prana ImmortalityDocumento18 páginasPrana ImmortalitySubramanya SeshagiriAinda não há avaliações
- Embro MCQ 2015Documento15 páginasEmbro MCQ 2015Tofik MohammedAinda não há avaliações