Escolar Documentos
Profissional Documentos
Cultura Documentos
Prohibition of Direct To Consumer Advertising
Enviado por
aholmes172Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Prohibition of Direct To Consumer Advertising
Enviado por
aholmes172Direitos autorais:
Formatos disponíveis
Title 21 – Food and Drugs
Chapter I – Food and Drug Administration - Department of Health and Human Services
Subchapter C – Drugs: General
Part 202 – Prescription Drug Advertising
Sec 202.1 – Prescription Drug Advertisements
(a) – Sec 202.1 is now repealed and replaced by Sec 202.3
Sec 202.2 – Definitions
(a) For the purposes of this section, prescription drug is defined as a finished
dosage form, e.g., tablet, capsule, or solution that contains an active drug
ingredient and is only available through a prescription written by a duly licensed
provider and dispensed in accordance with federal and state pharmacy practice
laws. For purposes of this section, prescription drug also means biological product
within the meaning of section 351(a) of the Public Health Service Act.
(b) For the purposes of this section, Direct to Consumer Advertising is defined as
providing material in the form of written, audio, or visual advertisements in
newspapers, magazines, general consumer publications, television, or websites
that promote a prescription drug, as defined above, with the intent to influence
consumers or healthcare providers to seek treatment with a prescription drug.
(c) For the purposes of this section, pharmaceutical sales representative is
defined as a professional employee of a drug application holder who educates
and informs healthcare professionals about prescription drugs including, but not
limited to, approved and unapproved uses, important safety information, clinical
trial data, and recommended doses.
(d) For the purposes of this section, drug application holder is defined as a
pharmaceutical company who holds the marketing application (New Drug
Application) for a prescription drug.(does this include generic drug
manufacturers?)
(e) For the purposes of this section, prescription drug sample is defined as a unit
of a prescription drug that is not intended to be sold and is intended to promote
the sale of the drug (21CFR203.3i).
Sec 202.3 – Prohibition of Direct to Consumer Advertising for Prescription Drugs
(a) Effective January 1st, 2010, sec 202.3 replaces Sec 202.1 of 21CFR202.
(b) Drug application holders may no longer pursue or engage in Direct to
Consumer Advertising of prescription drugs.
(c) Drug application holders’ pharmaceutical sales representatives can continue to
engage, interact with, and educate healthcare providers about prescription drugs
as long as they provide a fair and balanced profile for the prescription drug to
include:
(1) Full disclosure of the approved and unapproved uses for the
prescription drug and any supporting or detracting clinical trial data for
these uses.,
(2) Full disclosure of the safety profile of the drug, including information
on placebo controlled studies and similar competitor comparator drug
safety profiles.For the purposes of this section, full disclosure is to include,
but is not limited to, all known or observed side effects, prevalence of side
effects, and known contraindications.
(3) Full disclosure of financial interests of all individual research scientists
or research groups included in or in the conduct of clinical trial studies or in
the writing of journal articles based on said studies.
(d) Pharmaceutical sales representatives may no longer distribute prescription
drug samples as part of their interaction with healthcare providers, as this is
considered of a form of Direct to Consumer Advertising.
(e) Sec 202.3 will be fully enacted on January 1st 2010, drug application holders
failing to meet the requirements of Sec 202.3 will be in violation of this
regulation.
Você também pode gostar
- What's Your Diagnosis?Documento1 páginaWhat's Your Diagnosis?aholmes172Ainda não há avaliações
- Antitrust LawDocumento13 páginasAntitrust Lawaholmes172Ainda não há avaliações
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (890)
- Final HPM5001Documento9 páginasFinal HPM5001aholmes172Ainda não há avaliações
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- Fundus Slide ShowDocumento21 páginasFundus Slide Showaholmes172Ainda não há avaliações
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (399)
- Silver Lake Memorial Hospital OverviewDocumento8 páginasSilver Lake Memorial Hospital Overviewaholmes172Ainda não há avaliações
- Retina Today (Filters)Documento3 páginasRetina Today (Filters)aholmes172Ainda não há avaliações
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- A Simplified Severity Scale For Age-Related Macular Degeneration AREDS Report No 18Documento14 páginasA Simplified Severity Scale For Age-Related Macular Degeneration AREDS Report No 18aholmes172Ainda não há avaliações
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- Retina Eye Specialists: A Futuristic View BackgroundDocumento6 páginasRetina Eye Specialists: A Futuristic View Backgroundaholmes172Ainda não há avaliações
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- Ethics IncidentsDocumento4 páginasEthics Incidentsaholmes172Ainda não há avaliações
- Silver Lake Memorial Hospital OverviewDocumento8 páginasSilver Lake Memorial Hospital Overviewaholmes172Ainda não há avaliações
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Electronic Child Health Network Case Analysis Austin Holmes School of Public Health, New York Medical College HPM6058Documento6 páginasElectronic Child Health Network Case Analysis Austin Holmes School of Public Health, New York Medical College HPM6058aholmes172Ainda não há avaliações
- GUI - Final - Referencing - Approved - Oct 2020Documento18 páginasGUI - Final - Referencing - Approved - Oct 2020Proschool HyderabadAinda não há avaliações
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- LA & GA by SakshiDocumento43 páginasLA & GA by SakshiSakshi sdpcAinda não há avaliações
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- Neurotoxicity of Ecstasy (MDMA) : An OverviewDocumento10 páginasNeurotoxicity of Ecstasy (MDMA) : An OverviewArian JafariAinda não há avaliações
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2219)
- tcrm0301 003Documento11 páginastcrm0301 003paulAinda não há avaliações
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- Position Paper On Critical Care Pharmacy Services: 2020 UpdateDocumento22 páginasPosition Paper On Critical Care Pharmacy Services: 2020 UpdatePriscila Navarro MedinaAinda não há avaliações
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- Use of Antibiotic and Analgesic Drugs During LactationDocumento11 páginasUse of Antibiotic and Analgesic Drugs During LactationpinakshiAinda não há avaliações
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (265)
- Common Pharmacy Abbreviation & Dosage FormsDocumento14 páginasCommon Pharmacy Abbreviation & Dosage FormsKENNETH GENER JAMES SOMERAAinda não há avaliações
- Antacids H Antagonists Proton Pump InhibitorsDocumento49 páginasAntacids H Antagonists Proton Pump Inhibitorsmelvingodric_arceAinda não há avaliações
- EthicsDocumento21 páginasEthicsamitshahco50% (2)
- Notice To Importers NDS Submission Feb 2022Documento4 páginasNotice To Importers NDS Submission Feb 2022Daniyl JonesAinda não há avaliações
- Apticon Call For Papers - 27!04!2023Documento4 páginasApticon Call For Papers - 27!04!2023Kamani KaushikbhaiAinda não há avaliações
- Non Invasive Methods of Estimating Pharmacokinetic Parameters2Documento31 páginasNon Invasive Methods of Estimating Pharmacokinetic Parameters2Thilak Chandra50% (2)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- Active Substance Pharmaceutical Dosage FormDocumento2 páginasActive Substance Pharmaceutical Dosage FormkingAinda não há avaliações
- Drug Interactions With Anti-Asthma MedicationDocumento6 páginasDrug Interactions With Anti-Asthma MedicationSuryasumanthAinda não há avaliações
- Sem 4 Lectura 2 Inhibidores de Recaptación de SerotoninaDocumento18 páginasSem 4 Lectura 2 Inhibidores de Recaptación de SerotoninaJesús MoraAinda não há avaliações
- Dravyaguna Vigyan Paper ReviewDocumento8 páginasDravyaguna Vigyan Paper Reviewchauhan_892277982Ainda não há avaliações
- 4Q2014EXCELDocumento857 páginas4Q2014EXCELyash143565Ainda não há avaliações
- Lehne's Pharmacology for Nursing Care Test Bank Chapter 1Documento3 páginasLehne's Pharmacology for Nursing Care Test Bank Chapter 1Angel Beaudoin-AlfordAinda não há avaliações
- Dispensing Lab - Calculations and Practice of PharmacyDocumento36 páginasDispensing Lab - Calculations and Practice of PharmacyJoyce VillanuevaAinda não há avaliações
- PhenobarbitalDocumento3 páginasPhenobarbitalChristine Joy Pepito50% (2)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (119)
- Ajovy - Fremanezumab - Drug Information - UpToDateDocumento6 páginasAjovy - Fremanezumab - Drug Information - UpToDateDiana PhamAinda não há avaliações
- Antihistaminic & Antiallergic Drugs: Biosynthesis of HistamineDocumento18 páginasAntihistaminic & Antiallergic Drugs: Biosynthesis of HistamineSara MohamedAinda não há avaliações
- Usp 2008 P 2 Supplement 3Documento166 páginasUsp 2008 P 2 Supplement 3EstiPramestiningtyas100% (1)
- Notices For The Application of Plant Master File Form A PDFDocumento5 páginasNotices For The Application of Plant Master File Form A PDFAnandharaj AsaithambiAinda não há avaliações
- In Vitro in Vivo Correlation IMPORTANCE OF DISSOLUTION IN IVIVC PDFDocumento5 páginasIn Vitro in Vivo Correlation IMPORTANCE OF DISSOLUTION IN IVIVC PDFMehmet ÖzdemirAinda não há avaliações
- Evaluasi Keterseiaan Obat TRHP FormulariumDocumento96 páginasEvaluasi Keterseiaan Obat TRHP FormulariumAsetianiAinda não há avaliações
- Medication Routes Forms: SpeakingDocumento8 páginasMedication Routes Forms: SpeakingFiky NiswatiAinda não há avaliações
- Proper Disposal of Expired or Unwanted DrugsDocumento9 páginasProper Disposal of Expired or Unwanted Drugscarramrod2Ainda não há avaliações
- 1 Comparative Pharmacokinetics of Oral Ibuprofen FormulationsDocumento11 páginas1 Comparative Pharmacokinetics of Oral Ibuprofen FormulationsNishad PrabhuAinda não há avaliações
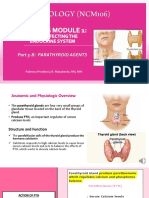
- Module 1 Part 5B. Parathyroid AgentsDocumento21 páginasModule 1 Part 5B. Parathyroid AgentsBSN2G- SABLA-ON LORRAINE ANNEAinda não há avaliações