Escolar Documentos
Profissional Documentos
Cultura Documentos
Umted States Patent 1191 1111 Patent Number: 4,594,090: Johnson (45) Date of Patent: Jun. 10, 1986
Enviado por
spotch2011Descrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Umted States Patent 1191 1111 Patent Number: 4,594,090: Johnson (45) Date of Patent: Jun. 10, 1986
Enviado por
spotch2011Direitos autorais:
Formatos disponíveis
Umted States Patent 1191
1111 Patent Number:
Johnson
[45]
[54]
HIGH NUTRIENT CONTENT FERTILIZERS
[211
Inventor:
4,315,763
2/ 1982 Stoller et a1. ..
6/1983
Lowder . . . . . .
. . . . ..
Wallace Johnson, Geraldme, Mont.
4,397,675
3/1933
Young , _ , _ ,
_ _ _ _ __ 71/23
59446
4,402,852 8/1983 Young
4,404,116 8/1983 Young
Appl- N0-= 786,996
-
Jun. 10, 1986
4,388,101
[76]
Date of Patent:
4,594,090
[22]
Med
Oct 15 1985
[51]
Int (14 ______________________ __ C05B 15/00; CQSC 9/00
....... .. 71/29
71/29
252/182
252/182
4,409,015 10/1983 Grace, Jr.
.... .. 71/28
4,445,925
5/1984
Young .
.... .. 71/28
4,447,253
5/1984 Young .
.... .. 71/28
4,456,463
6/1984 Stoller ................................... .. 71/29
[52] US. (:1. ......................................... .. 71/29; 7711/23;
Prim, Examiner__pems H_ Lander
[58]
Att rne , A
t, rFir Poll ck, V d Sande &
Pridyy gen 0
"'
[56]
Field of Search ........................ .. 71/29, 34, 36,40
References Cited
U-S' PATENT DOCUMENTS
[57]
ABSTRACI
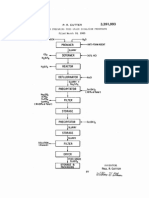
A method of making fertilizer comprising the steps of
2,814,556 11/1957 Christoffel ............................ .. 71/29
mixing an aqueous solution of sulfuric acid with diam
3,022,153 2/1962 Miller ....... ..
71/29
monium phosphate in a weight ratio of diammonium
3,326,666 6/1967 Walters, Jr
71/29
71/34
phosphate to sulfuric acid from about 3 to about 5, and
mixing the resulting reaction products with urea in a
31541730 V1971 51am 6} a1
Glitena" ' ' ' - - - - '
' - ' ' " 71/29
weight ratio of urea to sulfuric acid from about 7 to
3723086 3/1973 Poyne? et a1 '
3,713,802
" 71/29
about 10. The resulting fertilizer has low corrosion
3,918,952 11/1975
3,928,015 12/1975
Neumiller .... ..
Siegeletal.
.. 71/28
.. 71/28
4,116,664 9/1978 Jones ............ ..
71/29
4,310,343
4,134,75O
V1973
1/1979
1/
1982 Verdegaal
Norton et a1.
et al. ................. . 71/29
71/28'
to erti
dh
It 0 tt
t
b l
b to
P
es a
asasa ' 1 empera re W3
16 Claims, No Drawings
4,594,090
HIGH NUTRIENT CONTENT FERTILIZERS
FIELD OF THE INVENTION
This invention relates to liquid fertilizers having a 5
concentrated acidic fertilizers which have a very low
salt-out temperature and which are substantially non
corrosive are desirable.
SUMMARY OF THE INVENTION
high plant nutrient content, a low pH, and a low salting
out temperature, and to processes for making such fer
tilizers.
BACKGROUND OF THE INVENTION
Liquid fertilizers, in general, have some advantages
over solid fertilizers in that they can be handled more
easily and more readily applied in controlled amounts to
soils. Liquid fertilizers are especially advantageous in
areas which are irrigated because the fertilizer can
sulfuric acid in making the fertilizers; however, highly
It is accordingly one object of this invention to pro
vide an acidic liquid fertilizer having a high concentra
tion of plant nutrients and having a low salt-out temper
ature.
It is another object of this invention to provide an
acidic liquid fertilizer which combines a low pH and
corrosion inhibition without special additives.
It is still another object of this invention to provide a
method of making a low pH liquid fertilizer with sulfu- '
readily be applied to the soil through the irrigation
system. For economics in handling, storing, and trans
porting liquid fertilizers it is desirable for the fertilizer
. ric acid as one of the reagents wherein the temperature
tended to salt-out at low temperatures, which causes
fertilizer containing N,P2O5, and S in a concentration of
at least about 28% and having a salt-out temperature
below about 0 F. comprising the steps of (a) mixing an
aqueous solution of sulfuric acid with diammonium
is maintained within acceptable limits by control of the
concentrations and compositions of the reactants.
to have a high concentration of plant food components.
In accordance with this invention there has been
However, highly concentrated liquid fertilizers have 20 provided a method of producing a stable acidic liquid
problems in storage and in distributing the fertilizers.
The problems of salt-out are accentuated by the diffi
culty of redissolving the solids which have formed.
Attempts to alleviate the problems caused by salt-out
have been made in the prior art. For example, in U.S.
Pat. No. 4,315,763 Stoller describes a high analysis fer
tilizer reported therein not to salt-out at 0 C. In U.S.
phosphate, said aqueous solution containing sulfuric
acid in a concentration from about 10 to about 16% by
weight, said diammonium phosphate being present in an
amount from about 3 grams to about 5 grams per gram
Pat. No. 4,388,101 Lowder describes a liquid fertilizer
of
sulfuric acid; (b) mixing urea with the reaction prod
made from sulfuric acid, urea, and ammonia which is 30
ucts of sulfuric acid and diammonium phosphate, said
stated to be crystal-free when stored for one month at
urea being provided in an amount from about 7 grams to
32 C. However, liquid fertilizers having salt-out tem
about 10 grams per gram of the sulfuric acid which had
peratures signi?cantly lower than 32 F. are desirable.
been mixed with the diammonium phosphate in the
In many areas of the country which are irrigated the
soils are alkaline, and the preferred fertilizers for these 35 preceding step.
The fertilizer produced by this process has a high
soils have a low pH in order to increase the acidity of
concentration of plant nutrients and yet does not salt
the soil. Sulfuric acid is a conventional component used
out until an extremely low temperature, i.e. 0 F. or
to achieve a desirable pH for the fertilizer. However,
even 20 F. is reached. In addition, for a low pH
sulfuric acid is corrosive to materials such as mild steel
used in storing and dispensing fertilizers and efforts 40 material the fertilizer unexpectedly has little or no cor
rosive action on materials such as mild steel or alumi
have been made to inhibit the corrosive effect of fertiliz
ers based on sulfuric acid. However, at least some of
these efforts have required the use of additives which
function as corrosion inhibitors. For example, in U.S.
Pat. No. 4,402,852, Young discloses the use of a dialkyl 45
num.
DETAILED DESCRIPTION OF THE
INVENTION
thiourea as a corrosion inhibitor for sulfuric acid-urea
In the ?rst step of carrying out the invention, an
compositions in the presence of carbon steel, and in U.S.
Pat. No. 4,404,116, Young discloses the use of a cupric
aqueous solution of sulfuric acid in a concentration of
from about 10% to about 16% is mixed with diammo
nium phosphate in a weight ratio of diammonium phos
effect of urea-sulfuric acid mixtures on stainless steel. 50 phate to sulfuric acid of from about 3 to about 5. In the
preferred method of carrying out the invention the
A further problem in the manufacture of fertilizers
aqueous solution of sulfuric acid is mixed with the diam
using sulfuric acid arises from the exothermic nature of
monium phosphate at a temperature of from about 100
its reaction with other components used to make the
F. to about 140 F. Temperatures below about 100 F.
fertilizer. The exothermic reaction can result in unac- '
ceptably high temperatures during the process of mak 55 result in a substantial increase in the mixing time, and
ing the fertilizer.
temperatures above about 140 F. may result in the
This problem has been attacked in several different
formation of crystals in the mixture. In the preferred
ways in the prior art. For example, in U.S. Pat. No.
method of carrying out the invention, advantage is
4,310,343, Verdegaal et a1 discloses the use of a heat sink
taken of the heat of hydration of sulfuric acid by adding
to dissipate heat which builds up when making a liquid 60 sulfuric acid to water to form a hot aqueous solution of
fertilizer having a high nitrogen and sulfur content, and
sulfuric acid. For instance, mixing about a 33% solution
in U.S. Pat. No. 4,445,915, Young discloses a method of
of sulfuric acid at about 70 F. with enough water at
removing heat generated by the reaction of urea and
about 70 F. to produce a 14% solution of sulfuric acid
sulfuric acid by cooling the liquid phase by direct heat
will produce a mixture at 70 F. having a temperature of
exchange with air.
65 about 140 F.
The prior art discloses teachings which have been
The aqueous sulfuric acid solution which is mixed
used to overcome problems arising (1) from the use of
with the diammonium phosphate contains sulfuric acid
concentrated liquid fertilizers and (2) from the use of
in a concentration in the range of about 10% to about
ion-containing compound for reducing the corrosive
4,594,090
16% by weight, preferably in a concentration from
about 11% to about 14% by weight and most preferably
about 12% to about 13% by weight.
The dialkylphosphate is preferably provided in a
weight ratio of dialkylphosphate to sulfuric acid of
about 3.5 to about 4.5 and most preferably in a weight
ratio of about 4.
Although reference is made herein to diammonium
phosphate, the composition of the phosphate compound
4
EXAMPLE I
Ninety grams of 33% sulfuric acid is mixed with 150
grams of water and 102 grams of dry diammonium
5
phosphate analyzing 18-46-0 is added to the resulting
mixture. The sulfuric acid reacts exothermically with
the water and then with the diammonium phosphate
and the temperature rises from about 70 F. to about
130 F.
258 grams of granulated urea analyzing 46-0-0 is
added to the hot reaction products of the preceding
step. The dissolution of the urea causes the temperature
of the resulting invention to drop to about 100 F. and
may depart from that for the formula conventionally
ascribed to the compound as (NH4)2HPO4) in which
two ammonium ions per phosphate ion are shown and,
for example, may suitably range down to about 1.8
ammonium ions per phosphate ion. In the preferred 5 produces a liquid fertilizer analyzing 23-8-0-1.5, having
a pH of 2.5 to 3.0, and a salt-out temperature of less than
method of carrying out the invention, if less than two
0 F. The product is a clear green liquid.
ammonium ions are present per phosphate ion, suffi
The resulting product has low corrosive properties
cient additional phosphate compound is added to bring
the ammonium content to concentrations which would
and can be used even with mild steel.
be achieved by the use of pure diammonium phosphate. 20
EXAMPLE II
Mixing of the aqueous solution of sulfuric acid with
Eighty-four grams of sulfuric acid having a concen
the diammonium phosphate results in a temperature
tration of 33% were mixed with 174 grams of water and
increase from about 20 F. to about 30 F. For example,
dry diammonium phosphate in an amount of 132 grams
sulfuric acid and diammonium phosphate, each having
was
added to the resulting aqueous solution of sulfuric
an initial temperature of about 100 F., will produce a 25 acid, bringing the temperature of the mixture to about
mixture having a temperature after reaction of about
140 F. Two hundred and ten grams of urea were then
130 F.
added to the resulting mixture which reduced the tem
In the next step of the process, urea is added to the
perature to about 100 F. The resulting product ana
mixture produced by mixing diammonium phosphate
lyzed 20-10-O-1.5 had a pH of 1.5 to 2.75.
and sulfuric acid. This step is endothermic and the tem
perature of the solution will decrease by about 20 F. to
about 30 F. It has unexpectedly been found that the
mixture of sulfuric acid and diammonium phosphate
produced in the preceding step has the ability to dis
solve and/or react with relatively large amounts of urea
to produce a solution which is stable against salt-out and
EXAMPLE III
A 600 gram sample of a liquid fertilizer made as set
out in Example 11 was placed in a freezer maintained at
-20 F. and a visual examination of the sample was
made every hour. When the temperature of the liquid
reached 5 F., a few crystals started to appear, and at
which is unexpectedly non-corrosive. For example, it
10 F., the crystals became more obvious. However,
the crystals did not settle out. Even when the tempera
has been found that the resulting product can contain
'urea in a concentration of from about 7 to about 10 40 ture of the liquid reached 20" F., there was no salt
out.
grams of urea per gram of sulfuric acid. Furthermore,
the resulting solution, even though containing such a
high concentration of urea, is stable at temperatures as
low as 0 F. and even lower to 20 F. In the preferred
method of carrying out the invention urea in a weight
ratio of urea to sulfuric acid to about 7.5 to about 9 is
provided. The optimum weight ratio is about 8.
The water content of the liquid fertilizer may be in
the range of 32 to about 45% by weight, is preferably in
the range of 35 to about 40% by weight. In the pre
ferred method of carrying out this invention, the weight
EXAMPLE IV
In order to test the corrosiveness of the instant fertil
izer with that of another acidic, high analysis fertilizer,
two pieces of aluminum having the same size and
weighing 114 grams each were prepared. One was
placed in 600 grams of a liquid fertilizer analyzing
20-10-0-1.5 which was made in accordance with Exam
ple II and having a pH of 1.75 while the other was
placed in 600 grams of a 29-9-0-5 liquid fertilizer having
a pH of about 0.5.
Each sample was left in the fertilizer at about room
temperature for 21 days at the end of which visual ex
The concentration of nitrogen in the fertilizer is pref 55 amination of the samples showed existence of a residue
ratio of urea to diammonium phosphate is maintained
within the range of about 1.5 to about 3, and most pref
erably from about 1.7 to about 2.3.
on each sample. However, the sample which had been
erably in the range from about 18% to about 25%; the
in the fertilizer of the present invention showed about
concentration of P205 is preferably present in the range
10% of the amount of residue as the sample which had
from about 8% to about 13%; and, the concentration of
been in the other fertilizer.
S is preferably in the range from about 1% to about 2%, 60
At the end of the 21 day period, both aluminum sam
and most preferably from about 1.4% to about 1.7%.
ples were removed from the fertilizer solutions, washed
The pH preferably is in the range from about 1.5 to
in plain tap water, dried and weighed. The sample
about 3, and most preferably from about 1.5 to about
which had been in the 20-10-0-1.5 fertilizer weighed
2.75. The total concentration of N, P205 and S is prefer
113.5 grams and the one which had been in the 29-0-0-5
ably from 31% to about 36% and most preferably from 65 solution weighed 112 grams.
about 31% to about 33%.
Having thus described the invention the following
examples are offered to illustrate in more detail.
I claim:
1. A method of producing a stable acidic liquid fertil
izer containing N, P205 and S in a total concentration of
4,594,090
10% to about 16% by weight, and said diammo
nium phosphate being present in amount from
about 3 grams to about 5 grams per gram of sulfuric
acid; and
8. The method according to claim 1 wherein the
aqueous solution of sulfuric acid is at a temperature of
from about 100 F. to about 140.
9. The method according to claim 1 wherein the
weight ratio of urea to diammonium phosphate is from
about 5 to about 3.
10. The method according to claim 1 wherein the
weight ratio of urea to diammonium phosphate is about
at least about 28% and having a salt-out temperature
below about 0 F. comprising:
A. mixing an aqueous solution of sulfuric acid with
diammonium phosphate, said aqueous solution con
taining sulfuric acid in a concentration from about
1.7 to about 2.3.
B. mixing urea with the reaction products of said
aqueous solution of sulfuric acid and said diammo
11. The method according to claim 1 wherein the
aqueous solution of sulfuric acid contains sulfuric acid
in a concentration from about 11% to about 14% by
nium phosphate, said urea being provided in
weight, the diammonium phosphate is provided in a
tion from about 12% to about 13% by weight.
4. The method according to claim 1 wherein the
with urea in a weight ratio of urea to sulfate of about 8.
10
weight ratio of diammonium phosphate to sulfuric acid
amount from about 7 grams to about 10 grams per
15 from about 3.5 to about 4.5, and the product of the
gram of sulfuric acid which had been mixed with
reaction is mixed with urea in a weight ratio of urea to
diammonium phosphate in step A.
sulfuric acid from about 7.5 to about 9.
2. The method according to claim 1 wherein the
12. The method according to claim 1 wherein the
aqueous solution contains sulfuric acid in a concentra
aqueous solution of sulfuric acid contains sulfuric acid
tion from about 11% to about 14% by weight.
20 in a concentration from about 12% to about 13% by
3. The method according to claim 1 wherein the
weight, the diammonium phosphate is provided in a
weight ratio of diammonium phosphate to sulfuric acid
aqueous solution contains sulfuric acid in a concentra
of about 4, and the product of the reaction is mixed
13. A liquid fertilizer made in accordance with claim
1 having a total concentration of N, P205 and S from
nium phosphate in a weight ratio of diammonium phos
about 31% to about 36%.
phate to sulfuric acid from about 3.5 to abut 4.5.
14. A liquid fertilizer made in accordance with claim
5. The method according to claim 1 wherein the
1 having a total concentration of N, P205 and S of from
aqueous solution of sulfuric acid is mixed with diammo 30 31% to about 33%.
nium phosphate in a weight ratio of diammonium phos
15. A liquid fertilizer made in accordance with claim
1 which analyzes about 18% to about 25% nitrogen,
phate to sulfuric acid of about 4.
about 8% to about 13% P205 and about 1.4 to about
6. The method according to claim 1 wherein the
1.7% sulfur, has a salt-out temperature below about 0
reaction products of sulfuric acid and diammonium
35 F. and has a pH from about 1.5 to about 3.0.
phosphate are mixed with urea in a weight ratio of urea
aqueous solution of sulfuric acid is mixed with diammo
16. A liquid fertilizer made in accordance with claim
to sulfuric acid from about 7.5 to about 9.
7. The method according to claim 1 wherein the
1, which has a salt-out temperature below about 20 F.
and a pH of about 1.5 to about 2.75, and contains
N,P2O5 and S in a total concentration from about 31%
reaction products of sulfuric acid and diammonium
phosphate are mixed with urea in a weight ratio of urea
to sulfuric acid of about 8.
to about 33% by weight.
I
45
55
60
65
1!
it
it
UNITED STATES PATENT AND TRADEMARK OFFICE
CERTIFICATE OF CORRECTION
PATENT NO.
4,594,090
DATED
June 10, 1986
INVENTOR(S) 1
Wallace Johnson
It is certi?ed that error appears in the above-identified patent and that said Letters Patent
is hereby corrected as shown below:
In column 1, line 32, delete "32C" in favor of -32F--.
Signed and Scaled this
Sixteenth
Day of September 1986
[SEAL]
Anal:
DONALD J. QUIGG
AttestiugO?'icer
Comrofmuqdmlh
Você também pode gostar
- United States Patent (191: Inoue Et A) - (11) Patent Number: (45) Date of PatentDocumento4 páginasUnited States Patent (191: Inoue Et A) - (11) Patent Number: (45) Date of PatentShrutiAinda não há avaliações
- United States Patent 0 "Ice: Patented May 9, 1972Documento4 páginasUnited States Patent 0 "Ice: Patented May 9, 1972Nguyễn Thanh TùngAinda não há avaliações
- High Nitrogen Mixed FertilizerDocumento8 páginasHigh Nitrogen Mixed Fertilizerpakde jongkoAinda não há avaliações
- Arsenal Philadelphia, Pa. 19137: FrankfordDocumento23 páginasArsenal Philadelphia, Pa. 19137: FrankfordPutri PramodyaAinda não há avaliações
- 2.0 Production of Primary Metabolites 2.1 Production of Organic Acids 2.1.1 Production of Citric AcidDocumento22 páginas2.0 Production of Primary Metabolites 2.1 Production of Organic Acids 2.1.1 Production of Citric AcidBharathiAinda não há avaliações
- United States Patent 0: Patented August 16, 1966 2Documento4 páginasUnited States Patent 0: Patented August 16, 1966 2trinh xuan hiepAinda não há avaliações
- Us 4315763Documento11 páginasUs 4315763Thusith WijayawardenaAinda não há avaliações
- US4297290Process For Preparing Sorbitan EstersDocumento5 páginasUS4297290Process For Preparing Sorbitan Esterstahera aqeelAinda não há avaliações
- Unied States W 0: Patented Nov. 11, 1969Documento2 páginasUnied States W 0: Patented Nov. 11, 1969Yeisson MoraAinda não há avaliações
- UNITED Starts: Patented Apr. 16, 1935Documento2 páginasUNITED Starts: Patented Apr. 16, 1935shalsinia chantalAinda não há avaliações
- Copie de US2899444-1Documento4 páginasCopie de US2899444-1KHALED KHALEDAinda não há avaliações
- Translate Paten US5976485Documento24 páginasTranslate Paten US5976485Lenywulandari AyundaAinda não há avaliações
- Preparation of 150 ML of Ferrous Sulfate Solution Pedia BP: by (Name)Documento12 páginasPreparation of 150 ML of Ferrous Sulfate Solution Pedia BP: by (Name)Anonymous oRjLoaAinda não há avaliações
- United States Patent 0 ": AgricultureDocumento4 páginasUnited States Patent 0 ": AgricultureNitinPrachiJainAinda não há avaliações
- KjeldahlDocumento6 páginasKjeldahlCarlos Andrés MatizAinda não há avaliações
- Sulphuric Acid ManufactureDocumento5 páginasSulphuric Acid ManufactureLeonardo ColmenaresAinda não há avaliações
- US3014784Documento2 páginasUS3014784SatyamSahuAinda não há avaliações
- United States Patent (191: MercadeDocumento4 páginasUnited States Patent (191: MercadeSoufi BadrAinda não há avaliações
- A Further Investigation of the Symmetrical Chloride of Paranitroorthosulphobenzoic AcidNo EverandA Further Investigation of the Symmetrical Chloride of Paranitroorthosulphobenzoic AcidAinda não há avaliações
- Sulphuric Acids: Group Members: Samoi Oladipo Alliyah LindsayDocumento11 páginasSulphuric Acids: Group Members: Samoi Oladipo Alliyah Lindsaysamoi oladipoAinda não há avaliações
- Preparation of 150 ML of Ferrous Sulfate Solution Pedia BP: by (Name)Documento12 páginasPreparation of 150 ML of Ferrous Sulfate Solution Pedia BP: by (Name)Anonymous oRjLoaAinda não há avaliações
- Production of Sulphuric AcidDocumento3 páginasProduction of Sulphuric AcidShahid Mehmud100% (1)
- United States: Patent OfficeDocumento3 páginasUnited States: Patent Officemehul10941Ainda não há avaliações
- Research PaperDocumento10 páginasResearch PaperCatherine chaimaAinda não há avaliações
- US3689216Documento5 páginasUS3689216PABLO URIZ CEREZOAinda não há avaliações
- Removal of Fluorine From Wet Process Phosphoric AcidDocumento2 páginasRemoval of Fluorine From Wet Process Phosphoric AcidAdios AAinda não há avaliações
- Sulfuric Acid TreatmentDocumento9 páginasSulfuric Acid TreatmentShankar AcharAinda não há avaliações
- Preparation Sulfatel: Lynn ShoemakerDocumento3 páginasPreparation Sulfatel: Lynn ShoemakerMilan PetrikAinda não há avaliações
- Kinetics of Wood Saccharification Hydrolysis of Cellulose and Decomposition of Sugars in Dilute Acid at High Temperature.Documento10 páginasKinetics of Wood Saccharification Hydrolysis of Cellulose and Decomposition of Sugars in Dilute Acid at High Temperature.René MartínezAinda não há avaliações
- Ribotide StabilityDocumento3 páginasRibotide StabilityadityarmehtaAinda não há avaliações
- Sulfur and Sulfuric AcidDocumento24 páginasSulfur and Sulfuric AciddhavalAinda não há avaliações
- Production of Sulfuric AcidDocumento29 páginasProduction of Sulfuric Aciddeshaka11Ainda não há avaliações
- Us 3109732Documento4 páginasUs 3109732Ahmed RabeaAinda não há avaliações
- Balestrero 1986Documento4 páginasBalestrero 1986shenn0Ainda não há avaliações
- Soil Survey Standard Test MethodDocumento5 páginasSoil Survey Standard Test MethodMPK08Ainda não há avaliações
- H3PO4Documento23 páginasH3PO4Leo Edrik Cortez VidalAinda não há avaliações
- Sugar Cane Juice Sulphitation-ReaccionDocumento12 páginasSugar Cane Juice Sulphitation-ReaccionVILMA CAROLINA PORTILLO CHAVEZAinda não há avaliações
- Paten Sulfur Mixer PDFDocumento7 páginasPaten Sulfur Mixer PDFLily DianaAinda não há avaliações
- US3416887Documento6 páginasUS3416887khairulAinda não há avaliações
- Formula: H-O4-P.2Na: Sodium PhosphateDocumento3 páginasFormula: H-O4-P.2Na: Sodium PhosphateKeiffer Ann LopezAinda não há avaliações
- A Single-Solution Method For The Determination of Soluble Phosphate in Sea WaterDocumento6 páginasA Single-Solution Method For The Determination of Soluble Phosphate in Sea WaterA'yunil HisbiyahAinda não há avaliações
- Sulphonation Plant ChemithonDocumento36 páginasSulphonation Plant ChemithonKantilal Malwania100% (1)
- Content: The Determination of The Amount of Phosphate in A DetergentDocumento20 páginasContent: The Determination of The Amount of Phosphate in A DetergentAditya jain100% (10)
- History The Digestion Process: Organic N + H SO (NH) SO + H O + Co + Other Sample Matrix By-ProductsDocumento8 páginasHistory The Digestion Process: Organic N + H SO (NH) SO + H O + Co + Other Sample Matrix By-Products16_dev5038Ainda não há avaliações
- Matrix Acidizing of Sandstone4Documento5 páginasMatrix Acidizing of Sandstone4HelyaAinda não há avaliações
- Patent Office: 5 Claims. (CL 260-69)Documento2 páginasPatent Office: 5 Claims. (CL 260-69)Teleson MarquesAinda não há avaliações
- A THE Determination THE: SmallDocumento4 páginasA THE Determination THE: SmallharulyAinda não há avaliações
- Transparent Bar Soap Composition Comprising Glycerine Derivative US6656893 PDFDocumento15 páginasTransparent Bar Soap Composition Comprising Glycerine Derivative US6656893 PDFpertmasterAinda não há avaliações
- Storage: o - InzoDocumento4 páginasStorage: o - InzoOscar SobradosAinda não há avaliações
- July 20, 1954 J. L. Krieger 2,684,286: InventorDocumento4 páginasJuly 20, 1954 J. L. Krieger 2,684,286: InventorNurhafizah Abd JabarAinda não há avaliações
- Sir Zafar AssignmentDocumento15 páginasSir Zafar AssignmentTaha MohiuddinAinda não há avaliações
- The Determination of Sulfate and Sulfide Sulfur in Rocks or MineralsDocumento9 páginasThe Determination of Sulfate and Sulfide Sulfur in Rocks or Mineralssupendra phuyalAinda não há avaliações
- A Guide To Kjeldahl Nitrogen Determination Methods and ApparatusDocumento13 páginasA Guide To Kjeldahl Nitrogen Determination Methods and ApparatusNoranisza MahmudAinda não há avaliações
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresNo EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresNota: 5 de 5 estrelas5/5 (1)
- Legal Chemistry: A Guide to the Detection of Poisons, Examination of Tea, Stains, Etc., as Applied to Chemical JurisprudenceNo EverandLegal Chemistry: A Guide to the Detection of Poisons, Examination of Tea, Stains, Etc., as Applied to Chemical JurisprudenceAinda não há avaliações
- Plant and Animal Bio-Chemistry - Including Information on Amino Acids, Proteins, Pigments and Other Chemical Constituents of Organic MatterNo EverandPlant and Animal Bio-Chemistry - Including Information on Amino Acids, Proteins, Pigments and Other Chemical Constituents of Organic MatterAinda não há avaliações
- Water Softening with Potassium Chloride: Process, Health, and Environmental BenefitsNo EverandWater Softening with Potassium Chloride: Process, Health, and Environmental BenefitsAinda não há avaliações
- Chemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastNo EverandChemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastAinda não há avaliações
- Personal Tutor: 11 + MATHS Test 6Documento10 páginasPersonal Tutor: 11 + MATHS Test 6siddhant4uAinda não há avaliações
- EL119 Module 2Documento4 páginasEL119 Module 2Kristine CastleAinda não há avaliações
- Cell Signaling - The ComponentsDocumento7 páginasCell Signaling - The Componentsk10 Lớp Dinh DưỡngAinda não há avaliações
- Data Sheet 6GK5213-3BB00-2TB2: Transfer RateDocumento6 páginasData Sheet 6GK5213-3BB00-2TB2: Transfer RateClaudiu VlasceanuAinda não há avaliações
- Visco GraphDocumento4 páginasVisco GraphwamlinaAinda não há avaliações
- 329 Cryogenic Valves September 2016Documento8 páginas329 Cryogenic Valves September 2016TututSlengeanTapiSopanAinda não há avaliações
- Exercises: Use The Correct Form of Verbs in BracketsDocumento3 páginasExercises: Use The Correct Form of Verbs in BracketsThủy NguyễnAinda não há avaliações
- World War 1 NotesDocumento2 páginasWorld War 1 NotesSoarSZNAinda não há avaliações
- Micron Serial NOR Flash Memory: 3V, Multiple I/O, 4KB Sector Erase N25Q256A FeaturesDocumento92 páginasMicron Serial NOR Flash Memory: 3V, Multiple I/O, 4KB Sector Erase N25Q256A FeaturesAEAinda não há avaliações
- Police Log September 24, 2016Documento14 páginasPolice Log September 24, 2016MansfieldMAPoliceAinda não há avaliações
- Concept of InsuranceDocumento4 páginasConcept of InsuranceNazrul HoqueAinda não há avaliações
- Head and Neck Seminal Papers From Tata HospitalDocumento29 páginasHead and Neck Seminal Papers From Tata HospitalSudhir NairAinda não há avaliações
- Relativity Space-Time and Cosmology - WudkaDocumento219 páginasRelativity Space-Time and Cosmology - WudkaAlan CalderónAinda não há avaliações
- Income Tax - MidtermDocumento9 páginasIncome Tax - MidtermThe Second OneAinda não há avaliações
- Lagundi/Dangla (Vitex Negundo)Documento2 páginasLagundi/Dangla (Vitex Negundo)Derrick Yson (Mangga Han)Ainda não há avaliações
- Everyday Life - B1 - ShoppingDocumento7 páginasEveryday Life - B1 - ShoppingAmi BarnesAinda não há avaliações
- PESTEL AnalysisDocumento2 páginasPESTEL AnalysisSayantan NandyAinda não há avaliações
- Congenital Abnormalities of The Female Reproductive TractDocumento14 páginasCongenital Abnormalities of The Female Reproductive TractMary SheshiraAinda não há avaliações
- ITR-C (Instrument) 16cDocumento1 páginaITR-C (Instrument) 16cMomo ItachiAinda não há avaliações
- Agro Tech Foods LTDDocumento17 páginasAgro Tech Foods LTDAlmas KhanAinda não há avaliações
- Nursing ManagementDocumento5 páginasNursing Managementheron_bayanin_15Ainda não há avaliações
- TQ Science10 Q3 ST4Documento2 páginasTQ Science10 Q3 ST4mae cudal100% (1)
- Amazing RaceDocumento2 páginasAmazing Racegena sanchez BernardinoAinda não há avaliações
- Yemen Companies Contact DetailsDocumento5 páginasYemen Companies Contact DetailsYAGHMOURE ABDALRAHMAN78% (9)
- Router Board Performance TestsDocumento2 páginasRouter Board Performance TestsedkaviAinda não há avaliações
- Cover PageDocumento209 páginasCover PageABHISHREE JAINAinda não há avaliações
- Beamer Example: Ethan AltDocumento13 páginasBeamer Example: Ethan AltManh Hoang VanAinda não há avaliações
- Vocabulary: ExileDocumento5 páginasVocabulary: ExileWael MadyAinda não há avaliações
- Restricted Earth Fault RelayDocumento5 páginasRestricted Earth Fault Relaysuleman24750% (2)
- Awareness On Stock MarketDocumento11 páginasAwareness On Stock MarketBharath ReddyAinda não há avaliações