Escolar Documentos
Profissional Documentos
Cultura Documentos
Ann Oncol-2007-Aksoy-1904-6
Enviado por
Anonymous 3KRsaIDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Ann Oncol-2007-Aksoy-1904-6
Enviado por
Anonymous 3KRsaIDireitos autorais:
Formatos disponíveis
letters to the editor
Annals of Oncology
Triple-negative breast cancers are frequently defined as
a single group identifiable using routine clinical tests. They are
negative for estrogen receptor (ER), progesterone receptor
(PR), and the human epidermal growth factor receptor 2
(HER2), the so-called triple-negative breast cancers. This term
originated from hierarchical clustering analysis of gene
expression microarray studies [1, 2]. One of the five subtypes of
breast cancer identified by these analyses characteristically
expressed very low levels of ER and related genes and did not
have HER2 overexpression, the triple-negative phenotype. This
subtype also expressed many genes expressed in myoepithelial
or basal cells in the normal breast duct and the term basal like
was given. This subgroup accounts for 15% of all types of breast
cancer. Histologically, triple-negative breast cancers are poorly
differentiated and are characterized by an aggressive clinical
history. Since there are no specific treatment guidelines for this
subgroup, triple-negative breast cancers are managed with
standard treatment; however, such treatment leaves them
associated with a high rate of local and systemic relapse [3]. In
this study, we try to investigate some demographic, clinical, and
pathological characteristics of the triple-negative breast cancers
and comparing these with non-triple-negative breast tumors in
Turkish population. Standard histological and
immunohistochemical analysis were carried out for ER, PR,
and HER2 status. ER and PR are considered negative if
immunoperoxidase staining of tumor cell nuclei is <5%. HER2
was assessed through immunohistochemistry (IHC) or FISH.
IHC is scored on a qualitative scale from 0 to 3+, based on
interpretation of staining intensity, with 0 and 1+ classified
as negative, 2+ as borderline, and 3+ as positive. FISH is
scored on a quantitative scale with <2 copies of the HER2
1904 | letters to the editor
Volume 18 | No. 11 | November 2007
Downloaded from http://annonc.oxfordjournals.org/ by guest on April 24, 2016
Demographic, clinical, and
pathological characteristics of
Turkish triple-negative breast
cancer patients: single center
experience
letters to the editor
Annals of Oncology
is associated with an increased risk of developing basal-like
cancer [5]. Furthermore, early menarche in addition has
a stronger effect on the risk of basal-like cancers [6]. In our
study, we did not evaluate these risk factors. However,
interestingly we found a positive association between oral
contraceptive use and triple-negative breast cancer. This
finding needs further verification in larger clinical trials.
Basal-like cancers are typically high-grade tumors, with
a very high proliferative rate and central necrosis that present
with large primary tumors [7]. In accordance with the
literature, patients with triple-negative tumors in our study
population had more frequently high-grade tumors than
non-triple-negative tumors (52.9% versus 28.4%, P = 0.041).
However, this was not true in our study population for
tumor size. Studies have documented that basal-like cancers are
less likely to have metastasized to the axillary lymph nodes
(42% basal-like cancers in one series node positive versus
60% controls) [8]. In our cohort also, frequency of lymph node
metastasis was lower in patients with triple-negative tumors
than the others. Therefore, we speculate that triple-negative
tumors may prefer to disseminate to distant sites
hematogenously. Basal-like breast cancers have a different
pattern of distant metastasis, compared with ER-positive breast
cancer, with less frequent metastasis to bone and liver and more
frequent metastasis to lung and brain [8, 9]. Due to shorter
follow-up of patients in our study group, we are not able to
show metastatic pattern in our triple-negative breast cancer
patients.
Although adjuvant chemotherapy is highly effective in
the treatment of triple-negative cancers, the prognosis of
triple-negative cancers remains poor. More thorough
understanding of the biology and demographic characteristics
of triple-negative breast cancer and mechanisms of tumor
progression may allow the development of rational-targeted
treatment strategies. Preliminary preclinical and clinical results
in this area are indeed quite promising.
S. Aksoy, O. Dizdar, H. Harputluoglu & K. Altundag*
Table 1. Patient characteristics both in triple-negative and other groups
n = 160 (%)
Median age (range)
Menopausal status
Pre
Peri
Post
Oral contraceptive use
Yes
No
Grade
III
III
Lymph node
Positive
Negative
Lymphovascular invasion
Yes
No
Triple negative
The others
P value
17 (10.6)
44 (2657)
143 (89.4)
47.5 (2078)
0.2
12 (70.6%)
5 (29.4%)
61 (43.0%)
9 (6.3%)
72 (50.7%)
0.05
6 (64.7%)
11 (35.3%)
17 (12.2%)
122 (87.8%)
0.02
8 (47.1%)
9 (52.9%)
96 (71.6%)
38 (28.4%)
0.041
5 (29.4%)
12 (70.6%)
52 (38.5%)
83 (61.5%)
0.012
50 (38%)
81 (62%)
0.32
8 (47.1%)
9 (52.9%)
Volume 18 | No. 11 | November 2007
Department of Medical Oncology, Hacettepe University Institute of
Oncology, Ankara, Turkey
(*E-mail: altundag66@yahoo.com)
references
1. Perou CM, Sorlie T, Eisen MB et al. Molecular portraits of human breast tumours.
Nature 2000; 406: 747752.
2. Sorlie T, Perou CM, Tibshirani R et al. Gene expression patterns of breast
carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad
Sci U S A 2001; 98: 1086910874.
3. Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic
options. Lancet Oncol 2007; 8: 235244.
4. Carey LA, Perou CM, Livasy CA et al. Race, breast cancer subtypes, and survival
in the Carolina Breast Cancer Study. JAMA 2006; 295: 24922502.
5. Millikan RC, Newman B, Tse CK et al. Epidemiology of basal-like breast cancer.
Breast Cancer Res Treat 2007 Jun 20; [Epub ahead of print].
6. Yang XR, Sherman ME, Rimm DL et al. Differences in risk factors for breast cancer
molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers
Prev 2007; 16: 439443.
7. Silva LD, Clarke C, Lakhani SR. Basal-like breast cancer. J Clin Pathol 2007 May
11; [Epub ahead of print].
letters to the editor | 1905
Downloaded from http://annonc.oxfordjournals.org/ by guest on April 24, 2016
gene classified as negative. One hundred and sixty
consecutive breast cancer patients presenting at Hacettepe
University Institute of Oncology were evaluated. Most tumors
were ER+ (76%, 123/160), PR+ (74%, 118/160), and HER2+
(59%, 94/160). Of all study population, 10.6% (17/160)
had triple-negative tumors. Patients with triple-negative
tumors were younger than those with non-triple negatives
(44 versus 47.5 years P = 0.20) (Table 1). Most of the patients
with triple-negative tumors were premenopausal at diagnosis
(70.6% versus 43.0%, P = 0.050). Frequency of oral
contraceptive use was higher in patients with triple negative
than non-triple-negative breast cancer patients (35.3%
versus 12.2%, P = 0.02). Frequency of lymph node metastasis
was lower in patients with triple-negative tumors than
non-triple-negative tumors (29.4% versus 61.5%, P = 0.012).
T stage and status of the distant metastasis were not different
at diagnosis in patients with triple-negative tumor and
non-triple-negative group. Positive family history of breast
cancer and hormone replacement therapy was not different
in patients with triple-negative tumors and those with
non-triple-negative patients. Patients with triple-negative
tumors had more frequently high-grade tumors (grade III)
than non-triple-negative tumors (52.9% versus 28.4%,
P = 0.041). There was no difference in the frequency of
lymphovascular invasion between triple-negative and other
groups.
In our small cohort study population, we found that
triple-negative patients have younger age, a history of more
frequent use of oral contraceptives, more grade III tumors, and
less lymph node metastases compared with non-triple-negative
patients. Triple-negative breast cancers are more commonly
seen in younger, premenopausal women, in women of
African descent or Hispanic, and with lower socioeconomic
status [4]. Our findings are also consistent with the literature as
younger and more premenopausal patients were included in
triple-negative group. Pregnancy not followed by breast-feeding
letters to the editor
Annals of Oncology
8. Foulkes WD, Metcalfe K, Hanna W et al. Disruption of the expected positive
correlation between breast tumor size and lymph node status in BRCA1-related
breast carcinoma. Cancer 2003; 98: 15691577.
9. Rodriguez-Pinilla SM, Sarrio D, Honrado E et al. Prognostic significance of basallike phenotype and fascin expression in node-negative invasive breast
carcinomas. Clin Cancer Res 2006; 12: 15331539.
doi:10.1093/annonc/mdm487
Downloaded from http://annonc.oxfordjournals.org/ by guest on April 24, 2016
1906 | letters to the editor
Volume 18 | No. 11 | November 2007
Você também pode gostar
- Harrison's 17th EdDocumento3 páginasHarrison's 17th EdShila Lupiyatama50% (2)
- PlateletpheresisDocumento6 páginasPlateletpheresisEmhemed Amer TabibAinda não há avaliações
- Handbook of Qualitative Health Research For Evidence-Based PracticeDocumento569 páginasHandbook of Qualitative Health Research For Evidence-Based PracticeMariana LepinskyAinda não há avaliações
- OSCE - Health PromotionDocumento13 páginasOSCE - Health PromotionbabukanchaAinda não há avaliações
- Postpartum Hemorrhage FinalDocumento6 páginasPostpartum Hemorrhage Finalvarshasharma05Ainda não há avaliações
- Group 3 ThesisDocumento19 páginasGroup 3 ThesisSVPS100% (1)
- Triple-Negative Breast Cancer CHEMO PDFDocumento13 páginasTriple-Negative Breast Cancer CHEMO PDFVikash SinghAinda não há avaliações
- Poorer Survival Outcomes For Male Breast Cancer Compared With Female Breast Cancer May Be Attributable To In-Stage MigrationDocumento9 páginasPoorer Survival Outcomes For Male Breast Cancer Compared With Female Breast Cancer May Be Attributable To In-Stage MigrationabcdshAinda não há avaliações
- Epidemiology of Breast Cancer Subtypes in Two Prospective Cohort Studies of Breast Cancer SurvivorsDocumento13 páginasEpidemiology of Breast Cancer Subtypes in Two Prospective Cohort Studies of Breast Cancer SurvivorsZawali DzArticlesAinda não há avaliações
- Onco 13964Documento6 páginasOnco 13964skyyblue10Ainda não há avaliações
- Faculty of Pharmacy, AIMST University, Semeling, Bedong, Malaysia-08100Documento7 páginasFaculty of Pharmacy, AIMST University, Semeling, Bedong, Malaysia-08100Anonymous 51JDhTNCAinda não há avaliações
- Cancer Epidemiol Biomarkers Prev 2008 Phipps 2078 86Documento10 páginasCancer Epidemiol Biomarkers Prev 2008 Phipps 2078 86Muhammad MaulanaAinda não há avaliações
- (+) Author AffiliationsDocumento9 páginas(+) Author AffiliationsMuhammadShahzadAinda não há avaliações
- Breast Cancer Subtype Distribution Is Different in Normal Weight, Overweight, and Obese WomenDocumento7 páginasBreast Cancer Subtype Distribution Is Different in Normal Weight, Overweight, and Obese WomenValir HusleAinda não há avaliações
- Oncollogy 3Documento9 páginasOncollogy 3AG LarikAinda não há avaliações
- Early Breast CancaerDocumento17 páginasEarly Breast CancaerDwi Arnhilah Miranda100% (1)
- 2074WJMH - WJMH 39 506Documento10 páginas2074WJMH - WJMH 39 506MSAinda não há avaliações
- Major Risk Factors Affecting Tripple Negative Breast Cancer in Black WomenDocumento14 páginasMajor Risk Factors Affecting Tripple Negative Breast Cancer in Black Womenapi-449537789Ainda não há avaliações
- Chapter On1Documento8 páginasChapter On1Soul MirrorAinda não há avaliações
- Triple NegativeDocumento7 páginasTriple Negativet. w.Ainda não há avaliações
- Hormonal Contraceptives and Hormone Replacement Therapy As A Possible Risk Factor For Breast CancerDocumento6 páginasHormonal Contraceptives and Hormone Replacement Therapy As A Possible Risk Factor For Breast CancerIdman GushaendriAinda não há avaliações
- Literature Review CancerDocumento7 páginasLiterature Review Cancerafdtftloi100% (1)
- Breast Cancer Research PaperDocumento13 páginasBreast Cancer Research PapershaunAinda não há avaliações
- A Review of Triple-Negative Breast Cancer: Nick Patten. Last Light (Detail) - Oil On Canvas, 44" × 56"Documento4 páginasA Review of Triple-Negative Breast Cancer: Nick Patten. Last Light (Detail) - Oil On Canvas, 44" × 56"Kritik KumarAinda não há avaliações
- Bauer2007 PDFDocumento8 páginasBauer2007 PDFAdi W. YengAinda não há avaliações
- Breast CancerDocumento9 páginasBreast CancerRoxanita DavilaAinda não há avaliações
- Dissertation Ovarian CancerDocumento12 páginasDissertation Ovarian CancerWriteMyPaperForCheapCanada100% (1)
- Journal BedahDocumento15 páginasJournal BedahNovia NadhiraAinda não há avaliações
- Serum Levels of Sex Steroids in Benign and Malignant Disorders of Breast in Libyan Women Full ModelDocumento1 páginaSerum Levels of Sex Steroids in Benign and Malignant Disorders of Breast in Libyan Women Full ModelJagannadha Rao PeelaAinda não há avaliações
- Ca CervixDocumento17 páginasCa CervixNisa Aprilen GintingAinda não há avaliações
- Rakha Et Al. 2006 Prognostic Markers in Triple Negative Breast CancerDocumento8 páginasRakha Et Al. 2006 Prognostic Markers in Triple Negative Breast CancerdanishAinda não há avaliações
- Pathophysiology: Breast Cancer Risk FactorsDocumento44 páginasPathophysiology: Breast Cancer Risk FactorsMusthafa Afif WardhanaAinda não há avaliações
- Triple Negative Breast CancerDocumento15 páginasTriple Negative Breast CancerUjas PatelAinda não há avaliações
- A Research Proposal As A Partial Requirement in Delivery RoomDocumento16 páginasA Research Proposal As A Partial Requirement in Delivery RoomHoward John M. Ramiterre100% (1)
- Pi Is 0960977613002920Documento6 páginasPi Is 0960977613002920Qudamah JasemAinda não há avaliações
- Effect Od Systemic Adjuvant Treatment On The Risk of Metachronous Contralateral Breast CancerDocumento9 páginasEffect Od Systemic Adjuvant Treatment On The Risk of Metachronous Contralateral Breast CancermohamedhazemelfollAinda não há avaliações
- Ajol File Journals - 494 - Articles - 112408 - Submission - Proof - 112408 5833 311975 1 10 20150203Documento5 páginasAjol File Journals - 494 - Articles - 112408 - Submission - Proof - 112408 5833 311975 1 10 20150203KeHuyDietAinda não há avaliações
- American Cancer SocietyDocumento40 páginasAmerican Cancer Societybrunna_pultzAinda não há avaliações
- Edbook Am.2015.35.e66Documento8 páginasEdbook Am.2015.35.e66Lamchabbek NajouaAinda não há avaliações
- Advances in Screening, Diagnosis, and Treatment ofDocumento7 páginasAdvances in Screening, Diagnosis, and Treatment ofBastomy EkaAinda não há avaliações
- 410-Article Text-1440-1-10-20211018Documento7 páginas410-Article Text-1440-1-10-20211018silviatengkerAinda não há avaliações
- Breast Cancer PaperDocumento16 páginasBreast Cancer PaperTrudy CroweAinda não há avaliações
- Cancer of The Cervix and Oral Contraceptives:: Minimizing Oral Contraceptive RisksDocumento2 páginasCancer of The Cervix and Oral Contraceptives:: Minimizing Oral Contraceptive RisksRidlaAchmadAinda não há avaliações
- DJR 030Documento8 páginasDJR 030Muhammad MaulanaAinda não há avaliações
- Borderline Ovarian Tumors - UpToDateDocumento19 páginasBorderline Ovarian Tumors - UpToDatenoor hyAinda não há avaliações
- Review Article On Breast CancerDocumento9 páginasReview Article On Breast CancerMehtab AhmedAinda não há avaliações
- 5926 21334 1 PBDocumento3 páginas5926 21334 1 PBM. K. AdilAinda não há avaliações
- NIH Public Access: Author ManuscriptDocumento19 páginasNIH Public Access: Author ManuscriptChemistixAinda não há avaliações
- Aab OvariumDocumento3 páginasAab OvariumAgustinus FatollaAinda não há avaliações
- Gabriel Luciper in DetailsDocumento13 páginasGabriel Luciper in DetailsSuresh IndiaAinda não há avaliações
- Impact of Breast Cancer Subtypes On Prognosis of Women With Operable Invasive Breast Cancer: A Population-Based Study Using SEER DatabaseDocumento10 páginasImpact of Breast Cancer Subtypes On Prognosis of Women With Operable Invasive Breast Cancer: A Population-Based Study Using SEER DatabasesilviatengkerAinda não há avaliações
- 2 AbDocumento6 páginas2 AbSaheed AbdulkarimAinda não há avaliações
- Review: Abenaa M Brewster, Mariana Chavez-Macgregor, Powel BrownDocumento10 páginasReview: Abenaa M Brewster, Mariana Chavez-Macgregor, Powel BrownSaheed AbdulkarimAinda não há avaliações
- Differences in Breast Cancer Survival by Molecular Subtypes in The United StatesDocumento8 páginasDifferences in Breast Cancer Survival by Molecular Subtypes in The United StatesAndreza Patricia Marinho de Souza MartinsAinda não há avaliações
- Scand J Work Environ Health:3-7: Environmental Risk Factors of Breast CancerDocumento6 páginasScand J Work Environ Health:3-7: Environmental Risk Factors of Breast CancerKhush BhullarAinda não há avaliações
- Brief: Ovarian Cancers: Evolving Paradigms in Research and CareDocumento4 páginasBrief: Ovarian Cancers: Evolving Paradigms in Research and CareFrancesco MultinuAinda não há avaliações
- Tanvi Journal Tumor BuddingDocumento35 páginasTanvi Journal Tumor Buddingeishitamahajan005Ainda não há avaliações
- Introduction To Breast CancerDocumento4 páginasIntroduction To Breast CancerEzzati AizaAinda não há avaliações
- Breast Fine Needle Aspiration Cytology Reporting Icet13i2p54Documento6 páginasBreast Fine Needle Aspiration Cytology Reporting Icet13i2p54salijanstarAinda não há avaliações
- Godone 2018Documento21 páginasGodone 2018Roberta GodoneAinda não há avaliações
- Bachelor of Science in Nursing: NCMB 312 - : Related Learning ExperienceDocumento6 páginasBachelor of Science in Nursing: NCMB 312 - : Related Learning ExperienceMada mada DaneAinda não há avaliações
- 2 26 1624520686 1ijbtrdec20211Documento4 páginas2 26 1624520686 1ijbtrdec20211TJPRC PublicationsAinda não há avaliações
- Chumsri 2019Documento18 páginasChumsri 2019faris nagibAinda não há avaliações
- Breast Disease: Diagnosis and Pathology, Volume 1No EverandBreast Disease: Diagnosis and Pathology, Volume 1Adnan AydinerAinda não há avaliações
- Triple-Negative Breast Cancer: A Clinician’s GuideNo EverandTriple-Negative Breast Cancer: A Clinician’s GuideAntoinette R. TanAinda não há avaliações
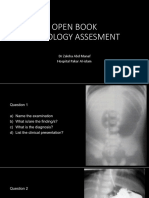
- Open Book Radiology Assesment: DR Zaleha Abd Manaf Hospital Pakar Al-IslamDocumento28 páginasOpen Book Radiology Assesment: DR Zaleha Abd Manaf Hospital Pakar Al-IslamChua Kai XiangAinda não há avaliações
- Legalizing MarijuanaDocumento3 páginasLegalizing MarijuanaFREE MOVIESAinda não há avaliações
- WHO 2019 nCoV Vaccines SAGE - Recommendation Inactivated 2023.1 EngDocumento10 páginasWHO 2019 nCoV Vaccines SAGE - Recommendation Inactivated 2023.1 EngMohamedRefaatMohamedAinda não há avaliações
- Pitfalls of Trauma Care: Dr. Nicole P. Hart Consultant Emergency Physician Associate Lecturer TraumaDocumento62 páginasPitfalls of Trauma Care: Dr. Nicole P. Hart Consultant Emergency Physician Associate Lecturer TraumaGiovanni HenryAinda não há avaliações
- NEW Risk Assessment Soilmec Piling Machine 110510Documento2 páginasNEW Risk Assessment Soilmec Piling Machine 110510mohamed ghalyAinda não há avaliações
- John 2016Documento3 páginasJohn 2016Mohammad Jofa Rachman PAinda não há avaliações
- Proposal of A Remote Monitoring System of Vital Signs For Heart Failure PatientsDocumento9 páginasProposal of A Remote Monitoring System of Vital Signs For Heart Failure PatientsAnonymous y3tPCbVrAinda não há avaliações
- PICO - Forming Answerable QuestionsDocumento4 páginasPICO - Forming Answerable QuestionsAugusto RosalesAinda não há avaliações
- UNIT 1. Nature of NursingDocumento43 páginasUNIT 1. Nature of NursingWerlyn Mae GonzagaAinda não há avaliações
- Checklist On Intravenous Insertion 1.1Documento2 páginasChecklist On Intravenous Insertion 1.1Geylla FaeldoniaAinda não há avaliações
- PeritonitisDocumento8 páginasPeritonitismuhammad ridwanAinda não há avaliações
- PPH4811-Assignment 01Documento16 páginasPPH4811-Assignment 01Ivine Sicelo SibekoAinda não há avaliações
- Vijayalakshmi S Kotrashetti, Alka D Kale, Mamata Hebbal, Seema R HallikeremathDocumento12 páginasVijayalakshmi S Kotrashetti, Alka D Kale, Mamata Hebbal, Seema R HallikeremathwelcometohellAinda não há avaliações
- Participants Midwifery Obstetrics PDFDocumento177 páginasParticipants Midwifery Obstetrics PDFNathaniel Cabatuan SalimbagatAinda não há avaliações
- Preeclampsia Diagnosis and ManagementDocumento15 páginasPreeclampsia Diagnosis and Managementanibal arenas velasquezAinda não há avaliações
- Abstract BookDocumento30 páginasAbstract BookAnonymous b0Jujx6kpAinda não há avaliações
- Iteration 1: Decomposition (How Would You Break Down Your Problem Into Sub-Problems?Documento10 páginasIteration 1: Decomposition (How Would You Break Down Your Problem Into Sub-Problems?Pandu HutagalungAinda não há avaliações
- Final Draft ProfileDocumento8 páginasFinal Draft Profileapi-244352427Ainda não há avaliações
- Audiometry Made Plug-And-PlayDocumento8 páginasAudiometry Made Plug-And-PlayKothapalli ChiranjeeviAinda não há avaliações
- Intl J Gynecology Obste - 2020 - Killeen - Examining The Use of The FIGO Nutrition Checklist in Routine AntenatalDocumento6 páginasIntl J Gynecology Obste - 2020 - Killeen - Examining The Use of The FIGO Nutrition Checklist in Routine AntenatalBenk Setsuna F. SeieiAinda não há avaliações
- Child Abuse and NeglectDocumento17 páginasChild Abuse and NeglectsamuelAinda não há avaliações
- PostnatalDocumento99 páginasPostnatalPlain GerlAinda não há avaliações
- Team Code-Amcc 10 Before The Hon'Ble Supreme Court of Dinda at New DelhiDocumento20 páginasTeam Code-Amcc 10 Before The Hon'Ble Supreme Court of Dinda at New DelhiUtkarsh singhAinda não há avaliações
- D7C C2Documento8 páginasD7C C2RamBabuMeenaAinda não há avaliações