Escolar Documentos
Profissional Documentos
Cultura Documentos
Rigorous Simulation and Design Plate Column PDF
Enviado por
Catherine CcasaDescrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Rigorous Simulation and Design Plate Column PDF
Enviado por
Catherine CcasaDireitos autorais:
Formatos disponíveis
Computers & Chemical Engineering, Vol. IO, No. 5, pp.
M-515,
Printed in Great Britain. All rights reserved
1986
Copyright 0
0098-1354/86 $3.00 + 0.00
1986 Pergamon Journals Ltd
RIGOROUS SIMULATION AND DESIGN OF COLUMNS
FOR GAS ABSORPTION AND CHEMICAL
REACTION-II
PLATE COLUMNS
L. DE LEYE and G. F. FROMENT
Laboratorium voor Petrochemische Techniek, Rijksuniversiteit, Gent, Belgium
(Received
22 January 1985; revision received 5 September 1985;
received for publication 20 January 1986)
1. INTRODUCTION
In Part I [l, this issue, pp. 4935041 of this paper the
mathematical modelling of absorption accompanied
by chemical reaction was developed both for simple
and complex cases and attention was focused upon
application to packed columns. Part II applies the
theory to the design or simulation of plate columns.
These are preferred to packed columns when widely
varying loadings may be expected. Also, large values
of the mass-transfer coefficients and large interfacial
areas can be achieved in traycolumns, which makes
them appropriate not only for the fast reactions
encountered in gas treating, but also, given the high
liquid hold-up, for the slow reactions encountered in
chemical-producing processes.
neous absorption and parallel reactions (Type 1A).
The variation of, the mole fractions of the absorbing
components in the gas phase and of the total gas flow
rate on plate k are given by
F
dyi
di!
l-yjp!E?
I= I Njly=O
=
MATHEMATICALMODELLING
and
(2)
while for non-absorbing
The boundary
The various flows and compositions of the gas and
liquid in the column and on a typical plate are shown
in Fig. 1. Gas and liquid feed or withdrawal on a
plate are also included. The components in the liquid
phase are numbered in the following order: components undergoing absorption and reaction, physically absorbed components, liquid-phase reactants,
reaction products involved in further reactions, final
reaction products and inert liquid-phase components.
When the total number of plates, the compositions,
flow rates, temperatures and pressures of the gas and
liquid feed streams and the flow rates of intermediate
gas or liquid withdrawals are given, the flow rates,
compositions, temperatures and pressures on each
plate have to be calculated. This is the simulation
problem. In the design mode the number of plates
required to achieve a specified absorption has to be
determined. This requires a number of iterations in
the column calculation.
In the model to be described, the gas is assumed to
be in plug flow, while the liquid on the plates is
completely mixed. Non-isothermal and non-isobaric
operation are considered. The equations below are
written for a fairly general example involving simulta505
forj=l,...,nA,
-Njly=i)AtR*
FYI= constant
2.
(1)
components,
for j = nA + 1, . . . , n,.
conditions
(3)
are
for 2 = O,yj=yjpk
F=@
forj=l,...,no.
and
(4)
for 2 = h, y,= yj,k
F = Fk
Pf and yFk are the gas flow rate and the mole
fractions in the gas entering the plate. These are
related to the quantities leaving the plate k + 1 in the
following way, accounting for side streams:
and
forj=l,...,no.
(6)
The equations for the flow rate and composition of
the liquid are derived, by way of example, for the
parallel reactions (33) and (34) (Type 1A) of Part I
[l]. Since the various reactions probably have
L. DE LEVE and G.
506
F. FROMENT
X ,,,;j=
.f-JL
1..
-La
X, .;j= 1 ,
Y, ,;j=l,...,nG
, nL
k-7; ,@,I,
Fk+lk+l T
Liquid
Gas
Fig. 1. Flows and compositions in the column and on a plate.
different reaction rates, the xj,k depend upon the
appropriate Hatta number.
For the absorbed components undergoing a very
fast reaction (appropriate Hatta number > 3),
xik=O
for the remaining
absorbed components,
forj=n,+l,...,n,.
forj=l,...,nv;
(10)
(7)
for those involved in a moderately fast reaction
(appropriate Hatta number between 0.3 and 3),
The mole fractions of reactants, reaction products
and inerts in the liquid on the plate are given by
X,,.k(G + WW,)
X/k(L+
Ww~)=xj,k-,L;_, +xw,,k WV,
+NjIy-.vL
=x,~k_,L;_,+xw,,kwk
A:Rh, - aj,jrj(l - A,y,)Rh,c,
forj=n,+l,...,n,+n,;
x {(@Y&-
(8)
-(G-I%-,
for the absorbed components undergoing a very slow
reaction (Ha c 0.3)
F,Y,,,) -
a/,/
[x,Ow~ + 4)
I-
+ W,XW,,,)l~
forj=n,+l,...
,+,+nR+nP
(11)
and
+y~kkFl~-Yj,kFk-Uj,jrj(l
forj=nv+n,+l,...,n,+nM+n,;
-A$,)Rh,C,
Xj,k(L;+
(9)
WWt)=Xj,k-IL;-I+X~,.~Wv~
forj=n,+n,+n,+l,...,n,.
(12)
507
Simulation of absorption and reaction in columns-II
The minus sign in equation (11) applies to the
reactants, the plus sign to the reaction products.
The absorption fluxes of the different components
are determined from
Njly-0 =
fi
Hi
~
ko.j(Pt)k + kL,j(Pt)k
forj=l,...,nA,
NjIy=o=
Hay
forj=l,...,nv,
~jl,=,=kG,j(Pt)k[Yj-H~(Xj.k)il
and from the application
tanh (Ha;)
(13)
of Ficks law for diffusion:
du,
- Djckdy
(20)
are not necessarily computed from the integrated
equations (16) and (17), but may also be obtained
through
y=o
forj=l,...,n,
HjXj,k
(14)
- cash (Ha;)
Njly-o=
and
N,J,,=,, = -
DjCk3
dY
tanh(Ha;)
ko,jG,)k + kL.j(Jt)k HaJ
Hj
forj=n,+l,...,nv+n,
YYL
forj=n,+l,...,n,.
(21)
(15)
and
For the determination of these fluxes, the concentration profiles of the absorbed components, undergoing fast reactions in the liquid film, have to be
computed from the following set of second-order
differential equations:
Djdzx=%r.
for j=
dy2
Ha!,k
Njly-yL=
,c (xj,k>i-xj,kcosh(Ha~)
LJ k
sinh (Ha;)
forj=nv+l,...,nv+n,
with
I,...
,nv+n,;
C, *
(16)
HI
and for the liquid-phase reactant participating
fast reactions,
in the
/=I
Hi(Pth
ck
and provided that Ha; is expressed by
Da+rFr,
with boundary
(22)
mj,,y+
1aj,,k,C$
- I (xi k)?J - (xK ,+)yDj
Haj =
conditions
kLJ
at
y=o
forj=l,...,nv
dx,= -- Njly=o
dy
(23)
and
forj=l,...,nv+n,
DjCk
-dxa = 0 for the liquid-phase reactant
dy
ticipating in the fast reactions
par(17)
kL.j
for j = nv + 1, . . . , n, + nM. (24)
andat
y=y,,
xj = 0
forj=l,...,nv
xj=xik
forj=nv+l,...,nv+n,
xa = x, k for the liquid-phase reactant
ticipating in the fast reactions.
par-
When the kinetic expressions of the nv very fast
and nM moderately fast reactions have the following
simple forms:
rj = kjC_?lC~R
for j = 1, . . . , nv
If one or more of the fast reactions have a more
general kinetic expression, the set of second-order
differential equations (16) has to be solved numerically. The integration is performed by means of a
variable-step finite-difference method for the solution
of boundary-value problems.
The fluxes of the remaining absorbed components,
undergoing a very slow reaction or which are only
physically absorbed, are given by
(18)
Njly-o=
and
rj = kjC,CtR
for j = nv + 1, . . . , n, + nM, (19)
Yj- HjXj*k
1
Hj
ko,j(Pt)k + kL,j(Pt)k
forj=nv+n,+l,...,n,.
the interfacial fluxes of the absorbed components that
undergo the fast reactions, obtained through the
approximate solutions of Onda et al. [2],
(25)
The temperature on a plate is determined from an
enthalpy balance around the plate:
508
L. DE LEYE and
G. F. FROMENT
with
G-IL;-,
? Xj,k-ICpL.,-Tk Fk f yj,kCpc;.j
j=l
Q$ = (-AH,b)(Ft_YFk- FkJj,k)
j=l
+ (WW, + Li) 5
,=I
t Tk+, (Fk+\-
Xj.kCpL,
VW,+)) 2
forj=l,...,n,+
Yj,k+lCpG.,
j-l
(27)
and
Q~k=(-AH~)~,{(~~Y~k-F,Yj.k)
+Tvk+,l/,k+,
j$
Yv,,,
+ I CPO., +
Twt
WV,
-[xj,k(L;+
OL
x
j=l
xw,.kcPL.,
Q: -,$,
Q;? - 5 Q;, (26)
,= I
W~,)-(~;-,~j,k-,
+ WVkXW,,,)I)
forj=l,...,n,.
start
YES
NO
Gas
withdr.
Estimate
ITER
k=l
DETERM.
PHYS.
kG
PLATE
PROP.
kL
CHARACT.
I
Haj,j
Determine
= 1 . . .
I-IR
NO
YES
Fig. 2(a)
(28)
Simulation of absorption and reaction in columns-11
Equation
3. A COMPUTER PROGRAM, A-Tray
(26) is written in the following form:
Tk_,A;+TkB;+Tk+,C;=D;
k=l,...,
N,
(29)
with A; = 0 and CN = 0, so that the coefficient matrix
of the set of non-linear algebraic equations (29) is
reduced to a tridiagonal matrix.
The pressure drop on a plate depends upon the
type of tray (sieve, bubble caps, valve, . . . , etc.).
Correlations are available in the literature and these
were incorporated into the program A-Tray.
Ha" .* j
3'
= l,n
V + "M
509
The second subprogram of A-Tower, specifically
dealing with plate columns is called A-Tray. It comprises three modules: one for physical absorption,
one for single absorption accompanied by a single
reaction, reversible or irreversible, and one for multiple absorption accompanied by a set of parallel or
consecutive reactions, with kinetic regimes ranging
from very slow to instantaneous and non-isothermal,
non-isobaric operation. It is clear from the previous
section that the calculations lead to the number of
xI
= l,n
V + "M
j,k
'k
I
Determine
kG'
PHYS.
Determine
PROP.
kG
kL
Plate
charact.
Plate
PHYS.
PROP.
kL
1
I
chat-act.
I
RUKUGILL
Num.
Eqs (l),(2)
;
y;:k
RUKUGILL
Num.
Eqe (l),(2)
Int.
= 1,nA
Eq
(3)
; j=n
';:k
Eqs
+ 1,n
l,n
(3)
; j = nA + l,nG
y;;k
Eqs
(5),(6)
(5),(e)
Fk+l
Fk+l
j = l,nG
'i,k+l'
_*
L'k
Eqs
'j,k'
j = 1,n
yj,ktl'
Eqs (11),(12)
Combin.
(11),(12)
j = nA t l,nL
2ndorder
Num. Integr.
Diff. eqs (16)
(8),(9),(10)
Eqs
'j,k'
Eqs
Ha"
F;"
F;"
Eq
y;:k
Int.
j = *v t l,nA
(23),(24)
j = l.nV
3'
Calculate
xI.
J*k
t nM
dc.
Ck
5-l
YYL
= 1,
"V + "M
= nv t
Eqs (8),(9),(10)
'j,k'
j = nv t l,nA
Calculate
Ck
d
4
Fig. 2(b)
l,nV
L. DE LEYEand G. F. FROMENT
510
real-not
theoretical-trays.
By way of example, the
algorithm used for the case whereby the absorption
is accompanied by Type 1A parallel reactions,
defined in Part I [1] of this paper, is shown in Fig. 2.
A tray-to-tray method
plate of the column. The
estimates of the unknown
various plates is reduced
is used, starting at the top
calculation of the successive
variables at the top and the
to the problem of solving a
NO
(APt)k-+.(Pt)k;
k = 1 ... N
M0
Estimate
j = 1 . . . nC-1
I
yj,l'
Estimate
j = 1 . . . nA
'j,l'
F1
L
TV
ITER
= ITER
t 1
b8
SSQ, = Z(
(x.
k)E
J9
(x.
k)C
(Ha" .jE
tZ(
(Xi
= Z(
k)E - (Xi,k)C
(x*,
+ Z(
(x.
=
Z(
k)E
(x.
k)C
Cc,),
('k)E
)E - (Xi,
+(
- ('k)C)2
(=k)E
J,k)'
('I.
)E
J,k
)
('j,k)E
-q2
.lgk
('j,k)E
SSQ,
(Ha .)
(Ha"j)E
('j,k)E
SSQ,
(=k)C
)
(CkjE - (CkjC 2
)2 + (
)
(=k)E
('k)E
Fig. 2(c)
Fig. 2(a-c). Flow chart of the algorithm for the simulation of a plate column in which absorption is
accompanied by a Type IA system of parallel reactions.
511
Simulation of absorption and reaction in columns---II
Table 3. Commuted results for the H,S-DEA
Table 1. Constructive details of the valve trays in
the H,S-DEA column
Property
Active area, n (m)
Relative free area on plate
Weir length (m)
Weir height (m)
Valve thickness (m)
0.4225
0.153
0.872
0.066
0.00188
L (kmol/h)
F (kmol/h)
YHB
xti,s
+wx-I
XHS;;;y
of non-linear algebraic equations. For this purpose the program contains Wegsteins method [3] and
the generalized secant method [4]. The application of
A-Tray to a couple of important industrial processes
is illustrated in the next section.
set
4. EXAMPLES OF THE APPLICATION
4.1. The absorption of H2S
ethanolamine (DEA) solution
in an aqueous di-
HS- + RNH; .
(30)
This reaction is instantaneous and reversible.
Data for the determination
of the equilibrium
constant of the reaction, K,, were taken from the
literature [7,8].
Densities and viscosities of the solution and
diffusivities of the reactants in the solution were
calculated out of the experimental values [9] and
literature data [lo, 111. For the determination of the
diffusivity of the H,S in water, Wilke-Changs correlation was used. This diffusivity was corrected for
the composition of the aqueous solution according to
the Stokes-Einstein relationship.
Table 2. Standard correlations in
the A-Tray program for the determination
of the mass-transfer
coefficients and the plate characteristics
Property
ko
k,
A:
h,
f,
&, h
Correlation
Stichlmair [I 21
Stichlmair [12]
Stichlmair [12]
Stichlmair (121
Stichlmair [I21
Glitsch Inc. [13]
Bottom
column
2208.8
161.69
0.13 x lo-
0.0
0.41 x lo-
0.0
0.0
7.47
318.15
535.68
0.442
0.367
0.153
0.371
2247.9
200.8
0.195
0.134 x 10-J
0.230 x IO-
0.173 x 10-l
0.173 x 10-l
7.590
321.5
292.45
0.443
0.387
0.169
0.328
No. of iterations: 4
CPU time used (Data General MV 6000): 25 s
OF A-Tray
In this example a DEA solution is used for the
removal of H,S from a refinery stream. The gas feed
has an average molecular weight of 24.9 kg/kmol and
contains 19.5 mol of Hz S. The flow rate, temperature
and pressure of the gas feed are 200.85 kmol/h,
318.15 K and 7.6 b, respectively. A 20 wt% DEA
solution at a temperature of 318.15 K is used as
solvent. Its flow rate is 2208.8 kmol/h. The column
has a diameter of 0.98 m and is equipped with 18
Glitsch Vl-ballast trays. The constructive details of
the plates are summarized in Table 1.
The absorption of H2S in this solution is accompanied by the following overall reaction [5,6]:
H, S + R,NH &
T (R)
A: (m2/m)
ko, HZ (kmollm h b)
F. ::)(mih)
F
LI
absorption column
Top
column
The solubility of H,S in the solution was taken
from Kent and Eisenberg [8].
The standard correlations incorporated in the program, which are listed in Table 2, were used for the
determination of the mass-transfer coefficients and
the different plate characteristics.
The initial estimates of temperature and pressure
on each tray were 3 18.15 K and 7.6 b. The mole
fraction of HIS at the top of the column was estimated to be 1 x lo-, the convergence tolerance was
set equal to 10-j. The results of the computations are
summarized in Table 3.
The variations of the mole fractions of H,S in the
gas phase, of DEA and the reaction products in the
liquid phase and of the gas and liquid flow rates along
the column are shown in Fig. 3.
Figure 4 shows the computed temperature and
pressure profiles in the column.
4.2. The simultaneous absorption of H,S and CO2 in
an aqueous NaOH solution
The absorption of H2S and CO2 in an aqueous
NaOH solution is applied in the purification of the
effluent resulting from the thermal cracking of naphtha for the production of ethylene. The absorption is
carried out in a plate column with two NaOH
circuits, one with a concentrated and one with a lean
caustic solution. The configuration of the tower is
schematically represented in Fig. 5.
The gas feed to the column,
F,, equals
5800 kmol/h. The feed temperature and pressure are
3 13.15 K and 12.8 b. The composition of the gas feed
is given in Table 4. 2510 kmol/h of an aqueous
solution containing 4 wt% of NaOH are fed at the
top of the column. 1500 kmol/h of the liquid solution
is withdrawn from tray 15 and 2300 kmol/h of a
1 wt% NaOH solution is added on the underlying
tray.
The column, with a diameter of 2.764m, is
equipped with 30 V,-Glitsch Ballast trays. The constructive details of these trays are given in Table 5.
L. DE LEYEand G. F. FROMENT
512
Gas
0
0
TOP
or
liquid
flaw
rate
(kmol/h)
1000
I
0.1 x10-
I
l-
2000
I
0.3 x10-
I
02x10-
I
x,
mole
t
0.4 x10-
I
fraction
5-
y,
mole
fraction
Fig. 3. Variation of the mole fractions of H,S in the gas phase, of DEA and the reaction products in
the liquid phase and of the gas and liquid flow rates along the column.
Total
TOD
pressure
(b)
8.0
7.5
Temperature
330
320
(K
Fig. 4. Temperature and pressure on each tray in the H,S-DEA
absorption column.
The absorption of H,S in an NaOH solution is
accompanied by the following overall reaction [14]:
H,S + NaOH 2
NaHS + H20.
This reaction is instantaneous
In strong
OH
solutions
(31)
and reversible.
CO2 is undergoing
the
following overall reaction [ 15, 161:
COz + 2NaOH -
Na2 CO3 + H,O.
(32)
Since NaOH and the salt products are completely
dissociated, reactions (31) and (32) can be presented
in ionic form:
H2S + OH- &
HS- + Hz0
(33)
CO:- + H,O.
(34)
and
Fig. 5. Schematic representation
CO2 + 20H-
column.
of the H,S-CO,-NaOH
Simulation of absorption and reaction in columns-11
Table 6. Compositions, flow rate and temperature
of the intermediate liquid withdrawal (W,,,) and
liquid feed (WV,,) in the H,S-CO,-NaOH
absorp
tion column
Table 4. Composition of gas feed
for the H,S-CO,-NaOH
absorption column
Component
Mole fraction
KS
Stream Wwll
0.00079
0.00055
0.28612
0.31337
0.07493
0.10218
0.0442
0.0171
0.16076
CO,
CH,
GH,
C,H,
C,H,
C,H,
C,H,s
H,
513
Stream IV,,,
1500
0.123 x lo-
W (kmol/h)
XHlS
xcol
2300
0.0
0.0
0.0
0.183 x lo-
0.602 x 10-J
0.710 x lo-*
314.63
&hOH
%w-IS
%&go,
T (K)
0.4529 x IO-
0.0
0.0
313.15
Table 5. Constructive details of the ballast trays in
the H,S-CO,-NaOH
absorntion column
Prooertv
Active area, C2(m*)
Relative free area on plate
Weir length (m)
Weir height (m)
Valve thickness (m)
Density material (kg/m)
The equilibrium
constant
Table 7. Computed
5.072
0.1869
2.259
0.05
0.00188
8169
K, of the first reaction is
results for the H$-CD-NaOH
column
L (kmol/h)
F (kmol/h)
YH2.s
Y,Z
+s
xcol
+&OH
Top
column
Bottom
column
2510
5792.2
0.103 x 10-r
0.132 x 10-s
0.0
0.0
0.1842 x 10-l
3314.6
5800
0.792 x 10-s
0.551 x lo-
0.903 x 10-s
0.0
0.551 x 10-Z
0.138 x 1O-2
0.930 x 10-a
12.79
313.78
523.1
0.381
0.378
0.313
0.410
0.170
0.226
39.3
+WiS
W-1
For the
K, , data
reaction
function
kCco, CoH_
determination of the equilibrium constant
of Edwards et al. [17] were used. The
rate coefficient k in equation (36), as a
of temperature and ionic strength of the
Flow
0
0.0
12.569
314.11
525.7
0.401
0.392
0.390
0.429
0.159
0.225
76.1
P, (b)
The kinetic expression of the reaction with CO2 has
the following form:
r =
0.0
XN.EO,
K1 = (HrS)(OH-)
absorption
T (K)
A ; (m2/m)
IC0.u2s(kmollm2 h b)
ko.,,, (kmol/m h b)
k,n,, (m/h)
k,, co1 (m/h)
hF (m)
el.
Haco2
No. of iterations: 8
CPU time used: 242 s
rote
(kmol/h)
2500
5000
I
0.1 x10-1
b
02x10-4
I
x, mole
fraction
5
NoOH
10
25
Bottom
0
0.1
x10-3
0.5
01
x10-3
y,
mole
x10-z
fraction
Fig. 6. Variations of the mole fractions of the different components
the gas and liquid flow rates along the H,S-CO,-NaOH
in the gas and liquid phases
absorption
column.
and
of
514
L. DE LEYEand G. F. FROMENT
Total
TOP
12 0
pressure
(b)
12.5
130
1
5
F = Total molar gas flow (kmol/h)
F, = Enhancement factor
Fk = Molar gas flow rate leaving plate k (kmol/h)
Hj = Henrys coefficient for absorbed component j
(b m3/kmol)
Ha, Ha = Hatta number, modified Hatta number
T
10
Temp
laquld
is
mtermed
feed
4
0).: 15
f;
a
20
-AH, = Heat of absorption of component j (kJ/kmol)
-AH? = Heat of reaction of reaction i (kJ/kmol)
& = Froth height on plate (m) .
Ki = Equilibrium constant of reaction j
T
25
Bottom 30
>\:
3,0
315
Temperature
Fig. 7. H$-CO,
C, = Total molar concentration in the liquid on
plate k (kmol/m)
cP= Specific heat (kJ/kmol K)
D = Molecular diffusivity (m2/h)
dk = Column diameter (m)
320
1K)
absorption in NaOH. Temperatures and
pressures along the column.
solution, is given by Hikita et al. [16] and Pinsent et
al. [18].
The simultaneous absorption of H,S and CO2 in
the NaOH solution is accompanied by a Type 1B
system of parallel reactions.
The diffusion coefficients of H,S and CO, in Hz0
were determined from experimental data [9, 191.
These coefficients were corrected for the composition
of the liquid using the Stokes-Einstein relationship.
For the determination of the diffusion coefficients of
the ionic species the correlation of Nernst-Haskell
[20] was used.
The solubilities of H,S and CO, in H,O were
computed from data by Edwards et al. [17]. They
were corrected for the ionic strength of the solution.
Again, the standard correlations (see Table 2) in
the program were used for the determination of the
mass-transfer coefficients and plate characteristics.
The initial estimates of temperature and pressure
on each tray were 313.15 K and 12.8 b. The mole
fractions of H,S and CO2 at the top of the column
were estimated to be 1 x lOen and 1 x 10m6. The
convergence tolerance was set equal to lo-. The
computed results are summarized in Tables 6 and 7.
The reaction with CO2 is in the very fast regime.
The variations of the mole fractions of the different
components in the gas and liquid phases and of the
gas and liquid flow rates along the column are shown
in Fig. 6. Fig. 7 shows the computed temperatures
and pressures along the column.
NOMENCLATURE
A, = Absorbed component j
a = Stoichiometric coefficient
A, = Gas-liquid interfacial area per unit liquid
volume (m2/m3)
A: = Gas-liquid interfacial area per m3 froth on the
plate (m2/m3)
a; = Interfacial area per unit of packed column
(mlm)
C, = Molar concentration
of component
A
(kmol/m)
kj = Reaction-rate coefficient of reaction j
coefficient
for
mass-transfer
absorbed component A, (kmol/m2 h b)
k , *. = Liquid-side
mass-transfer
coefficient of
-_I absorbed component A, (m/h)
L = Volumetric liquid flow rate (m3/h)
L = Molar liquid kow rate (kmoljhj
L&= Molar flow rate of liquid feed to column
(kmol/h)
L; = Molar flow rate of liquid stream leaving plate
k (kmol/h)
mj,, = Reaction order with respect to component j
in reaction I
h4 = Molecular weight (kg/kmol)
m = Reaction order
Njl,-0 = Interfacial flux of component j per unit
gas-liquid interfacial area (kmol/m2 h)
n, = Number of absorbing components
n, = Number of reactions in the liquid phase
no = Total number of components in gas phase
nL = Total number of components in liquid phase
nM= Number of gas-phase components involved in
moderately fast reactions
np = Number of reaction products in the liquid
phase
na = Number of reactants in liquid phase
n, = Number of gas-phase components involved in
very slow reactions
nv = Number of gas-phase components involved in
very fast reactions
p = Partial pressure (b)
pt = Total pressure (b)
P, = Product j
r = Reaction rate (kmol/m h)
Rj = Liquid-phase reactant j
Q$ = Total heat of absorption of component j on
plate k (kJ/h)
QF = ;f;;;,heat
of cooling taken away from plate
k o, A, = Gas-side
QTk = T$$)heat
of reaction of reaction j on plate k
Tk = Temperature on plate k (K)
T,, = Temperature of intermediate gas feed to plate
k (K)
T,,
= Temperature
plate k (K)
of intermediate
liquid feed to
V,, = Flow rate of intermediate gas feed to plate k
(kmol/h)
V,,
= Flow rate of intermediate
from plate k (kmol/h)
gas withdrawal
X, = Mole fraction of component j in the liquid
bulk
.x~,~= Mole fraction of component j in the bulk of
the liquid stream leaving plate k
x~,,~ = Mole fraction of component j in the intermediate liquid feed to plate k
y = Coordinate perpendicular to the gas-liquid
interface (m)
Simulation of absorption and reaction in columns-II
yr, = Location of reaction front of reaction j (m)
yo = Gas-film thickness (m)
y, = Mole fraction of component j in the bulk of
the gas phase
yik = Mole fraction of component j in the bulk of
the gas stream leaving plate k
y, = Liquid-film thickness (m)
J+,,~ = Molar fraction of component j in the intermediate gas feed to plate k
z = Axial coordinate in the froth on the plate (m)
Greek symbols
eL = Liquid hold-up
of packing or fraction of
liquid in the froth
pL = Liquid density (kg/m3)
R = Cross-section of tower (m2)
R, = Active area of plate (m)
Subscripts
A, = With respect to absorbed component j
b = In the bulk of the gas or liquid phase
Cj = With respect to the consecutive component j
G=Gas
i = At gas-liquid interface
j = Co&one& index
k = Plate number
L = Liquid
Pj= With respect to product j
RI= With respect to reactant j
REFERENCES
L. De Leye and G. F. Froment, Comput. them. Engng
10, 493 (1986).
K. Onda, E. Sada, T. Kobayashi and M. Fujine, Chem.
Engng Sci. 25, 1023 (1970).
J. H. Wegstein, Communs Ass. comput. Mach. 1, 9
(1958).
A. W. Westerberg, H. P. Hutchison, R. L. Motard and
P. Winter, Process Flowsheeting, pp. 54-65. Cambridge
Univ. Press, Cambs. (1979).
5. C. Ouwerkerk, Hydrocarbon Process. 57(4), 89 (1978).
6. A. E. Cornelissen, Trans. Inst. them. Engrs 58, 242
(1980).
7. Handbook of Physical Constants, revised edn; Geol. Sot.
Am. Mem. 97, Sect. 18 (1966).
8. R. L. Kent and B. Eisenberg, Hydrocarbon Process.
55(2), 87 (1976).
9. W. J. Thomas and I. A. Furzer, Chem. Engng Sci. 17,
115 (1962).
10. Y. M. Tseng and A. R. Thompson, J. them. Engng Data
9(2), 264 (l-964).
11. J. A. Riddick and W. B. Bunger, Organic Solvents, 3rd
edn. Wiley Interscience, New York (1970).
12. J. Stichlmair, Grundlagen der Dimensionierung des
GaslFlussiakeit-Kontaktapparates.
13.
14.
15.
16,
17.
Superscripts
G = In gas phase
L=
in =
out =
eq =
abs =
R=
C=
In liquid phase
At inlet
At outlet
Equilibrium value
Absorption
Reaction
Cooling
515
18.
Bodenkolonne.
Verlag Chemie, Weinheim-(1978).
Glitsch Inc.. Ballast Tray Design Manual, Bull. NO.
4900. Dallas, Tex. (1978):
G. Astarita, Mass Transfer with Chemical Reaction.
Elsevier, Amsterdam (1967).
P. V. Danckwerts, Gas-Liquid Reactions. McGrawHill, New York (1970).
H. Hikita, S. Asai and T. Takatsuka, Chem. Engng J.
11, 131 (1976).
T. J. Edwards, J. Newman and J. M. Prausnitx, AIChE
JI 21(2), 248 (1977).
B. R. W. Pinsent, L. Pearson and F. J. W. Roughton,
Trans. Farad. Sot. 52, 1512 (1956).
19. R. H. Perrv and C. H. Chilton, Chemical Engineers
Handbook, Chap. 3, 5th edn. McGraw-Hill, New York
(1973).
20. R. C. Reid, J. M. Prausnitz and T. K. Sherwood, The
Properties of Gases and Liquids. McGraw-Hill, New
York (1977).
Você também pode gostar
- Character AnalysisDocumento1 páginaCharacter AnalysisDaniel GolovliovAinda não há avaliações
- MET Sample Test ADocumento28 páginasMET Sample Test AEdwin Arias Marin67% (3)
- Answer Key For ECCE Sample Test, Form B: Listening Grammar Vocabulary ReadingDocumento1 páginaAnswer Key For ECCE Sample Test, Form B: Listening Grammar Vocabulary ReadingCatherine CcasaAinda não há avaliações
- Fishbone Map PDFDocumento1 páginaFishbone Map PDFgianfranc2111Ainda não há avaliações
- Fishbone Map PDFDocumento1 páginaFishbone Map PDFgianfranc2111Ainda não há avaliações
- Gas Absorption With Chemical Reaction in Packed PDFDocumento5 páginasGas Absorption With Chemical Reaction in Packed PDFCatherine CcasaAinda não há avaliações
- Emergency Preparedness LessonsDocumento2 páginasEmergency Preparedness LessonsCatherine CcasaAinda não há avaliações
- 305 Manual TM-5 6Documento21 páginas305 Manual TM-5 6Catherine CcasaAinda não há avaliações
- Vilmain Et Al PDFDocumento31 páginasVilmain Et Al PDFCatherine CcasaAinda não há avaliações
- Thermodynamics ContentsDocumento690 páginasThermodynamics ContentsCatherine CcasaAinda não há avaliações
- Files Admin Articles CISOil-Gas-Sakhalin PDFDocumento3 páginasFiles Admin Articles CISOil-Gas-Sakhalin PDFCatherine CcasaAinda não há avaliações
- Elliott Centrifugal Compressor Specifications PDFDocumento2 páginasElliott Centrifugal Compressor Specifications PDFCatherine CcasaAinda não há avaliações
- Plastic Deformation of Glassy Polymers: Correlation Between Shear Activation Volume and Entanglement DensityDocumento7 páginasPlastic Deformation of Glassy Polymers: Correlation Between Shear Activation Volume and Entanglement DensityCatherine CcasaAinda não há avaliações
- 2005 Sep. Purif. Technol. TerMaatHDocumento15 páginas2005 Sep. Purif. Technol. TerMaatHHVu NguyenAinda não há avaliações
- What's Correct For My Application A Centrifugal or Reciprocating Compressor PDFDocumento10 páginasWhat's Correct For My Application A Centrifugal or Reciprocating Compressor PDFRapee PuaksungnoenAinda não há avaliações
- Design of Industrial Asorption Reactive PDFDocumento14 páginasDesign of Industrial Asorption Reactive PDFCatherine CcasaAinda não há avaliações
- 2005 Sep. Purif. Technol. TerMaatHDocumento15 páginas2005 Sep. Purif. Technol. TerMaatHHVu NguyenAinda não há avaliações
- Chung Thesis 2 PDFDocumento153 páginasChung Thesis 2 PDFCatherine CcasaAinda não há avaliações
- Plastic Deformation of Glassy Polymers: Correlation Between Shear Activation Volume and Entanglement DensityDocumento7 páginasPlastic Deformation of Glassy Polymers: Correlation Between Shear Activation Volume and Entanglement DensityCatherine CcasaAinda não há avaliações
- Onda Mass Transfer Coefficients Between PDFDocumento7 páginasOnda Mass Transfer Coefficients Between PDFCatherine CcasaAinda não há avaliações
- What's Correct For My Application A Centrifugal or Reciprocating Compressor PDFDocumento10 páginasWhat's Correct For My Application A Centrifugal or Reciprocating Compressor PDFRapee PuaksungnoenAinda não há avaliações
- Rigorous Simulation and Dessign Packing Column PDFDocumento12 páginasRigorous Simulation and Dessign Packing Column PDFCatherine CcasaAinda não há avaliações
- What's Correct For My Application A Centrifugal or Reciprocating Compressor PDFDocumento10 páginasWhat's Correct For My Application A Centrifugal or Reciprocating Compressor PDFRapee PuaksungnoenAinda não há avaliações
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (894)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (399)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (119)
- Northstar NSB 190ft HT RedDocumento2 páginasNorthstar NSB 190ft HT RedSahand Aras100% (1)
- Quantum-Mechanical Aspects of The L. Pauling's Resonance Theory.Documento4 páginasQuantum-Mechanical Aspects of The L. Pauling's Resonance Theory.Bezverkhniy VolodymyrAinda não há avaliações
- Sample Paper For ChemistryDocumento23 páginasSample Paper For ChemistryAmit joshiAinda não há avaliações
- ACRYREX® CM-211: Chi Mei CorporationDocumento3 páginasACRYREX® CM-211: Chi Mei CorporationPhuoc Thinh TruongAinda não há avaliações
- ROCKWOOL© Technical InsulationDocumento36 páginasROCKWOOL© Technical InsulationHaytham ElsayedAinda não há avaliações
- Sugar (Sucrose) (C12h22o11)Documento12 páginasSugar (Sucrose) (C12h22o11)Karl SiaganAinda não há avaliações
- Reservoir Drive Mechanisms PDFDocumento28 páginasReservoir Drive Mechanisms PDFWassef MB100% (1)
- 85 TPH CFBC Boiler Operation and Maintenance ManualDocumento152 páginas85 TPH CFBC Boiler Operation and Maintenance ManualAamirMalik100% (1)
- Adhesives: Standard Terminology ofDocumento12 páginasAdhesives: Standard Terminology ofJOHN MARTINAinda não há avaliações
- Safety Data Sheet: Masteremaco S 5400ciDocumento10 páginasSafety Data Sheet: Masteremaco S 5400ciSolomon AhimbisibweAinda não há avaliações
- Shell Momentum Balance 1Documento16 páginasShell Momentum Balance 1Kevwe Macaulay -GbogidiAinda não há avaliações
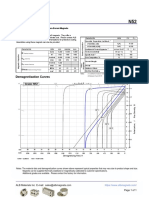
- N52 Grade Neodymium Magnets DataDocumento1 páginaN52 Grade Neodymium Magnets DataSteve HsuAinda não há avaliações
- Re CessnaSingle 1996on Structural Repair MM SESR04Documento167 páginasRe CessnaSingle 1996on Structural Repair MM SESR04chipocludo7av2100% (4)
- Marine PurifierDocumento30 páginasMarine PurifierJayDelosSantos100% (1)
- RP 08 - Dehydrogenase Activity in ChloroplastsDocumento4 páginasRP 08 - Dehydrogenase Activity in ChloroplastsAlfred SangAinda não há avaliações
- New Approaches To Halogen Free Polyolefin Flame ReDocumento8 páginasNew Approaches To Halogen Free Polyolefin Flame Reabilio_j_vieiraAinda não há avaliações
- Abaco Moody PDFDocumento1 páginaAbaco Moody PDFAxo Pijo CopónAinda não há avaliações
- LugalvanG35TechnicalInformation PDFDocumento4 páginasLugalvanG35TechnicalInformation PDFJakin RookAinda não há avaliações
- Vortex Quantum SeriesDocumento34 páginasVortex Quantum SeriesmiguelcAinda não há avaliações
- Selective Laser SinteringDocumento24 páginasSelective Laser SinteringRahul GandhiAinda não há avaliações
- Mazahar Publication PDFDocumento18 páginasMazahar Publication PDFNur Aini IktikhafsariAinda não há avaliações
- Anderol General BrochureDocumento12 páginasAnderol General BrochureepesanoAinda não há avaliações
- ATP WorksheetDocumento5 páginasATP WorksheetRyan De AlloAinda não há avaliações
- Patent UV Refurbish BrochureDocumento20 páginasPatent UV Refurbish BrochureAnonymous HNs2dr76jEAinda não há avaliações
- Energy Balance and Thermo PresentationDocumento83 páginasEnergy Balance and Thermo Presentationca2n27Ainda não há avaliações
- Hygroscopic and Nonhygroscopic MaterialDocumento9 páginasHygroscopic and Nonhygroscopic Materialiresa_nuratAinda não há avaliações
- Mark Scheme (Results) Summer 2015: GCE Chemistry (6CH01/01) The Core Principles of ChemistryDocumento21 páginasMark Scheme (Results) Summer 2015: GCE Chemistry (6CH01/01) The Core Principles of ChemistryAmeenIbrahimAinda não há avaliações
- Clay Mineral Cements in SandstonesDocumento3 páginasClay Mineral Cements in Sandstonesandrea.cipagautaAinda não há avaliações
- Onion Cell Structure Under MicroscopeDocumento2 páginasOnion Cell Structure Under MicroscopeAnirudh100% (1)
- Experimental Lab Report 1Documento10 páginasExperimental Lab Report 1api-274857931100% (10)