Escolar Documentos
Profissional Documentos
Cultura Documentos
United States: (12) Patent Application Publication (10) Pub. No.: US 2015/0018589 A1
Enviado por
Lily DianaTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
United States: (12) Patent Application Publication (10) Pub. No.: US 2015/0018589 A1
Enviado por
Lily DianaDireitos autorais:
Formatos disponíveis
US 20150018589A1
(19) United States
(12) Patent Application Publication (10) Pub. No.: US 2015/0018589 A1
Duff et al.
(54)
(43) Pub. Date:
MANUFACTURE OF BUTADIENE FROM
ETHYLENE
Publication Classi?cation
(51)
(71) Applicant: TPC Group LLC, Houston, TX (US)
(72) Inventors: Joseph G. Duff, League City, TX (U S);
Jan. 15, 2015
Int. Cl.
C07C 5/48
(2006.01)
C07C 2/06
(2006.01)
(52) us CL
Clifford A- Maat, Pearland, TX (US);
CPC
Michael 0- Nu, Pearland, TX (Us);
USPC ........................................................ .. 585/326
C07C 5/48 (2013.01); C07C 2/06 (2013.01)
Mark P. Kaminsky, Friendswood, TX
(US)
(57)
ABSTRACT
(21) App1_ NO; 14/324,428
A method of producing butadiene includes: (1) dimeriZing
ethylene to butene followed by (2) oxidatively dehydrogenat
(22) Filed:
ing the butene to butadiene and (3) recovering the butadiene
by (i) absorbing the product With a hydrocarbon absorber oil
Jul. 7, 2014
Related US Application Data
(60)
and (ii) stripping a crude product stream from the absorber
Provisional application NO' 61/844,463, ?led on JUL
011. The absorber 0111s selected so as to be effectlve to seques
10, 2013.
ter ethylene dimerization-derived impurities from the system.
US 2015/0018589 A1
MANUFACTURE OF BUTADIENE FROM
ETHYLENE
Jan. 15,2015
SUMMARY OF INVENTION
[0007] There is provided in accordance with the present
invention a method of producing butadiene by way of (1)
CLAIM FOR PRIORITY
dimerizing ethylene to butene followed by (2) oxidatively
[0001] This application is based upon US. Provisional
Application No. 61/844,463, ?led Jul. 10, 2013 ofthe same
dehydrogenating the butene to butadiene and (3) recovering
the butadiene by (i) absorbing the product with a hydrocarbon
title, (Attorney Docket No. TPC-13-4A), the priority of
which is hereby claimed and the disclosure of which is incor
porated herein by reference.
TECHNICAL FIELD
[0002]
The present invention relates to the manufacture of
butadiene by way of dimerizing ethylene to butene followed
by oxidative dehydrogenation of the butene to butadiene and
recovery of the butadiene produced with a hydrocarbon
absorber oil.
BACKGROUND
absorber oil and (ii) stripping a crude product stream from the
absorber oil, (4) wherein the hydrocarbon absorber oil is
effective to sequester ethylene dimerization-derived impuri
ties from the system, should they be present in the reactor
e?luent. In accordance with the invention, undesirable ethyl
ene dimerization-derived impurities which are not combusted
in the oxidative dehydrogenation zone are absorbed into the
absorber oil and may be removed from the absorber oil from
time to time as necessary or simply stripped with the C4s
during continuous operation of a butadiene production sys
tem and separated out in a conventional puri?cation train.
[0008] In another embodiment, there is provided a process
for co-producing butadiene and butene-1 from ethylene.
[0003] It is known in the art to dimerize ethylene to butene
and use the recovered butene for manufacturing butadiene.
BRIEF DESCRIPTION OF DRAWING
US. Pat. No. 3,728,415 to Arganbright discloses producing
[0009]
butenes by dimerizing ethylene with a catalyst including pal
erence to the drawing wherein FIG. 1 is a schematic diagram
The invention is described in detail below with ref
ladium oxide with molybdenum oxide or tungsten oxide and
illustrating operation of an oxidative dehydrogenation pro
using the product for dehydrogenation to make butadiene.
[0004] Other references of interest include the following:
raw material.
cess to make butene and butadiene based on ethylene as the
US. Pat. Nos. 3,911,042 and 3,969,429 to Belov et al. which
DETAILED DESCRIPTION
disclose titanium/aluminum catalyzed dimerization of ethyl
ene and note the product is useful for making butadiene; US.
Pat. No. 7,488,857 to Johann et al. which discloses coproduc
tion of butadiene and butene-1 from butane; and United States
Patent Application Publication No. US 201 1/ 0288308 to
Grasset et al. which discloses ethylene dimerization with
nection with the FIGURE for purposes of illustration, only.
The invention is de?ned in the appended claims. Terminology
used throughout the speci?cation and claims herein are given
titanium/aluminum catalyst.
[0005] It is proposed in Japanese Patent Publication 2011
[0011] Unless otherwise indicated, butadiene or BD
refers to 1,3 butadiene or mixtures comprising 1,3 butadiene.
[0010]
The invention is described in detail below in con
their ordinary meanings.
148720 to manufacture butadiene from ethylene by way of
[0012] Consisting essentially of and like terminology
dimerizing ethylene followed by oxidative dehydrogenation
using speci?ed catalysts to minimize impact of various impu
refers to the recited components and excludes other ingredi
ents which would substantially change the basic and novel
characteristics of the composition. Unless otherwise indi
cated or readily apparent, a composition consists essentially
of the recited components when the composition or article
includes 90% or more by weight of the recited components.
That is, the terminology excludes more than 10% of unrecited
rities. The method proposed includes the following steps (I)
and (II): a step (I) for producing n-butene essentially free of
isobutene by dimerizing ethylene at a reaction temperature of
150 to 400 C. in the presence of a catalyst consisting of
nickel, alumina, and silica having a nickel content of 0.0001
to 1 wt. %; and a step (II) for producing butadiene by per
forming an oxidative dehydrogenation reaction on the
n-butene obtained in said step (I) with oxygen at a reaction
temperature of 300 to 600 C. in the presence of a complex
metal oxide comprising molybdenum and bismuth as essen
components.
[0013] Ethylene dimerization-derived impurities means
and includes impurities such as ethylene trimers (C6), ethyl
ene tetramers (C8), diole?ns such as butadiene, isoprene, and
heavier diole?ns, as well as acetylenic by-products of ethyl
tial ingredients.
ene dimerization.
[0006] Impurities such as isobutene associated with con
ventional processing are problematical as noted in the 201 1
148720 Publication. Additional impurities such as trimer
[0014] Ethylene is dimerized into n-butenes suitable foruse
in connection with the present invention by a variety of cata
lytic processes. One suitable method is to utilize a homoge
neous catalyst system which includes a nickel compound
(C6), tetramer (C8), diole?n (butadiene, isoprene, and
heavier diole?n) and acetylenic by-products of ethylene
dimerization likewise present challenges to ef?cient opera
tion of a butadiene manufacturing process. See also United
States Patent Application Publication No. US 2014/0088332
of Rolland which relates to butadiene manufacture from eth
ylene using nickel and zirconium dimerization catalysts to
make butenes as well as United States Patent Application
Publication No. US 2014/0088331 also of Rolland which
relates to butadiene manufacture from ethylene using nickel
and titanium dimerization catalysts to make butenes.
such as nickel phosphine oxide and an alkyl aluminum co
catalyst such as ethyl aluminum dichloride. Such processes
produce predominantly 2-butenes. See, for example, U. S. Pat.
No. 5,162,595 to Wu, the disclosure of which is incorporated
by reference.
[0015] Alternatively, ethylene is dimerized into n-butenes
suitable for use in connection with the present invention
through the use of a homogeneous catalyst system which
includes an organometallic titanium catalyst. In general, such
processes include a titanium organometallic complex with at
US 2015/0018589 A1
Jan. 15,2015
least one alkoxide ligand and an alkyl aluminum co-catalyst
to produce predominantly 1-butene as is seen, for example, in
United States Patent Application Publication No. US 2011/
0288308 of Grasset et al., noted above, the disclosure of
which is incorporated herein by reference. One suitable cata
[0019] A typical process of the invention includes dimeriZ
ing ethylene to provide a butene rich hydrocarbonaceous
feed, superheating said hydrocarbonaceous butene rich feed
to a temperature of at least about 204 C. (400 F), mixing
said hydrocarbonaceous butene rich feed with superheated
lytic system includes titanium tetrabutoxide and triethyl alu
steam and an oxygen rich gas to form a reactor feed stream,
minum. Titanium-based dimerization processes may be rela
tively selective, such as the Alphabutol process and are
reported to reduce fractionation costs when 1-butene of rela
reacting said reactor feed stream over a ferritic oxide catalyst,
tively high purity is required:
thereby forming a butadiene enriched product stream. Suit
able ferritic oxidative dehydrogenation catalysts are also
described in Miklas, METHOD OF ACTIVATING ZINC-FERRITE OXI
DATIVE DEHYDROGENATION CATALYST; US. Pat. No. 3,953,370;
Apr. 27, 1976, which relates to use of steam at a temperature
of from 371-7040 C. (700-13000 F.) to activate a Zinc ferrite
oxidative dehydrogenation catalyst for preparation of butadi
LnM +
>
LnM
lButene
i,_,
2 02H,
M I Metal
L, I Ligand
[0016] Substantial puri?cation to reduce ethylene dimer
ization-derived impurities in the butenes produced by ethyl
ene dimerization to less than 100 ppm is ordinarily required
for most uses of the product butenes. Even at these levels, the
impurities can accumulate when present in a raw material, act
ene from C4-C8 hydrocarbons as well as Bajars et al; DEHY
DROGENATION WITH MAGNESIUM FERRTTE; US. Pat. No. 3,284,
536; US. Pat. No. 4,083,844 to Purdy entitled CALCIUM
OXIDE MODIFIED ZINC FERRITE OXIDATIVE DEHY
DROGENATION CATALYSTS AND USE as well as CATA
LYTIC OXIDATIVE DEHYDROGENATION PROCESS;
US. Pat. No. 4,658,074, the disclosures of which are incor
porated herein by reference.
[0020] The oxidative dehydrogenation catalyst bed is pre
heated to a temperature which is suf?cient to initiate the
oxidative dehydrogenation reaction The butadiene rich reac
tor ef?uent, Details as to feed compositions and operating
temperatures appear in Welch et al., Buladiene via oxidative
as catalyst poisons and be detrimental to system operation.
dehydrogenation, Hydrocarbon Processing, November 1978,
[0017] In accordance with the invention generally, ethylene
pp. 131-136, the disclosure of which is incorporated herein by
dimerization-derived impurities present in feed to the oxida
tive dehydrogenation reactor are either combusted in the reac
tor or fed forward to the butadiene reactor ef?uent stream
wherein they are assimilated into the absorber oil, by being
absorbed by the oil. The impurities are removed from the
system as necessary. Ethylene dimerization-derived impuri
reference.
[0021]
The butadiene enriched product stream exiting the
reactor is cooled through a quench column, in which heat is
removed from the butadiene enriched product stream and
steam content thereof condensed.
[0022] After passing through the quench column, the buta
ties of 100 ppm or more in the butenes are tolerated in the feed
diene enriched product stream directed to a scrubber, and
to the oxidative dehydrogenation unit in the inventive pro
ultimately, an absorber column by absorption into a compat
cess.
ible absorption oil, which is adapted to preferentially absorb
[0018] The ethylene dimerization-derived impurities pro
duced by way of ethylene dimerization and remaining in the
butadiene and other C4s as well as ethylene dimerization
reactor ef?uent are absorbed along with C4s in the product
stream using a suitable absorber oil, typically a hydrocarbon
tor ef?uent.
oil as described hereinafter. The butenes fed to an oxidative
referred to as lean oil) used in the absorption step can suitably
be para?inic, or a mixture of para?ins and aromatics,
dehydrogenation reactor may thus contain relatively signi?
cant levels of ethylene dimerization-derived impurities as
noted above. The impurities in the reactor ef?uent are
absorbed into a suitable absorber oil along with other C4s.
The ethylene dimerization-derived impurities may be
removed from the oil during routine processing of the oil with
derived impurities present in oxidative dehydrogenation reac
[0023]
Suitable fresh absorber oils (also sometimes
although particularly superior results are obtained using oils
which are richer in, or possibly even entirely, vinyl cyclohex
ene (butadiene dimer). Suitable absorber oils are tolerant to
and assimilate impurities. One preferred class of oils is a
paraf?nic oil having the composition shown in Table 1.
absorber off-gas, removed as stripper distillate, or removed as
lean oil re-run heavies. Alternatively, the impurities can be
TABLE 1
stripped from the oil with C4s and removed by later puri?ca
Absorber Oil Composition 1
tion by conventional means. Some impurities are compatible
with the absorber oil and need not be removed at all. In
extreme cases, butenes may even be fed directly from a dimer
ization unit with reduced or even no removal of ethylene
dimerization-derived impurities to an oxidative dehydroge
nation unit to form butadiene if so desired; however, care
should be taken to avoid very high levels of impurities since
some may burn under reaction conditions and reduce yields
signi?cantly if levels are too high. The oxidative dehydroge
nation production system uses a hydrocarbon absorber oil
which is tolerant to ethylene dimerization-derived impurities
and readily absorbs the impurities.
A Normalized Weight Percent
Cnr
3
4
5
6
7
8
9
10
Naph.
IPar.
nPar.
0.34
0.53
.08
0.14
.96
4.47
0.94
.071
4.67
9.80
0.22
1.37
5.70
Cycl Ol.
0.05
Arom.
Total
0.03
.026
1.81
0.08
1.07
7.02
20.28
US 2015/0018589 A1
Jan. 15,2015
TABLE 1-continued
TABLE 1-continued
Absorber Oil Composition 1
11
12+
8.76
Poly
8.94
Total
33.21
21.37
2336
7.12
Absorber Oil Composition 1
0.20
37.45
23.36
C Summary ofComponents
8.94
50.91
15.35
0.24
0.29
100.00
B Normalized Volume Percent
Benzene
TOt?-l Aromatics
Total Ole?ns
0.00 LV %
0.26 LV %
0.23 LV %
Total Saturates
99.51 LV %
Total Oxygen
Cnr
Naph.
IPar.
nPar.
Cycl Ol.
Arom.
0.00 Wt %
Total
Where:
C-nr = Carbon Number
Naph. = Naphthene
6
7
0.35
0.63
0.09
1.10
8
9
10
11
0.71
4.61
9.38
8.23
0.15
1.02
0.24
1.48
4.65
21.73
6.06
7.47
12+
2.08
0.09
0.04
0.19
1.11
7.14
20.37
37.62
0.03
0.23
23.75
POly
7-85
Total
31M
5102
23.75
1635
023
026
I-Par- = ISO-Paraf?n
n-Par. = n-Paraf?n
Cycl Ol. = Cycle-ole?n
Amm. =Ammatic
[0024]
Good results are also obtalned When the fresh
7-85
absorber oil is primarily Espersol 250, an aromatic naphtha
10000
product With a boiling range of 90 C. to 150 C. (200 F. to
300 F.) having the composition shown in Table 2 (Celsius
Boiling Points provided in Table 2A).
TABLE 2
Absorber Oil Composition 2
Component
Benzene
Cyclohexane
Methyl Cyclohexane
Toluene
2,2,4Trimethyl Pentane
Vinyl Cyclohexane
Ethyl Cyclohexane
M&PXylene
OXylene
Styrene
Propyl Benzene
Butyl Benzene
Heavies (Assume 2M
Molecular
N.B.
Speci?c
Chroma.
Assumed
Mole
Vol.
Weight
Point ( F.)
Gravity
Wt %
78.11
84.16
176.2
178
213.7
231
236.1
262.1
269.2
281
291
294
318.6
361.4
466
0.8845
0.783
0.774
0.872
0.696
0.8335
0.788
0.867
0.885
0.911
0.862
0.864
1.029
6
3
1
12
1
3
1
19
17
10
1
4
22
5
2
1
13
2
5
1
20
18
12
2
6
13
6.8
2.5
1.1
15
1.9
4.9
0.9
20.1
18.1
12.3
1.8
4.8
9.7
5
2.3
1.1
13.2
2.6
5.3
1.1
20.4
18
11.6
2.1
6.1
11.2
98.18
92.13
114.23
108.18
112.22
106.16
106.16
104.14
120.19
134.21
142.2
Naphthalene)
TABLE 2A
Absorber Oil Composition 2 (Celsius Boiling Points)
N.B.
Component
Molecular
Weight
Point
( C.)
Speci?c
Gravity
Benzene
Cyclohexane
78.11
84.16
80.11
81.1
0.8845
0.783
Methyl Cyclohexane
Chroma. Assumed
%
Wt %
6
3
5
2
98.18
100.9
0.774
Toluene
2,2,4Trimethyl Pentane
92.13
114.23
111
113.4
0.872
0.696
12
1
13
2
Vinyl Cyclohexane
Ethyl Cyclohexane
M&PXylene
OXylene
Styrene
Propyl Benzene
Butyl Benzene
Heavies (Assume 2M
108.18
112.22
106.16
106.16
104.14
120.19
134.21
142.2
127.8
131.8
138
144
146
159.2
183
241
0.8335
0.788
0.867
0.885
0.911
0 862
0 864
1.029
3
1
19
17
10
1
4
22
5
1
20
18
12
2
6
13
Naphthalene)
Mole % Vol. %
6.8
2.5
1.1
15
1.9
4.9
0.9
20.1
18.1
12.3
1 8
4.8
9.7
5
2.3
1.1
13.2
26
53
1.1
20.4
18
11.6
2 1
6.1
11.2
US 2015/0018589 A1
Jan. 15,2015
[0025] After passing through the absorber column, the
may be combined or interchanged either in whole or in part.
absorber oil having butadiene and other C4s as well as ethyl
ene dimerization-derived impurities dissolved therein is
directed to a degasser tower where carbon dioxide, residual
that the foregoing description is by way of example only, and
nitrogen and hydrogen are removed, the absorber oil being
passed thence to a stripper wherein butadiene product and
other C4s dissolved in the absorber oil is stripped out and
forwarded to further puri?cation.
[0026] One preferred embodiment of the present invention
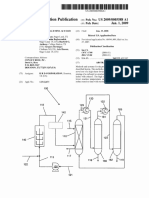
is a co-production system 5 shown schematically in FIG. 1.
Furthermore, those of ordinary skill in the art will appreciate
is not intended to limit the invention.
1. A method of producing butadiene from an ethylene raw
material feed comprising:
providing ethylene to a homogeneous reaction medium
housed in a dimerization reactor;
dimeriZing ethylene to butene in the homogeneous reaction
medium to provide a hydrocarbonaceous butene rich
Ethylene is provided to a liquid ?lled reactor 10 containing a
feed with ethylene dimerization-derived impurities
titanium/aluminum homogeneous catalyst via line 12
wherein butene-1 is produced from the ethylene. The
therein;
mixing said hydrocarbonaceous butene rich feed with
butene-1 is provided to a butene-rich product stream 14 as
well as a butene-1 rich feed stream 16 with ethylene dimer
drogenation reactor feed stream and superheating said
ization-derived impurities.
[0027]
Stream 14 is puri?ed and butene-1 (>99%) is recov
ered therefrom, while stream 16 is mixed with steam 18 and
an oxygen rich gas 20 and provided to an oxidative dehydro
genation unit as part of a reaction/quench/compression sec
steam and an oxygen rich gas to form an oxidative dehy
oxidative dehydrogenation reactor feed stream to a tem
perature of at least 204 C. (400 F.),
feeding the oxidative dehydrogenation reactor feed stream
to an oxidative dehydrogenation reactor;
oxidatively dehydrogenating said reactor feed stream in the
tion 22 after superheating in a superheater 24. Output 26 is
enriched in butadiene and contains butene-1.
[0028] Stream 26 is fed to absorber, degasser and stripper
dehydrogenation catalyst thereby forming a butadiene
enriched product stream;
units indicated at 28 and a crude butadiene stream 30 is
feeding the butadiene enriched product stream to a C4
absorber wherein C4 s including butadiene and ethylene
recovered. Stream 30 is a majority by weight butadiene and is
further puri?ed by conventional means in order to provide
butadiene of greater than 99% purity, while other C4s are
recycled or otherwise recovered.
[0029] In the various embodiments of the invention, the
oxidative dehydrogenation catalyst may be a ferritic catalyst,
such as a ferritic oxide catalyst consisting essentially of:
oxygen, a major proportion of iron; a minor proportion of
Zinc; and smaller amounts of manganese; phosphorus, and the
residue of a nitrate free calcium precursor. The process may
be operated wherein the molar ratio of oxygen to butene in the
oxidative dehydrogenation reactor feed stream is from 0.4:1
oxidative dehydrogenation a reactor over an oxidative
dimerization-derived impurities which may be present
in oxidative dehydrogenation reactor ef?uent are
absorbed into a compatible absorption oil;
providing the absorption oil to a stripper in which C4
volatiles including butadiene are desorbed and stripped
from said absorption oil under reduced pressure to pro
vide a crude product stream; and
recovering butadiene from said crude product stream.
2. The method according to claim 1, wherein the hydrocar
bonaceous feed stream has greater than 100 ppm of ethylene
dimerization-derived impurities.
the oxidative dehydrogenation reactor feed stream feed
3. The method according to claim 1, wherein the hydrocar
bonaceous feed stream has greater than 200 ppm of ethylene
stream is from 0.5:1 to 16:1.
dimerization-derived impurities.
[0030]
4. The method according to claim 1, wherein the hydrocar
bonaceous feed stream has greater than 300 ppm of ethylene
to 08:1 and/ or wherein the molar ratio of steam to butene in
The homogeneous reaction medium in the dimer
ization reactor may comprise a homogeneous catalyst com
dimerization-derived impurities.
prising a nickel compound and an alkyl aluminum co-catalyst
or the homogeneous catalyst may comprise a nickel phos
phine oxide and ethyl aluminum dichloride in which case the
butenes in the oxidative dehydrogenation reaction feed
5. The method according to claim 1, wherein the hydrocar
bonaceous feed stream has greater than 100 ppm of ethylene
stream are predominantly 2-butenes.
dimerization-derived impurities.
[0031]
The homogeneous reaction medium in the dimer
ization reactor may comprise a homogeneous titanium/alu
minum catalyst such as a homogeneous titanium organome
tallic complex with at least one alkoxide ligand and an alkyl
aluminum co-catalyst such as titanium tetrabutoxide and tri
ethyl aluminum, in which cases the butene in the oxidative
dehydrogenation reactor feed stream is predominantly
1 -butene.
[0032] While the invention has been described in detail,
modi?cations within the spirit and scope of the invention will
be readily apparent to those of skill in the art.
dimerization-derived impurities and less than 1% ethylene
6. The method according to claim 1, wherein the absorber
oil comprises one or more compounds selected from the
group consisting of toluene, xylenes, styrene and naphtha
lenes.
7. The method according to claim 1, wherein the absorber
oil contains over 90% para?inic compounds.
8. The method according to claim 1, wherein the absorber
oil consists essentially of compounds with atmospheric pres
sure boiling points of from 75 C. to 250 C.
9. The method according to claim 1, wherein the absorber
oil consists essentially of an aromatic naphtha product with
tions, discussed above in connection with the Background
an atmospheric pressure boiling range of from 90 C. to 150
C.
10. The method according to claim 1, wherein the absorber
and Detailed Description, the disclosures of which are all
oil consists essentially of vinyl cyclohexene.
[0033]
In view of the foregoing discussion, relevant knowl
edge in the art and references, including co-pending applica
incorporated herein by reference, further description is
deemed unnecessary. In addition, it should be understood that
aspects of the invention and portions of various embodiments
11. The method according to claim 1, wherein said butadi
ene enriched product stream is treated with an AAR catalyst
bed.
US 2015/0018589 A1
12. The method according to claim 1, wherein said ethyl
ene dimerization-derived impurities include one or more
impurities selected from ethylene trimers (C6); ethylene tet
ramers (C8); diole?ns such as butadiene, isoprene, and
heavier diole?ns; as well as acetylenic by-products of ethyl
ene dimerization.
Jan. 15,2015
feeding the oxidative dehydrogenation reactor feed stream
to an oxidative dehydrogenation reactor;
oxidatively dehydrogenating said reactor feed stream in the
oxidative dehydrogenation reactor over an oxidative
dehydrogenation catalyst thereby forming a butadiene
enriched product stream;
13. A method of co-producing butene-l and butadiene
from an ethylene raW material feed comprising:
providing ethylene to a homogeneous reaction medium
feeding the butadiene enriched product stream to a C4
absorber Wherein C4 s including butadiene and ethylene
including a homogeneous titanium/aluminum catalyst
absorbed into a compatible absorption oil;
providing the absorption oil to a stripper in Which C4
volatiles including butadiene are desorbed and stripped
from said absorption oil under reduced pressure to pro
vide a crude product stream; and
recovering butadiene from said crude product stream.
14. The method according to claim 13, Wherein the homo
geneous titanium/aluminum catalyst comprises a titanium
organometallic complex With at least one alkoxide ligand and
an alkyl aluminum co-catalyst.
15. The method according to claim 13, Wherein the homo
geneous titanium/aluminum catalyst comprises titanium tet
rabutoxide and triethyl aluminum.
housed in a dimerization reactor;
dimeriZing ethylene predominantly to butene-l in the
homogeneous reaction medium to provide (i) a hydro
carbonaceous butene-l rich feed With ethylene dimer
ization-derived impurities therein and (ii) a butene-l
rich product stream;
WithdraWing and purifying the butene-l rich product
stream and recovering butene-l therefrom;
mixing said hydrocarbonaceous butene-l rich feed With
steam and an oxygen rich gas to form an oxidative dehy
drogenation reactor feed stream and superheating said
oxidative dehydrogenation reactor feed stream to a tem
perature of at least 2040 C. (400 F.),
dimerization-derived impurities Which may be present
in oxidative dehydrogenation reactor ef?uent, are
Você também pode gostar
- Us 20120035390Documento18 páginasUs 20120035390sariAinda não há avaliações
- Combustion of Pulverised Coal in a Mixture of Oxygen and Recycled Flue GasNo EverandCombustion of Pulverised Coal in a Mixture of Oxygen and Recycled Flue GasAinda não há avaliações
- Productoion of PetDocumento14 páginasProductoion of PetAryan KumarAinda não há avaliações
- Advances in Biofeedstocks and Biofuels, Volume 2: Production Technologies for BiofuelsNo EverandAdvances in Biofeedstocks and Biofuels, Volume 2: Production Technologies for BiofuelsLalit Kumar SinghAinda não há avaliações
- European Patent Application: Process For Producing Dialkyl Carbonate and DiolDocumento21 páginasEuropean Patent Application: Process For Producing Dialkyl Carbonate and DiolRitam GhoshAinda não há avaliações
- Synthetic Natural Gas: From Coal, Dry Biomass, and Power-to-Gas ApplicationsNo EverandSynthetic Natural Gas: From Coal, Dry Biomass, and Power-to-Gas ApplicationsTilman J. SchildhauerAinda não há avaliações
- United States: (12) Patent Application Publication (10) Pub. No.: US 2014/0250770 A1Documento10 páginasUnited States: (12) Patent Application Publication (10) Pub. No.: US 2014/0250770 A1Keysler PonceAinda não há avaliações
- Process Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersNo EverandProcess Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersAinda não há avaliações
- Ethy Acetate Production US20140012037A1Documento41 páginasEthy Acetate Production US20140012037A1sariAinda não há avaliações
- Hydrogen Production TechnologiesNo EverandHydrogen Production TechnologiesMehmet SankirAinda não há avaliações
- PET PatentDocumento17 páginasPET PatentRiza Shinta RAinda não há avaliações
- Operator's Guide to General Purpose Steam Turbines: An Overview of Operating Principles, Construction, Best Practices, and TroubleshootingNo EverandOperator's Guide to General Purpose Steam Turbines: An Overview of Operating Principles, Construction, Best Practices, and TroubleshootingNota: 5 de 5 estrelas5/5 (1)
- EP2003159B1 Process For Production of Polyethylene TerephthalateDocumento19 páginasEP2003159B1 Process For Production of Polyethylene Terephthalatenurul_marifahAinda não há avaliações
- Pendukung 1LIU ET ALL 2018 US20180201568A1Documento8 páginasPendukung 1LIU ET ALL 2018 US20180201568A1nysa arientikaAinda não há avaliações
- Sustainable Energy Conversion for Electricity and Coproducts: Principles, Technologies, and EquipmentNo EverandSustainable Energy Conversion for Electricity and Coproducts: Principles, Technologies, and EquipmentAinda não há avaliações
- United States Patent: Reimann Patent No.: Date of PatentDocumento5 páginasUnited States Patent: Reimann Patent No.: Date of PatentFernando Beer FrenkelAinda não há avaliações
- GC/LC, Instruments, Derivatives in Identifying Pollutants and UnknownsNo EverandGC/LC, Instruments, Derivatives in Identifying Pollutants and UnknownsAinda não há avaliações
- Patent Asam Asetat 2015Documento22 páginasPatent Asam Asetat 2015Nyimas Ulfatry100% (1)
- Advances in Biofeedstocks and Biofuels, Volume 1: Biofeedstocks and Their ProcessingNo EverandAdvances in Biofeedstocks and Biofuels, Volume 1: Biofeedstocks and Their ProcessingLalit Kumar SinghAinda não há avaliações
- Process For Ethyl Acetate ProductionDocumento9 páginasProcess For Ethyl Acetate ProductionMuhammad Yanuar AnantaAinda não há avaliações
- U.S. Patent 4,293,314: Gelled Fuel-Air Explosive October 6, 1981.No EverandU.S. Patent 4,293,314: Gelled Fuel-Air Explosive October 6, 1981.Ainda não há avaliações
- European Patent SpecificationDocumento13 páginasEuropean Patent SpecificationWSERAinda não há avaliações
- Practical Synthetic Organic Chemistry: Reactions, Principles, and TechniquesNo EverandPractical Synthetic Organic Chemistry: Reactions, Principles, and TechniquesAinda não há avaliações
- United States Patent (19) 11 Patent Number: 6,093,845: Van Acker Et Al. (45) Date of Patent: Jul. 25, 2000Documento8 páginasUnited States Patent (19) 11 Patent Number: 6,093,845: Van Acker Et Al. (45) Date of Patent: Jul. 25, 2000falya aronaAinda não há avaliações
- Process Design Strategies for Biomass Conversion SystemsNo EverandProcess Design Strategies for Biomass Conversion SystemsAinda não há avaliações
- TEPZZ - 44765B - T: European Patent SpecificationDocumento16 páginasTEPZZ - 44765B - T: European Patent Specificationzweisteine777Ainda não há avaliações
- European Patent Specification: Printed by Jouve, 75001 PARIS (FR)Documento23 páginasEuropean Patent Specification: Printed by Jouve, 75001 PARIS (FR)Minh HoàngAinda não há avaliações
- TOLUENEDocumento7 páginasTOLUENEAlzahra AliAinda não há avaliações
- EP0659722B1Documento7 páginasEP0659722B1putraAinda não há avaliações
- US3398124Documento3 páginasUS3398124Siti RahmaliaAinda não há avaliações
- EC - EP2374803A1 - Method For Purifying Ethylene Carbonate, Process For Producing Purified Ethylene Carbonate, and Ethylene CarbonateDocumento19 páginasEC - EP2374803A1 - Method For Purifying Ethylene Carbonate, Process For Producing Purified Ethylene Carbonate, and Ethylene CarbonateListya Eka AnggrainiAinda não há avaliações
- EP2518094B1Documento11 páginasEP2518094B1Như HồAinda não há avaliações
- Us 2005187374Documento8 páginasUs 2005187374Huub van AertAinda não há avaliações
- Pet 4Documento36 páginasPet 4Martin GeorgeAinda não há avaliações
- United States: (12) Patent Application Publication (10) Pub. No.: US 2010/0197959 A1Documento19 páginasUnited States: (12) Patent Application Publication (10) Pub. No.: US 2010/0197959 A1sariAinda não há avaliações
- Us 7601311Documento10 páginasUs 7601311Văn Đại - BKHNAinda não há avaliações
- United States Patent (19) : Schmidt Et AlDocumento6 páginasUnited States Patent (19) : Schmidt Et AlArash AbbasiAinda não há avaliações
- United States: (12) Patent Application Publication (10) Pub. No.: US 2009/0163744 A1Documento8 páginasUnited States: (12) Patent Application Publication (10) Pub. No.: US 2009/0163744 A1Zaky YanwarAinda não há avaliações
- EP0873291B1Documento12 páginasEP0873291B1duy.ho188Ainda não há avaliações
- FP T 2016 MethaneDocumento26 páginasFP T 2016 MethaneAgung PrabowoAinda não há avaliações
- United States Patent: (10) Patent No.: US 7,910,784 B2Documento5 páginasUnited States Patent: (10) Patent No.: US 7,910,784 B2Chandra AdityaAinda não há avaliações
- US20100249366A1Documento5 páginasUS20100249366A1DenisAinda não há avaliações
- TEPZZ 4 8 - ZZA - T: European Patent ApplicationDocumento23 páginasTEPZZ 4 8 - ZZA - T: European Patent ApplicationMaru LinAinda não há avaliações
- Acido Propionico ImpurezasDocumento14 páginasAcido Propionico Impurezasromina orellanaAinda não há avaliações
- Production of Vinyl Chloride From Ethylene Dichloride by Pyrolysis Aspen Model DocumentationDocumento14 páginasProduction of Vinyl Chloride From Ethylene Dichloride by Pyrolysis Aspen Model DocumentationParaiba da Paraiba100% (2)
- United States: (12) Patent Application Publication (10) Pub. No.: US 2009/0005588 A1Documento10 páginasUnited States: (12) Patent Application Publication (10) Pub. No.: US 2009/0005588 A1sariAinda não há avaliações
- US3903185Documento6 páginasUS3903185Muhammad Akbar FahleviAinda não há avaliações
- Purezas PTADocumento7 páginasPurezas PTAAdrian vilariño gonzalezAinda não há avaliações
- Hydraulic System Cooling Water Treatment TCEP Solution 500mmolar Process AnalyzersDocumento11 páginasHydraulic System Cooling Water Treatment TCEP Solution 500mmolar Process Analyzersbenjy8769Ainda não há avaliações
- US5364986Documento4 páginasUS5364986Alam IlhamAinda não há avaliações
- European Patent Application: Hydroxyl Polyester Resins With High TGDocumento8 páginasEuropean Patent Application: Hydroxyl Polyester Resins With High TGAlexander Franco CastrillonAinda não há avaliações
- Turton AppBDocumento114 páginasTurton AppBamms9988Ainda não há avaliações
- EP2952536A2Documento45 páginasEP2952536A2Yahya AlmundzirAinda não há avaliações
- Us6147263 PDFDocumento6 páginasUs6147263 PDFأصلان أصلانAinda não há avaliações
- United States Patent: GraceyDocumento5 páginasUnited States Patent: GraceyahmadsaysAinda não há avaliações
- 1,3 Butadiene ProductionDocumento26 páginas1,3 Butadiene Productionasharab70100% (2)
- Soal TOEFL - Reading ComprehensionDocumento3 páginasSoal TOEFL - Reading ComprehensionElizabeth PoedjionoAinda não há avaliações
- Fulbright Master Application Form 1Documento10 páginasFulbright Master Application Form 1Print WarnetAinda não há avaliações
- Lampiran 1perhitungan Tugas KhususDocumento3 páginasLampiran 1perhitungan Tugas KhususLily DianaAinda não há avaliações
- 1Documento12 páginas1Lily DianaAinda não há avaliações
- LAMPIRAN 5 Flowsheet Unit SynloopDocumento1 páginaLAMPIRAN 5 Flowsheet Unit SynloopLily DianaAinda não há avaliações
- Danone at A GlanceDocumento21 páginasDanone at A GlanceGudang ErsopAinda não há avaliações
- Lampiran 4 Quenching Design 105 DDocumento1 páginaLampiran 4 Quenching Design 105 DLily DianaAinda não há avaliações
- Furfural PatentDocumento5 páginasFurfural PatentLily DianaAinda não há avaliações
- Paten Sulfur Mixer PDFDocumento7 páginasPaten Sulfur Mixer PDFLily DianaAinda não há avaliações
- Membrane Filtration HandbookDocumento129 páginasMembrane Filtration Handbookmario_nqn89% (9)
- Pertemuan 02 Rancangan Pabrik (14 Oktober 2015)Documento15 páginasPertemuan 02 Rancangan Pabrik (14 Oktober 2015)Lily DianaAinda não há avaliações
- Pertemuan 05 Rancangan Pabrik (04 November 2015)Documento15 páginasPertemuan 05 Rancangan Pabrik (04 November 2015)Lily DianaAinda não há avaliações
- LAMPIRAN 5 Flowsheet Unit SynloopDocumento1 páginaLAMPIRAN 5 Flowsheet Unit SynloopLily DianaAinda não há avaliações
- Msds Molten SulphurDocumento9 páginasMsds Molten SulphurLily DianaAinda não há avaliações
- Moist Air PropertiesDocumento12 páginasMoist Air PropertiesLily DianaAinda não há avaliações
- Us5108731 PDFDocumento12 páginasUs5108731 PDFLily DianaAinda não há avaliações
- Paten Kak DeviDocumento33 páginasPaten Kak DeviLily DianaAinda não há avaliações
- Us 20140309468Documento4 páginasUs 20140309468Lily DianaAinda não há avaliações
- PaperDocumento10 páginasPaperLily DianaAinda não há avaliações
- Proses Wsa 2 PDFDocumento2 páginasProses Wsa 2 PDFLily DianaAinda não há avaliações
- 10195en Rev6 PDFDocumento8 páginas10195en Rev6 PDFLily DianaAinda não há avaliações
- Pertemuan 05 Rancangan Pabrik (04 November 2015)Documento15 páginasPertemuan 05 Rancangan Pabrik (04 November 2015)Lily DianaAinda não há avaliações
- Konversi Sulfur Di Proses Pembakaran 1 PDFDocumento6 páginasKonversi Sulfur Di Proses Pembakaran 1 PDFLily DianaAinda não há avaliações
- Wsa Condenser PDFDocumento21 páginasWsa Condenser PDFLily DianaAinda não há avaliações
- c01Documento21 páginasc01Mustafa BakırAinda não há avaliações
- PDF Abstrak Id Abstrak-20376774Documento1 páginaPDF Abstrak Id Abstrak-20376774Lily DianaAinda não há avaliações
- Us 20150147266 A 1Documento18 páginasUs 20150147266 A 1Lily DianaAinda não há avaliações
- Us 8138371Documento10 páginasUs 8138371Lily DianaAinda não há avaliações
- Topsoe Wet Gas Sulphuric Acid (WSA) TechnologyDocumento8 páginasTopsoe Wet Gas Sulphuric Acid (WSA) Technologytsaleh100% (1)
- Topsoe Wet Gas Sulphuric Acid (WSA) TechnologyDocumento8 páginasTopsoe Wet Gas Sulphuric Acid (WSA) Technologytsaleh100% (1)
- Asme B18.2.1 PDFDocumento37 páginasAsme B18.2.1 PDFJUAN C100% (1)
- Periodic Table Color CodedDocumento1 páginaPeriodic Table Color CodedETHAN HENG ZENG AN MoeAinda não há avaliações
- C6.1.6 - Extracting CopperDocumento5 páginasC6.1.6 - Extracting Copperwcv2rs255jAinda não há avaliações
- Converting Dive Tanks For Oxygen ServiceDocumento12 páginasConverting Dive Tanks For Oxygen ServiceRichmond DiveclubAinda não há avaliações
- 1001 Tut Set T119Documento44 páginas1001 Tut Set T119Chirisuu PantsuAinda não há avaliações
- Lithium: Choosing The Right Analysis MethodDocumento2 páginasLithium: Choosing The Right Analysis MethodDavid Bolívar FigueroaAinda não há avaliações
- Technical IndexDocumento4 páginasTechnical IndexNgân LêAinda não há avaliações
- ASME IIC SFA5.16 - TiDocumento10 páginasASME IIC SFA5.16 - TitragaldabasAinda não há avaliações
- Inorganic Nomenclature Worksheet 345 FormulasDocumento7 páginasInorganic Nomenclature Worksheet 345 FormulasKonstantinos KosmidisAinda não há avaliações
- CHM256 - Tutorial 6Documento2 páginasCHM256 - Tutorial 6Fatimah Azzahrah0% (1)
- Acid Base For StudentsDocumento15 páginasAcid Base For StudentsPlan studyAinda não há avaliações
- Iron and Steel IndustryDocumento23 páginasIron and Steel IndustryArpan MAinda não há avaliações
- Chem Assignment OneDocumento13 páginasChem Assignment Oneapi-453111357Ainda não há avaliações
- Appendix III: Equivalent Weight of Substances Required in Volumetric Analysis - Engineering360Documento3 páginasAppendix III: Equivalent Weight of Substances Required in Volumetric Analysis - Engineering360Waleed EmaraAinda não há avaliações
- Diccionario de Términos Geológicos Minero Español InglésDocumento163 páginasDiccionario de Términos Geológicos Minero Español InglésAlejandra Neira GonzálezAinda não há avaliações
- Class Test 1 Rate of Reaction For Edexcel A2 ChemistryDocumento7 páginasClass Test 1 Rate of Reaction For Edexcel A2 Chemistryjeffydaniel1972Ainda não há avaliações
- Chapter - 3 Development of Agglomerated FluxesDocumento36 páginasChapter - 3 Development of Agglomerated Fluxesvikram singhAinda não há avaliações
- Extraction of Copper From Bacterial Leach Liquor of A Low Grade Chalcopyrite Test Heap Using LIX 984N-CDocumento4 páginasExtraction of Copper From Bacterial Leach Liquor of A Low Grade Chalcopyrite Test Heap Using LIX 984N-Cpmanquera89Ainda não há avaliações
- AmgDocumento9 páginasAmgSatishReddyAinda não há avaliações
- 2002 RD 1 Questions tcm18-190750Documento10 páginas2002 RD 1 Questions tcm18-190750LouiseflemingAinda não há avaliações
- Laboratory Name: Accreditation Standard Certificate Number Page No Validity Last Amended OnDocumento35 páginasLaboratory Name: Accreditation Standard Certificate Number Page No Validity Last Amended OnDebabrata MajhiAinda não há avaliações
- High Fire Glazes ExcerptDocumento24 páginasHigh Fire Glazes ExcerptFractalzAinda não há avaliações
- Miwah NI43-101 ReportDocumento131 páginasMiwah NI43-101 ReportAgussanyAinda não há avaliações
- Test Grease ConductorDocumento22 páginasTest Grease ConductorGirinda BayuAinda não há avaliações
- Astm CatalogDocumento13 páginasAstm CatalogjswldeepAinda não há avaliações
- GTAW and Power SourcesDocumento18 páginasGTAW and Power SourcesRavi Kumar SinghAinda não há avaliações
- MSTC Corporate - PresentationDocumento45 páginasMSTC Corporate - PresentationsherwinmitraAinda não há avaliações
- Chemistry Project For Class 12Documento13 páginasChemistry Project For Class 12gauravkhanna1996Ainda não há avaliações
- Solution StoichDocumento4 páginasSolution Stoichapi-296307501Ainda não há avaliações
- Synthesis of Phenyl-2-PropanoneDocumento14 páginasSynthesis of Phenyl-2-Propanonescrewyoureg89% (18)
- Sodium Bicarbonate: Nature's Unique First Aid RemedyNo EverandSodium Bicarbonate: Nature's Unique First Aid RemedyNota: 5 de 5 estrelas5/5 (21)
- Process Plant Equipment: Operation, Control, and ReliabilityNo EverandProcess Plant Equipment: Operation, Control, and ReliabilityNota: 5 de 5 estrelas5/5 (1)
- A New Approach to HAZOP of Complex Chemical ProcessesNo EverandA New Approach to HAZOP of Complex Chemical ProcessesAinda não há avaliações
- Guidelines for Chemical Process Quantitative Risk AnalysisNo EverandGuidelines for Chemical Process Quantitative Risk AnalysisNota: 5 de 5 estrelas5/5 (1)
- Functional Safety from Scratch: A Practical Guide to Process Industry ApplicationsNo EverandFunctional Safety from Scratch: A Practical Guide to Process Industry ApplicationsAinda não há avaliações
- Physical and Chemical Equilibrium for Chemical EngineersNo EverandPhysical and Chemical Equilibrium for Chemical EngineersNota: 5 de 5 estrelas5/5 (1)
- Lees' Process Safety Essentials: Hazard Identification, Assessment and ControlNo EverandLees' Process Safety Essentials: Hazard Identification, Assessment and ControlNota: 4 de 5 estrelas4/5 (4)
- Principles and Case Studies of Simultaneous DesignNo EverandPrinciples and Case Studies of Simultaneous DesignAinda não há avaliações
- Well Control for Completions and InterventionsNo EverandWell Control for Completions and InterventionsNota: 4 de 5 estrelas4/5 (10)
- The HAZOP Leader's Handbook: How to Plan and Conduct Successful HAZOP StudiesNo EverandThe HAZOP Leader's Handbook: How to Plan and Conduct Successful HAZOP StudiesAinda não há avaliações
- Coupled CFD-DEM Modeling: Formulation, Implementation and Application to Multiphase FlowsNo EverandCoupled CFD-DEM Modeling: Formulation, Implementation and Application to Multiphase FlowsAinda não há avaliações
- High Pressure Phase Behaviour of Multicomponent Fluid MixturesNo EverandHigh Pressure Phase Behaviour of Multicomponent Fluid MixturesAinda não há avaliações
- Distillation Design and Control Using Aspen SimulationNo EverandDistillation Design and Control Using Aspen SimulationNota: 5 de 5 estrelas5/5 (2)
- First U.K. National Conference on Heat Transfer: The Institution of Chemical Engineers Symposium Series, Volume 2.86No EverandFirst U.K. National Conference on Heat Transfer: The Institution of Chemical Engineers Symposium Series, Volume 2.86Ainda não há avaliações
- Fundamentals of Risk Management for Process Industry EngineersNo EverandFundamentals of Risk Management for Process Industry EngineersAinda não há avaliações
- Fun Facts about Hydrogen : Chemistry for Kids The Element Series | Children's Chemistry BooksNo EverandFun Facts about Hydrogen : Chemistry for Kids The Element Series | Children's Chemistry BooksAinda não há avaliações
- Biotechnology of Metals: Principles, Recovery Methods and Environmental ConcernsNo EverandBiotechnology of Metals: Principles, Recovery Methods and Environmental ConcernsAinda não há avaliações
- Nuclear Energy in the 21st Century: World Nuclear University PressNo EverandNuclear Energy in the 21st Century: World Nuclear University PressNota: 4.5 de 5 estrelas4.5/5 (3)
- The Stress Analysis of Pressure Vessels and Pressure Vessel Components: International Series of Monographs in Mechanical EngineeringNo EverandThe Stress Analysis of Pressure Vessels and Pressure Vessel Components: International Series of Monographs in Mechanical EngineeringS. S. GillNota: 3.5 de 5 estrelas3.5/5 (3)
- Coulson and Richardson’s Chemical Engineering: Volume 2B: Separation ProcessesNo EverandCoulson and Richardson’s Chemical Engineering: Volume 2B: Separation ProcessesAjay Kumar RayAinda não há avaliações