Escolar Documentos
Profissional Documentos
Cultura Documentos
Paraben Esters Review
Enviado por
NoemiDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Paraben Esters Review
Enviado por
NoemiDireitos autorais:
Formatos disponíveis
623
Journal ofFood Protection, Vol. 44, No.8, Pages 623-6321,August 1981)
Copyright, International Association of Milk, Food, and Environmental Sanitarians
Antimicrobial Activity of Non-Halogenated
Phenolic Compounds
P.M. DAVIDSON!"' and A. L. BRANDEN2
Department ofFood Technology and Science, University of Tennessee, P.O. Box 1071, Knoxville, Tennessee 37901 and Department ofFood
Science and Technology, Washington State University, Pullman, Washington 99164
(Received for publication August 29, 1980)
ABSTRACT
Phenol and its non-halogenated derivatives have been used
for over 100 years as antiseptics to control growth of
microorganisms. Their importance in controlling microbial
growth in foods, however, has been recognized only recently.
Phenolic compounds important in foods may be classed
conveniently into three categories. First, there are those
compounds currently approved for use in foods. This group
includes methyl, propyl, and heptyl esters of p-hydroxybenzoic
acid. Naturally occurring phenolic derivatives comprise the
second category. Simple alkyl. hydroxy- and methoxy-phenol
derivatives to complex polyphenols are included in this diverse
group. The third type is food additives which are antimicrobials
but are currently approved for other uses. The phenolic
antioxidants are the only compounds in this category which
have been tested thoroughly for their antimicrobial effectiveness. Each of these classes of phenolic compounds has widely
varying inhibitory powers against certain bacteria, fungi and
viruses. Their mode of action has been studied but has not been
elucidated fully. A review of research on the spectrum of
antimicrobial activity of these compounds as well as their
proposed mechanism is presented.
Addition of chemicals, either intentionally or unintentionally, to preserve foods has taken place since the time
of the ancient Chinese (4). However, very few approved
compounds are presently available for controlling growth
of toxigenic or spoilage microorganisms in foods. Due to
the complex toxicological testing procedures necessary
for approving new antimicrobials, current research in
this area has been involved with characterizing and
finding new applications for currently approved antimicrobials. Two areas of research which have received
less attention are the identification and evaluation of
antimicrobials occurring naturally in foods and the
screening of previously approved food additives for
antimicrobial activity. Phenolic compounds, i.e., compounds with a phenol nucleus, are somewhat unique in
that they may be found in all three phases of current
1
University a/Tennessee.
'Washington State University.
research on antimicrobials in foods. Individual phenolic
compounds have been identified which represent
currently approved antimicrobials, naturally occurring
antimicrobials and antimicrobials which are food
additives approved for other uses.
The purpose of this paper is to review the
antimicrobial activity of phenol and its non-halogenated
derivatives with a special emphasis on food-related
microorganisms. The mechanism of antimicrobial action
of these compounds and future research outlooks will
also be addressed.
HISTORICAL PERSPECTIVE
Use of phenol (Fig. 1) as an antimicrobial agent has its
roots in the early history of microbiology. Although I. P.
Semmelweis pioneered the use of lime chloride as an
antiseptic in 1850 for obstetrical care, it wasn't until
Joseph Lister's work that adoption of antiseptic
techniques became standard practice (12,56). In 1867,
Lister reported on the use of phenol (carbolic acid) in
treating compound fractures and for use in surgery.
Lister, with Pasteur's principles in mind, had shown that
phenol prevented or at least reduced the chances of
bacterial infections subsequent to wounds and surgery
(12). He went so far as to state that phenol was an
antiseptic that had no equal (60). Use of phenol as an
antimicrobial in antiseptics and disinfectants has
declined steadily since the time of Lister, most probably
due to its high toxicity and low antimicrobial activity.
Phenol has been replaced by more effective antimicrobial
compounds, including many phenol derivatives. Now
phenol is used primarily to compare the antimicrobial
effectiveness of other more effective compounds. The
ratio of the antimicrobial activity of a particular
disinfectant to phenol under standard conditions is
defined as the "phenol coefficient" (6).
Use of phenol as an antimicrobial in foods is
prohibitive due to its toxicity. Although phenol probably
has never been added to foods intentionally, except in
extremely minute amounts, it is present in many foods
(52) and there are instances in which phenol plays a
practical role in preservation of foods. For example, two
JOURNAL OF FOOD PROTECTION, VOL. 44, AUGUST 1981
624
DAVIDSON AND BRANEN
propyl and heptyl esters of para-hydroxybenzoic acid
(parabens). The methyl (21 CFR 184. 1490) and propyl
(21 CFR 184. 1670) esters are generally regarded as safe
(GRAS) at a maximum concentration of 0.1% each. The
n-heptyl (21 CFR 172. 145) ester is approved at a
maximum concentration of 12 ppm in fermented malt
beverages (beers) and 20 ppm in certain non-carbonated
soft drinks and fruit-based beverages.
The methyl and propyl parabens are normally used
simultaneously in food products in a 2 or 3 to 1 ratio.
They have been used in preservation of baked goods,
beverages, food pastes, flavor extracts, fruit products,
artificially-sweetened jams and jellies, olives, pickles and
syrups. The recommended use concentrations range
from 0.02-0.1 %, depending upon the product (15).
The primary advantage of the parabens over benzoic
acid is their wider effective pH range. Whereas the
optimum range for benzoic acid is 2.5-4.0, the parabens
are effective at pH 3-8 (1, 15).
OH
PHENOL
Figure 1.
The structure ofphenol.
compounds which are major contributors to the
preservation offoods by wood smoke have been shown to
be cresol (methyl phenol) and phenol (30).
APPROVED FOOD ADDITIVES
In the United States at the present time, only three
antimicrobial phenolic compounds are permitted for
direct addition to food products (18). These are methyl.
Bacteria
The first reports on the antimicrobial effectiveness of
the parabens came from studies in Europe by
Sabalitschka and co-workers in the 1920's, as reported
by Prindle and Wright (60). Since that time, several
comprehensive studies have dealt with the antibacterial
and antifungal activities of various esters of the
parabens. Aalto et al. (J) and Sokol (77) were the first
researchers to determine the minimal inhibitory concentrations of the methyl, ethyl, propyl, and butyl esters,
using current testing techniques (Table 1). In general,
they found that inhibition of gram-positive bacteria was
proportional to the molecular weight of the ester used. In
contrast, inhibition of gram-negative bacteria was not
necessarily related to the ester chain length. Aalto et al.
(1) also found that the propyl paraben was 2-8 times
more effective than phenol or sodium benzoate in
TABLE l. Concentrations in ~Jglml of esters ofp-hydroxybenzoic acid necessary for total inhibition ofgrowth of various bacteria.
Concentration (J..tg/ml)
Bacterium
Methyl
Ethyl
Propyl
Butyl
Bacillus cereus
2000
1000
125
400
250
500
400
500
400
500
400
200
8000
4000
1000
400
1000
250
1000
63
Bacillus subtilis
Sarcina lutea
2000
4000
1000
1000
Staphylococcus aureus
4000
1000
Streptococcus lactis
Clostridium botulinum
Pseudomonas aeruginosa
Pseudomonas .fragi
Escherichia coli
Salmonella typhosa
Klebsiella pneumoniae
Enterobacter
1200
4000
4000
2000
1000
2000
1000
2000
1000
500
1000
Heptyl
Ref.
12
38
63
125
12
125
1
1
38
l
12
38
12
38
63
77
57
8000
55
4000
38
1000
125
4000
JOURNAL OF FOOD PROTECTION. VOL 44. AUGUST 1-}81
625
ANTIMICROBIAL ACTIVITY OF PHENOLIC COMPOUNDS
preventing growth of both gram-negative and -positive
bacteria at pH 6.8.
Jurd et at. (38) tested the antimicrobial activity of the
propyl and heptyl esters against selected bacteria
tTable 1). They showed that 400 ~-tg propyl paraben/ml
inhibited all the bacteria tested, gram-positive as well as
-negative strains. The heptyl ester was even more
effective, inhibiting all strains of bacteria at 12 ~-tglml.
The authors also demonstrated that propyl paraben was
at least twice as effective as potassium sorbate in
inhibiting all strains at pH 7.0.
Several recent studies have examined the antimicrobial activity of the parabens against individual bacterial
strains. Freese et al. (31) studied the effect of the
;Jarabens on vegetative cells of the gram-positive
bacterium Bacillus subtilis. They found the following
growth inhibition percentages, using the concentrations
ndicated (> 100% inhibition indicated lysis of cells):
methyl paraben at 10 Mm, 115%; ethyl paraben at
3 Mm, 80%; propylparaben at 1 Mm, 142%; and butyl
paraben at 0.5 Mm, 150%. Growth inhibition was found
to be reversible when the inhibitor was removed.
Moustafa and Collins (55) found that 0.4% (4000
,:.tg/ml) propyl paraben totally inhibited growth of the
gram-negative food spoilage microorganism Pseudomonas fragi in lactose-yeast extract broth at 13 C. No
growth was observed after 30 h at 0.4o/o, whereas 0.2%
propyl paraben actually seemed to stimulate growth of
the microorganism. Martin et aL (53) reported no
inhibition in growth of Alcaligenes viscolactis at 0.06%
(600 ~-tg/ml) propyl paraben in skim milk. Pierson et al.
\57) demonstrated total inhibition of Staphylococcus
aureus at 500 !Jg propyl paraben/ml whereas 300 !.J.g of
the same compound/ml mereiy delayed growth of
Salmoneila typhimurium. The n-heptyl ester of p-hydroxybenzoic acid was found to be very effective for
inhibiting microorganisms involved in the malo-lactic
fermentation ofwines (13).
The antimicrobial activity of the parabens against
anaerobic sporeformers has been shown to be variable.
Klindworth et al. (45) found that vegetative cells of
Clostridium perfringens were inhibited by 31.8% with
0.05% (500 1-'g/ml) of a 3:1 methyl-propyl paraben
mixture in fluid thioglycollate medium at 45 C. Robach
TABLE 2.
and Pierson (63) reported on the inhibitory effect of
methyl and propyl paraben against spores of Clostridium
botuiinum NCTC 2021. In a thiotone-yeast extract glucose medium, bacterial growth was totally inhibited
by 200 IJ.g propyl and 1200 1-'g methyl paraben/ml. Toxin
production of C. botulinum was inhibited by 100 1-'g
propyl and 1000 !Jg of methyl paraben/ml. Although the
parabens demonstrated antimicrobial activity in laboratory media, their inhibitory activity against C. bmulinum
in food systems has been reported to be low (76).
Molds
Parabens are most effective as antifungal agents
(Table 2). Concentrations of methyl and propyl paraben
necessary for complete inhibition of molds and yeasts are
from 2 to 1250 times lower than for bacteria (1,38). Aalto
et al. (J) also found that the methyl, ethyl, propyl, and
butyl esters were more effective than 11 more complex
esters in consistently preventing fungal growth. Other
studies have shown, however, that the hexyl, heptyl and
octyl esters are as, or more, effective than the shorter
chain esters (13,38). Jurd et al. (38) showed that the
propyl and heptyl esters were about 2-8 times more
effective than potassium sorbate at pH 5.6.
In summary, the parabens vary widely in their
antimicrobial effectiveness against bacteria, molds and
yeasts. As with many phenolics, they are more effective
against gram-positive bacteria and molds than against
gram-negative bacteria. Since the spectrum of antimicrobial activity for these compounds in laboratory media is
fairly well defined, more work needs to be conducted on
their possible application in food products.
NATURALLY OCCURRING PHENOLIC COMPOUNDS
Phenolic compounds are widespread in nature (36)
and as a result are found in many food systems. These
compounds have been isolated from alcoholic and
non-alcoholic beverages, meat and poultry products,
dairy products, vegetables, nuts and an assortment of
miscellaneous food products (52, 73). The antimicrobial
activity of many naturally occurring phenolic compounds
has been investigated by several workers.
Simple phenol derivatives
Hundreds of isomers and combinations of alkyl,
Concentrations in #).glml ofesters ofp-hydroxybenzoic ac!d necessary ~~tfJ!(ll inhibition ofgrowth of various fungi.__
Concentration
Fungi
Aspergillus flavus
Aspergillus niger
Penicillium digitatum
Penicillium chrysogenum
Rhizopus nigricans
Alternaria sp.
Candida albicans
Saccharomyces cerevisiae
Torula uti/is
Methyl
1000
500
500
1000
1000
Propyl
200
200
63
200
125
100
125
125
200
200
200
JOURNAL OF FOOD PROTECTION. VOL. 44, AUGUST 1981
Heptyl
Ref.
38
1.38
1
38
1
50-100
38
1
1
100
25
38
38
38
626
DAVIDSON AND BRANEN
hydroxy and methoxy derivatives of phenol are possible.
According to Maga (52), simple phenolic derivatives are
present in a variety of foods. Many of these simple
derivatives have been tested for their antimicrobial
activity. The purpose of most tests was not to determine
possible applications of these compounds but simply to
compare their antimicrobial activity to that of pure
phenol. Therefore, a detailed discussion of the antimicrobial capabilities of the simple phenolic compounds would
be of little value. Instead, a general discussion of the
findings will be presented as a basis for information
presented on more complex phenolic compounds.
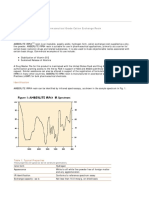
The addition of n-alkyl groups onto the phenol nucleus
has been found to increase its antimicrobial activity (44)
(Fig. 2). The phenol coefficient is proportional to the
chain length of the alkyl group against S. aureus a
gram-positive bacterium. In contrast, against Salmonella
typhi, a gram-negative organism, the phenol coefficient
decreases after then-amyl (5 C) derivative is reached.
3.0
1-
2.5
ILl
1f
2[)
1.5
0z
1.0
...
.....
....:z:
...
I!)
0.5
0
.....
0~----,----.,----.----.----.----.-----.
15
29
43
MW
57
ALKYl
71
85
99
CHAIN
Figure 2. The effect ofn-aklyl chain length of phenol on the
inhibition q{Staphylococcus aureus and Salmonella typhosa as
measured by the phenol coefficient. From: Prindle and Wright
(60).
The effect of the benzene ring position of the n-alkyl
derivatives of phenol on its antimicrobial activity is
somewhat controversial. Prindle and Wright (60)
reported that the antimicrobial activity of the individual
isomers of cresol (methyl phenol) and xylenol (dimethyl
phenol) are equal. Leifertova et al. (51) however, found
the p-cresol isomers to be most effective against bacteria
and molds while the 3,5 xylenol and 2,5 xylenol isomers
were most effective against molds and bacteria,
respectively.
Suter (83) summarized other important points which
could be made concerning the activity of simple phenol
derivatives. Normal alkyl derivatives were reported to be
more effective than branched chain derivatives. He also
reported that separation of alkyl groups from the phenol
nucleus by an oxygen (e.g., methoxy groups) decreased
their activity. Polyhydroxy-phenol derivatives have been
found to be generally less effective than pure phenol
(fable 3). Of the dihydroxy derivatives, only hydroquinone (p-hydroxyphenol) has any appreciable antimicrobial activity. Interestingly, hydroquinone is generally
more effective against gram-negative than -positive
bacteria (51,83). The trihydroxy derivatives have been
reported to have little activity. However, Leifertova et al.
(51) found some inhibition of Escherichia coli, Pseu
domonas aeruginosa and Bacillus subtilis by pyrogallol
(2, 3 hydroxyphenol). Addition of alkyl groups to
polyhydroxyphenol derivatives increased their activity to
some extent (83).
TABLE 3. Antimicrobial activity of hydroxy derivatives of
phenol at 37 C. a
Phenol coefficient
Compound
Catechol
Resorcinol
Hydroquinone
Phloroglucinol
Pyrogallol
Salmonella
Typhosa
Staphylococcus
aureus
0.87
0.40
.0.58
0.40
$).44
0
0
12
0
0
aFrom: Suter (83).
Hydroxycinnamic acid derivatives
Hydroxycinnamic acid derivatives are common in
fruits, vegetables, grains, coffee, tea, soy flour and
molasses at concentrations up to 1.7o/o of the total weight
of some foods. Compounds may be present at
concentrations high enough to provide protection for
plants against microbial invasion (35. 78). Several of the
derivatives and related compounds have been tested for
their antimicrobial activity .
Bacteria. Grodzinska-Zachwieja and Kahl (33) studied
the antimicrobial activity of an ethyl acetate extract of
chicory roots (Cichorium intybus L.). The extract was
found to contain a mixture of chlorogenic (3-caffeyl-quinic) acid and isochlorogenic (dicaffeyl-quinic) acids. A
disc assay was used to compare the sensitivity of 21
species of bacteria to the chicory extract and two
antibiotics. All gram-positive species were sensitive to
75 mg of extract per disk and in one instance the extract
was more effective than 10 units of penicillin. Of the 19
gram-negative species 16 showed either medium or
strong sensitivity to 75 mg of the extract. Compared to
50 fig of chloromycetin per disk, 11 of 19 species were
inhibited the same or to a greater extent by the chicory
extract. They concluded that the active portion of the
chlorogenic and isochlorogenic acid molecules was
caffeic acid, a hydroxycinnamic acid. Leifertova et a!.
(51) studied the effect of several hydroxycinnamic acid
derivatives against 6 bacterial strains: Staphylococcus
pyogenes, Staphylococcus epidermidis, B. subtilus, E.
coli, Proteus rettgeri. and Pseudomonas aeruginosa.
Only a 1% solution of 2.5 dihydroxycinnamic acid had
any appreciable activity against the bacteria in an agar
diffusion assay. Baranowski and Davidson (unpublished
results) found variable inhibition of S. aureus and
Pseudomonas fluorescens, using chlorogenic, caffeic,
ferulic and p-coumaric acids at 1000 flg/ml.
Fungi. Most of the studies on the hydroxycinnamic
acid derivatives have been involved with their anti-fungal
activity. Valle (86) reported that caffeic acid was
inhibitory to Fusarium nivale at a concentration of
JOURNAL OF FOOD PROTECTION. VOL. 44. AUGUST 1981
ANTIMICROBIAL ACTIVITY OF PHENOLIC COMPOUNDS
500 1-Lg/ml. Chlorogenic acid, which contains equimolar
amounts of caffeic and quinic acids, was inhibitory to the
organism only at 1000 1-Lg/ml. Valle concluded that the
active fraction of chlorogenic acid was the caffeic acid
moiety. Another cinnamic acid derivative, ethyl p-methoxy cinnamate, isolated from Curcurma zedoaria of the
tumeric family, was inhibitory to Trichophyton rubrum,
Aspergillus niger and Saccharomyces cerevisiae at
10 j.lg/ml.
Sikovec (70, 71) studied the effect of three hydroxycinnamic acid derivatives (chlorogenic, isochlorogenic
and caffeic) and two phenolic acids (gallic and ellagic) on
the aerobic respiration of S. cerevisiae. He tested the
compounds individually at 200 1-Lg/ml and mixtures of all
the compounds at 200 j.lg/ml and 800 j.lg/ml. The
mixture of phenols at 800 j.lg/ml reduced respiration
(C0 2 production) to about half that of the control.
Individually the phenols were only slightly inhibitory
with ellagic acid (a phenolic acid) being the most
inhibitory. As in other reports, caffeic acid was found to
be more inhibitory than the chlorogenic acids.
Baranowski et al. (5) made a similar study on the
antimicrobial effect of caffeic, chlorogenic, p-coumaric
and ferulic acids on growth of S. cerevisiae at 32 C,
pH 3.5. Caffeic and chlorogenic acid had little effect on
the organism at 1000 1-1g/ml. In the presence of
p-coumaric, however, 100 1-Lg/ml delayed growth initiation, and above 250 j.lg/ml, inhibition was proportional
to the concentration present. The organism was
completely inhibited by 1000 j.lg of p-coumaric/ml.
Ferulic acid was the most effective growth inhibitor
tested. At 50 j.lg/ml, this compound extended the lag
phase of S. cerevisiae and at 250 j.lg/ml growth of the
organism was completely inhibited. The degree of
inhibition was noted to be inversely related to the polarity
of the compounds. The authors suggested that these
naturally occurring compounds might interfere with
fermentation of fruits by this yeast.
In contrast to other studies, Leifertova et al. (51) found
little inhibition of nine species of yeast and molds by the
hydroxycinnamic acid derivatives. They tested 10
derivatives, including caffeic, p-coumaric and ferulic
acids, at 300 ppm (J.tg/ml), using a disc assay system.
With one exception (caffeic acid weakly inhibited the
mold Penicillium notatum), none of the compounds
demonstrated any antifungal activity. The differences in
the results obtained in this study compared to the
previously discussed studies may have been due to the
antimicrobial assay technique, the microorganisms used
or the concentrations of the compounds employed.
Because of the low solubility of the hydroxycinnamic acid
derivatives in polar solvents, it is possible that there was
little diffusion of the test compounds into the artificial
medium (5).
Viruses. The antiviral activity of caffeic acid was
investigated by Grodzinska-Zachwieja et al. (34). They
showed that 100 jAM of caffeic acid inhibited replication
of the DNA viruses (vaccinia and adenovirus) in tissue
627
culture. The RNA viruses tested were much less affected.
A concentration of 300 jAM was necessary to inhibit
replication of parainfluenza type 3 virus and no
inhibition of poliomyletis type 1 virus was shown even at
300 jAM of caffeic acid.
Anthocyanins and anthocyanidins
Anthocyanins are a group of water-soluble pigments
which occur naturally in many fruits. These compounds
consist of an aglycone (anthocyanidin) portion esterified
to one or more sugars. Many anthocyanidins are known
but only six are important in foods: pelargonidin,
cyanidin, delphinidin, peonidin, petunidin and malvidin
(17). All of the anthocyanidins are derivatives of the
flavylium cation and have various degrees of hydroxylation and methoxylation.
Masquelier (54) was the first to recognize the
inhibitory powers of the anthocyanins. He found that red
wine had considerable antimicrobial activity against
several bacteria, including S. typhi and E. coli. Further
experiments revealed that the antimicrobial activity of
the wine was lost upon decolorization with charcoal,
showing that neither ethanol nor organic acids were the
active fractions. Masquelier concluded that most of the
antimicrobial activity was contained in the anthocyanin
fraction with some contribution from caffeic acid. He
also found no inhibition of bacterial growth by the wine
must, which led to the theory that anthocyanins were
hydrolyzed during fermentation to release the active
inhibitor, anthocyanidin. Malvidin was postulated to be
the most active antimicrobial.
Pratt et al. (59) evaluated the anthocyanins from
grapes and strawberries as inhibitors against the
anaerobic sporeformer P.A. 3679, E. coli, and Lactobacillus acidophilus. Delphinidin-3-monoglycoside and
pelargonidin-3-monoglycoside inhibited growth of P.A.
3679 by 99% and of L. acidophilus by 30% at SO mg%
(500 j.lg/ml). E. coli was not inhibited by pelargonidin-3monoglycoside or cyanidin-3-monoglycoside at 10 mg%
(100 1-Lg/ml). They observed that growth of PA 3679 was
initially stimulated by the presence of the anthocyanins
but was depressed after 8 days of growth. They agreed
with the findings of Masquelier (54) that the aglycone
was the active antimicrobial fraction and that it was
being hydrolyzed from the anthocyanin to inhibit growth.
In a related study, Powers et al. (58) studied the effect
of anthocyanins and anthocyanidins on E. coli, S. au reus,
and Lactobacillus casei, using a disc assay system. They
found both inhibitory and stimulatory effects, using
pelargonidin-3-monoglycoside (P-Mg). delphinidin-3monoglycoside (D-Mg), malvidin-3,5-diglycoside (M-Dg),
malvidin-3-monoglycoside, malvidin-3, 5-diglycoside,
malvidin aglycone and delphinidin aglycone at 3-15 mg
per disc. Only P-Mg, D-Mg and M-Dg demonstrated any
inhibition of the microorganisms. They proposed that the
mode of action of these compounds was related to a
change in the oxidation-reduction potential or the
chelation of essential minerals.
JOURNAL OF FOOD PROTECTION, VOL. 44. AUGUST 1981
628
DAVIDSON AND BRANEN
Other naturally occurring compounds
Green olives contain a potent inhibitor of the lactic
acid bacteria which are normally associated with
fermentation of the product (2iJ, The inhibitor has been
identified as a phenolic compound, either the glycoside
oleuropein or its phenolic aglycone (28,39,40). Juven et
al. (39) found that purified oleuropein was inhibitory to
the following microorganisms (minimum inhibitory
concentration ranges jig/mi): Lactobacillus plantarum
(330-1000), Leuconostoc mesenteroides (330-6 70), P.
fluorescens (170-330), and B. subtilus (670-1000). No
inhibition of the following organisms was demonstrated
at 1000 jig/ml: Enterobacter aerogenes, E. coli, Candida
albicans or S. cerevisiae. In further studies using a disc
assay, 0.2o/o of the compound inhibited a species of
Rhizopus and Geotrichum candidum (40). No inhibition
of growth was demonstrated at the same concentration
against various species of Candida, Pichia or Saccharomyces.
Turbovsky et al. (85) found that wine yeasts, wild
yeasts and vinegar bacteria were unaffected or affected
only slightly by tannins at concentrations as high as
20,000 jig/mi. Schanderl (66), however, reported that
2000 jig of tannin/ml halted wine yeast fermentations
earlier than a control. Gallic acid, caffeic acid and quinic
acid at 100jig/ml were also found to delay fermentation
in the presence of 6 o/o added alcohol. Singleton and Esau
(73) stated that the increased antimicrobial activity of
wines at 8-10 years was due to the formation of tannins.
They predicted that the antibacterial effect of red and
white wines would be proportional to the amount of
flavonoid tannin present.
Konowalchuk and Speirs (46-49) published a series of
reports on the antiviral activity of various fruits and fruit
juices. Extracts of blueberries, crabapples, cranberries,
grapes, peaches. plums, pomegranates, raspberries,
strawberries, red wines, grape juice, apple juice and tea
were all found to inactivate poliovirus. Strawberry
extract, grape juice and apple juice were also reported to
inactivate coxsackievirus, echovirus, reovirus and herpes
simplex virus. They concluded that the primary viral
TABLE 4. Concentrations
Microorganism
inhibitors in these products are the polyphenolic
compounds. tannins. Tannic acid was found to have the
greatest antiviral activity against echovirus, poliovirus
and herpes simplex virus (46). Recently, Cliver and
Kostenbader (16) confirmed the work of Konowalchuk
and Speirs (4i} on the antiviral activity of grape juice.
They found, however, that the inhibitory factor of grape
juice did not degrade viruses and that the effect was
reversible. Therefore, the virus was capable of being
reactivated to cause infections.
Schraufstatter (6iJ investigated the antimicrobial
effect of chalcone, flavanone, flavone and flavonol. All
compounds except flavonol had a bacteriostatic effect on
S. aureus. Natural sources of these compounds were also
found to have slight bacteriostatic activity against the
microorganism.
PHENOLIC ANTIOXIDANTS
Phenolic antioxidants are most commonly used to
prevent oxidative rancidity associated with lipids and
lipid-containing products. These compounds have
recently been found to function as antimicrobials as well
as antioxidants.
Bacteria
Initial investigations of the antimicrobial activity of
phenolic antioxidants involved their use against various
bacterial species (Table 4). Ward and Ward (91) were the
first to investigate the effect of butylated hydroxytoluene
(BHT) against the gram-negative food poisoning
bacterium Salmonella senftenberg. They found little
inhibition of the microorganism by l.Oo/o BHT in brillant
green agar. S. typhimurium growth has been found to be
totally inhibited by 150 ppm (jtg/ml) butylated hydroxyanisole (BHA) in trypticase soy broth at 32 C (22) and
400 ppm BHA in nutrient broth at 37 C (14). Differences
in susceptibility are most likely due to strains. media,
temperature or technique of testing (22).
The growth of other gram-negative bacteria has been
found to be inhibited to various extents by BHA.
Enteropathogenic E. coli was found to be totally
inhibited by 400 ppm of BHA at 37 C in nutrient broth,
inhibit various bactena in laboratory media.
Antiox
Cone.
(J..cg/ml)
Ref.
91
BRT
>10.000
14.57
400
BRA
22
150
BRA
14
400
BRA
Escherichia coli
69
400
BRA
62
so
BHA
Vibrio parahaemo{vticus
20
>400
BRA
Pseudomonas .fragi
20
100
BRA
Pseudomonas jluorescens
14.22
150
BRA
Staphylococcus au reus
3.57
200
BRA
3
150
BHT
22.61
25
TBRQ
45
150
BRA
Clostridium perfringens
64
50
BRA
Clostridium botulinum
-------------------------------------------------------------Salmonella senftenherg
Salmonella typhimurium
JOURNAL OF FOOD PROTECTION. VOL. 44. AUGUST 19t,J
ANTIMICROBIAL ACTIVITY OF PHENOLIC COMPOUNDS
in a study by Chang and Branen (14). This same
concentration only extended the lag phase of E. coli
grown in trypticase soy broth at 35 C. in tests by Shih and
Harris (69). Growth of the marine food poisoning
bacterium Vibrio parahaemolyticus 04:K11 was shown
to be totally inhibited by as little as 50 ppm of BRA in
trypticase soy broth while 400 ppm of BRA were
required for the same effect in crab meat homogenate
(62). P .. fragi, a common food spoilage bacterium, was
found to be resistant to concentrations as high as
400 ppm of BRA in trypticase soy broth when grown at
7 and 26 C (20). In contrast, P. Jluorescens, a related
bacterium, was totally inhibited by 100 ppm of BRA at
7 C under the same conditions (20).
The gram-positive bacteria are, in general, more
susceptible to the growth-inhibiting effects of phenolic
antioxidants. S. aureus growth has been found to be
inhibited by 150 ppm of BRA (14,22) and 25 ppm of
tertiary butyl hydroquinone (TBHQ) (22,61). Ayaz et al.
(3) showed that enterotoxin production was inhibited
totally by 100 and 150 ppm of BHT and BRA,
respectively. Stern et al. (79) demonstrated that 100 ppm
of BRA was totally inhibitory to S. au reus in the presence
of 3% NaCI at pH 7.0. In the presence of 7% NaCI,
50 ppm ofBHA prevented growth of the organism.
The gram-positive anaerobic sporeformers C. per
ftingens and C. botulinum are highly susceptible to
BRA. Klindworth et al. (45) showed that 150 ppm of
BRA totally inhibited growth of three strains of C.
perfringens in Fluid Thioglycollate Medium at 37 C.
Robach and Pierson (64) found inhibition of growth and
toxin production by three strains of C. botulinum in
prereduced thiotone yeast-extract glucose medium at
37 C by 25-50 ppm BRA. Butylated hydroxytoluene was
less effective against C. botulinum, requiring 200 ppm
for complete inhibition.
Inhibition of general food spoilage microflora has also
been investigated. Trelease and Tompkin (84) found
500 ppm of BRA to be totally inhibitory to microorganisms from spoiled frankfurters. Erickson and Tompkin
(25) reported TBHQ to be inhibitory to the microflora of
soy flakes and fresh meat at 100-200 ppm in a broth
medium.
Phenolic antioxidants have been found to have
antimicrobial activity against other microorganisms,
including mycoplasmas. For example, in an artificial
medium 10 ppm BHT was reported to inhibit Mycoplasma synoviae at 37 C (87). A level of 400 ppm BHT in
the diet however, afforded chickens no protection from
this microorganism.
Fungi
The antifungal activity of these compounds has been
well documented. Mycelial growth and aflatoxin
production by Aspergillus parasiticus were totally
inhibited by 250 ppm BRA (1000 ppm for spores) in a
glucose-salts medium (14). Fung et al. (32) reported that
BRA, but not BHT, was able to inhibit growth and
aflatoxin production by Aspergillus flavus on a solid
629
medium. Similar results were obtained by Beuchat and
Jones (10), using heated and unheated conidia of A.
jlavus. At 100 ppm of BHA, colony formation by heated
and unheated conidia was totally prevented, whereas no
inhibition was demonstrated at 1000 ppm BHT. Ahmad
(2) found 200 ppm BRA to be 100% inhibitory to species
of Geotrichum, Penicillium and Aspergillus in a
glucose-salts medium. Presence of a subinhibitory level
of BRA (0.6 ppm) was shown to have a synergistic effect
in combination with amphotericin B, an antifungal
antibiotic, against the pathogenic yeasts Candida
albicans and Candida parapsilosis (7).
Viruses
Lipid-containing viruses are susceptible to very low
concentrations of BHT. The bacteriophage 0 6 and
herpes simplex virus were reported to be 50o/o inactivated
by as little as 10 4 - w-s M BHT, whereas poliovirus
which contains no lipid was not affected (74). Wand a et
al. (90) reported that BHT acted by preventing 0 6 from
attaching to its host cell. In contrast, Cupp et al. (19)
showed that bacteriophage PM2 inhibition by BHT was
due to complete disruption of the virus particle. Kim et
al. (43) reported that the lipid-containing human and
murine cytomegaloviruses and Semliki Forest Virus were
all inhibited by 40-320 ppm BHT. Vaccinia virus, which
also contains lipid, was not found to be inhibited. They
theorized that BHT inhibition was species-dependent.
Snipes and Keith (75) have recently reported that a
4-carbon n-alkyl derivative of BHT cleared lesions in
mice caused by herpes simplex virus much faster that
untreated controls.
Surak et al. (80,81) investigated the effects of BRA and
BHT on the protozoan Tetrahymena pyriformis. They
showed that 20 ppm of BRA inhibited growth of the
microorganism by SO o/o.
A variety of environmental conditions have been found
to affect the antimicrobial activity of the phenolic
antioxidants. The most dramatic effect on activity has
been shown to be in the presence of lipids. Robach et al.
(62) were the first to demonstrate a decrease in the
antimicrobial activity of BRA against V. parahaemolyticus in the presence of lipids of a crab meat homogenate.
The concentration necessary for complete inhibition of
the organism was eight times higher in the crab meat
homogenate (400 ppm) than in a nutrient broth
(50 ppm). Corn oil significantly decreased the activity of
BHT against both C. perfringens and A.flavus (2,45).
Two theories have been given for the decrease in
antimicrobial activity of BRA in the presence of lipid.
First, since the antimicrobial properties of BHA may
be related to its antioxidant powers, the antimicrobial
activity may be lost in preventing autoxidation of lipids.
Second, since BRA is hydrophobic, the compound may
solubilize in the lipid phase of the system and be
unavailable to act on microorganisms. Lamanna et al.
(50) reported that the addition of factors which tend to
increase the solubility of hydrophobic antimicrobials in
aqueous systems was equivalent to decreasing the
JO VRNAL OF FOOD PROTECTION VOL. 44, AUGUST 1981
630
DAVIDSON AND BRANEN
concentration of the antimicrobial available for killing.
There may also be a combination of factors which causes
the reduced effectiveness; however, none of these theories
has been tested as yet.
Another major factor contributing to activity is
temperature. Wanda et al. (90) was the first to note that
no inactivation of 0 6 bacteriophage occurred at 0 C with
0.03 mM BHT, whereas at 25 C the same concentration
inactivated 99.99% of the phage. Davidson and Branen
(20) showed the same phenomenon using BHA and P.
fluorescens at 1 and 26 C. It has been theorized that the
lower temperatures decrease the activity of the phenolic
antioxidants by reducing their solubility into the lipid
portion of the microorganisms (90).
The effects of the pH on antimicrobial activity have
been somewhat contradictory. Klindworth et al. (45)
found that C. peifringens was inhibited to a greater
extent at pH extremes of the range from 5.5-8.5. Beuchat
and Jones (10) also reported that BHA was more effective
in preventing outgrowth of A. jlavus conidia at pH 3.5
than at 5.5. In contrast, both S. aureus and A. jlavus
were found to be most sensitive at pH values near
neutrality in other studies (2,3).
In general, the phenolic antioxidants have a synergistic
effect when used in combination with other known
antimicrobials. In the presence of potassium sorbate,
both BHA and TBHQ have some synergistic antimicrobial activity against S. aureus in trypticase soy broth
(22.62). Combinations of BHA and potassium sorbate
have also been shown to have a synergistic effect against
S. typhimurium (22). Against C. perfringens, BHA was
shown to have at least additive antimicrobial activity with
nitrite, sorbic acid and parabens (45). Pierson et al. (57)
showed that BHA in combination with propyl paraben
had slightly higher antimicrobial activity than the
compounds used alone. No attempt was made in this
study to measure synergism.
MECHA.lllliSM OF ANTIMICROBIAL ACTIVITY
The mechanism of all antimicrobials falls into one or
more ofthree general categories: (a) reaction with the cell
membrane to cause increased permeability with resultant
loss of cellular constituents, (b) inactivation of essential
enzymes and (c) destruction or functional inactivation of
genetic material. Reversibility by the cell of these
mechanisms determines whether ultimate bacteriostasis
or lethality occurs (89).
The reactive portion of antimicrobial phenolic
compounds appears to be the free hydroxyl group (60).
Substitution of various side groups onto a phenol nucleus
may simply modify the reactivity of the compounds with
a resultant increase or decrease in antimicrobial activity.
Most researchers agree that the actual mechanism of
inhibition by all phenolic compounds involves a reaction
with the cell membrane of inactivation of essential
cellular enzymes or a combination ofthe two.
In early studies on phenol, it was reported that higher
concentrations precipitated all cellular proteins, whereas
lower concentrations selectively inhibited essential
enzymes (60). Fogg and Lodge (29) were the first to
propose that the mechanism of phenol inhibition was
enzyme inactivation. They found that the rate-limiting
step in inhibition was the penetration of the compound
into the cell, which was related to its lipid solubility.
Roberts and Rahn (65) studied the effects of phenol on
selected eyzymes of E. coli. They found that dehydrogenase and oxidase activities were most affected by
inhibitory concentrations of phenol.
More recently, investigations have centered on the
effect of phenol on cell membranes. Vas (88) proposed
that phenol attacked the cytoplasmic membrane of
microorganisms, causing the release of intracellular
constituents. Judis (37) studied the release of radioactively labeled compounds from E. coli in the presence
of phenol and some halogenated derivatives. It was found
that up to SO% of the Na glutamate-3,4- 1 "C and 12% of
the NaH 232 P0 4 were lost by E. coli in the presence of
phenolics. It was concluded that the loss was due to a
weakening or destruction of the permeability barrier of
the cell membrane. Bernheim (8) proposed that phenol
reacted primarily with the phospholipid component of
the cell membrane of P. aeruginosa. The phenol then
caused an increase in the permeability of the cell
membrane. Phenol was later shown to cause rapid
swelling of P. aeruginosa cells, which further substantiates this theory (9). Kaye and Proudfoot (42) investigated
the effect of phenolics on phosphatidyl ethanolamine
monolayers. They found that various phenolics caused
disruption of the integrity of the monolayers in direct
correlation with their antimicrobial activities.
Investigations into the mechanism of antimicrobial
activity of other phenolic compounds have also been
related to their effect on membranes. Juven et al. (41)
investigated the mechanism of action of the antimicrobial phenolic glycoside, oleuropein, on L. plantarum.
They found leakage of radioactively labeled glutamate,
potassium, and phosphorus from the organism in the
presence of oleuropein. They found no effect on the
glycolysis rate. This led to the conclusion that oleuropein
caused membrane permeability changes. In contrast,
Freese et a!. (31) demonstrated that the parabens
inhibited transport of substrates into the cell. They
reported that methyl, ethyl, propyl and butyl parabens
inhibited uptake of L-~erine by membrane vesicles and
inhibited growth, oxygen uptake and ATP production by
whole cells of B. subtilis.
Work conducted to elucidate the mechanism of
inhibition by the phenolic antioxidants strongly suggests
a reaction with the cell membrane. Surak et al. (81)
demonstrated that the phenolic antioxidant BHT caused
leakage of intracellular contents from T. pyrifomzis.
Wanda et al. (90) suggested inhibition of viruses by BHT
was caused by disruption of the viral membrane
(envelope). Davidson and Branen (21) recently found that
BHA caused leakage of 1"C-labeled intracellular
constituents from P. fragi and P. fluorescens. Other
studies showed that BHT causes disruption of the axial
JOURNAL OF FOOD PROTEC110N, VOL. 44. AUGUST 1981
631
ANTIMICROBIAL ACTIVITY OF PHENOLIC COMPOUNDS
symmetry (perturbation) and increased fluidity of lipid
alkyl chains of artificial phospholipid structures (24, 72).
Sgaragli et al. (68) found that phenolic antioxidants
caused a release of proteins from rat liver mitochondria
and lysosomes.
The mechanism of inhibition by phenolic antioxidants
may also be related to inactivation of essential enzymes
or interference with genetic functions. BRA and TBHQ
have been found to inhibit DNA, RNA and protein
synthesis (80,82). TBHQ has also been found to inhibit
metabolism of acetate.
It must be noted that microbial inhibition by phenolic
compounds may also be directly related to their aromatic
ring structure. For example, toluene has been shown to
cause considerable cytoplasmic membrane damage in E.
coli (23,92).
The actual mechanism of inhibition by these
compounds may be a combination of damage to the
cytoplasmic membrane and enzyme inhibition. Simple
leakage of intracellular compounds may contribute to
inhibition but, according to Lamanna et al. (50), it
usually is not the total cause for inactivation. Since many
enzyme systems of microorganisms are membranebound (50), a change in the lipid portion of the
membrane may theoretically affect the activity of those
enzymes. For example, Exterkate (26) recently showed
that membrane-perturbing organic solvents caused a
decrease in the activity of membrane-bound peptidases
of Streptococcus cremoris HP. More research needs to be
directed toward determining the exact mode of action of
phenolic compounds.
FUTURE STUDIES
Research on the antimicrobial activity of phenolic
compounds is needed in several areas. More work needs
to be done on defining the antimicrobial activity of the
parabens in foods. Research on the antimicrobial activity
of nitrites and sorbates has increased in recent years
while studies on other compounds have lagged. In light
of the fact that so few antimicrobial compounds are
approved for use in foods, we need to know as much as
possible about each compound.
The naturally occurring phenolic compounds have
been practically ignored as far as their antimicrobial
activity is concerned. Studies should be conducted on the
antimicrobial activity of the isolated compounds as well
as their activity in combinations. Information is also
needed on naturally occurring compounds in combination with environmental factors to determine the overall
potential for growth of spoilage or toxigenic microorganisms in foods. Additional research is needed on the
mechanism of action ofthese compounds.
Studies on the phenolic antioxidants as antimicrobials
need to be directed toward their possible application in
food systems. Initially, the effects of various food
components, especially lipids, on the antimicrobial
activity of the phenolic antioxidants should be more
clearly defined. Testing is also needed to develop the
most eftlcient method of applying these compounds to
foods to obtain maximal antimicrobial effectiveness.
Some possible application methods have been discussed
by Branen et al. (11). The phenolic antioxidants would
probably be most effective as antimicrobials in foods
with a reduced lipid content; however, these compounds
may also be useful as surface antimicrobials (by spraying
or dipping) on foods with higher lipid levels.
REFERENCES
1. Aalto, T. R., M. C. Firman, and N. E. Rigler. 1953. p-Hydroxybenzoic acid esters as preservatives. I. Uses, antibacterial and antifungal studies, properties and determination. J. Amer. Pharm.
Assoc. 42:449-457.
2. Ahmad, S. 1979. Inhibition of mold gro\\ih by butylated hydroxyanisole. M.S. Thesis. Washington State University. Pullman, WA.
3. Ayaz, M., L. 0. Luedecke, and A. L. Branen. 1980. Antimicrobial
effect of butylated hydroxyanisole and butylated hydroxytoluene
on Staphylococcus au reus. J. Food Prot. 43:4-6.
4. Ayres, I. C., J. 0. Mundt, and W. E. Sandine.1980. Microbiology
offoods. W. H. Freeman and Co. San Francisco. 708 p.
5. Baranowski, J. D., P. M. Davidson, C. W. Nagel, and A. L.
Branen. 1980. Inhibition. of Saccharomyces cerevisiae by naturally
occurring hydroxycinnamates. J. Food Sci. 45:592-594.
6. Bass. G. K. 1977. Methods of testing disinfectants. pp. 49-77. In
S. S. Block (ed.) Disinfection, sterilization, and preservation,
2nd ed. Lea and Febiger, Philadelphia.
7. Beggs, W. H., F. A. Andrews, and G. A. Sarosi. 1978. Synergistic
action of amphotericin B and antioxidants against certain
opportunistic yeast pathogens. Antimicrobial. Agents Chemother.
13:266-270.
8. Bernheim, F. 1972. The effect of chloroform, phenols, alcohols
and cyanogen iodide on the swelling of Pseudomonas aeruginosa
in various salts. Micro bioi. 5:143-149.
9. Bernheim, F. 1974. Effect of aromatic hydrocarbons, alcohols,
ketones and aliphatic alcohols on cell swelling and potassium
efflux in Pseudomonas aeruginosa. Cytobios 11:91-95.
10. Beuchat, L. R., and W. K. Jones. 1978. Effects of food preservatives and antioxidants on colony formation by heated conidia
ofAspergillusflavus. ActaA!imentaria 7:373-384.
11. Branen, A. L., P. M. Davidson, and B. Katz. 1980. Antimicrobial properties of lipids and phenolic antioxidants. Food
Technol. 34:42-53.
12. Brock, T. D. 1961. Milestones in microbiology. Prentice-Hall,
Inc. Englewood Cliffs, N.J. 273 p.
13. Chan, L., R. Weaver, and C. S. Ough. 1975. Microbial inhibition
caused by p-hydroxybenzoate esters in wine. Amer. J. Enol.
Viticulture 26:201-207.
14. Chang, H. C.. and A. L. Branen. 1975. Antimicrobial effects of
butylated hydroxyanisole (BHA). J. Food Sci. 40:349-351.
15. Chichester, D. F., and F. W. Tanner. 1972. Antimicrobial food
additives. pp. 115-184. In T. E. Furia (ed.) Handbook of food
additives. CRC Press, Cleveland, OH.
16. Cliver, D. 0., and K. D. Kostenbader. 1979. Antiviral effectiveness of grape juice. J. Food Prot. 42:100-104.
17. Clydesdale, F. M., and F. J. Francis. 1976. Pigments. pp. 385-426.
In 0. R. Fennema (ed.) Principles of food science. Part I. Food
chemistry. Marcel Dekker, Inc. New York.
18. Code of Federal Regulations. 1977. Title 21-Food and Drugs,
Parts 100-199. General Services Admin. Washington, D.C. 832 p.
19. Cupp, J., P. Wanda, A. Keith, and W. Snipes. 1975. Inactivation
of the lipid-containing bacteriophage PM2 by butylated hydroxytoluene. Antimicrobial Agents Chemother. 8:698-706.
20. Davidson, P. M ., and A. L. Branen. 1980. Inhibition of two
psychrotrophic Pseudomonas species by butylated hydroxyanisole.
J. Food Sci. 45:1603-1606.
21. Davidson, P. M.. and A. L. Branen. 1980. Antimicrobial
mechanisms ofbutylated hydroxyanisole against two Pseudomonas
species. J. Food Sci. 45:1607-1613.
22. Davidson, P. M .. C. J. Brekke, and A. L. Branen. 1980. Anti-
JOURNAL OF FOOD PROTECTION, VOL. 44. AUGUST 1981
632
DAVIDSON AND BRAN EN
microbial acuvtty of butylatcd hydroxyanisole, tertiary butylhydroquinone and potassium sorbate used in combination. 1. Food
Sci. (ln press).
23. DeSmet, M. J., J. Kingma, and B. Witholt. 1978. The effect of
toluene on the structure and permeability of the outer and cytoplasmic membranes of Escherichia coli. Biochim. Biophys. Acta
506:64-80.
24. Eletr, S., M. A. Williams, T. Watkins, and A. D. Keith. 1974.
Perturbations of the dynamics of lipid alkyl chains in membrane
systems: effect on the activity of membrane-bound enzymes.
Biochim. Biophys. Acta 339:190-201.
25. Erickson, D. R., and R. B. Tompkin. 1977. Antimicrobial and
antirancidity agent. U.S. Patent 4,044,160. Aug. 23, 1977.
26. Exterkate, F. A. 1979. Effect of membrane perturbing treatments
on the membrane-bound peptidases of Streptococcus cremoris
HP. J. Dairy Res.46:473-484.
27. Fleming, H. P .. and J. L. Etchells. 1967. Occurrence of an
inhibitor of lactic acid bacteria in green olives. Appl. Microbial.
15:1178-1184.
28. Fleming, H. P., W. M. Walter, and J. L. Etchells. 1969. Isolation
of a bacterial inhibitor from green olives. Appl. Microbial.
18:856-860.
29. Fogg, A. H., and R. M. Lodge. 1945. The mode of antibacterial
action of phenols in relation to drug fastness. Trans. Faraday Soc.
41:359-365.
30. Frazier, W. C., and D. C. Westoff. 1978. Food microbiology.
McGraw-Hill Book Co. New York. p. 163.
31. Freese, E., C. W. Sheu, and E. Galliers. 1973. Function of
lipophilic acids as antimicrobial food additives. Nature 241:
321-325.
32. Fung, D. Y. C., S. Taylor, and J. Kahan. 1977. Effects of
butylated hydroxyanisole (BHA) and butylated hydroxytoluene
(BHT) on growth and aflatoxin production of Aspergillus flavus
J. Food Safety 1:39-51.
33. Grodzinska-Zachwieja, Z., and W. Kahl. 1966. Bacteriostatic
action of chicory (Cichorium intybus L.) H. The action of chlorogenic and isochlorogenic add. Acta Bioi. Crac. Ser. Bot. 9:87-95.
34. Grodzinska-Zachwieja. Z., I. Zgomiak-Nowosielska, M. Marciszewska, and A. Gatkiewicz. 1976. Antiviral activity of caffeic
acid in vitro studies. Acta Bioi. Crac. Ser. Bot. 19:29-37.
35. Harbome, J. B., and J. J. Comer. 1961. Plant polyphenols 4.
Hydroxycinnamic acid-sugar derivatives. Biochem. J. 81:242.
36. Harbome, J. B.. and N. W. Simmonds. 1964. The natural
distribution of the phenolic aglycones. pp. 77-127. In J. B.
Harborne (ed.) Biochemistry of phenolic compounds. Academic
Press. London.
37. Judis. J. 1963. Studies on the mechanism of action of phenolic
disinfectants. II. Patterns of release of radioactivity from
Escherichia coli labeled by growth on various compounds. J.
Pharm. Sci. 52:126-131.
38. Jurd. L., A. D. King, K. Mihara, and W. L. Stanley. 1971. Antimicrobial properties of natural phenols and related compounds. I.
Obtusastyrene. Appl. Micro bioi. 21:507-510.
39. Juven. B.. Z. Samish, and Y. Henis. 1968. Identification of
oleuropein as a natural inhibitor of lactic fermentation of green
olives. Israel J. Agric. Res. 18:137-138.
40. Juven, B., and Y. Henis. 1970. Studies on the antimicrobial
activity of olive phenolic compounds. J. Appl. Bacterial. 33:721.
41. Juven, B., Y. Henis. and B. Jacoby. 1972. Studies on the
mechanism of the antimicrobial action of oleuropein. J. Appl.
Bacterial. 35:559-567.
42. Kaye. R. C., and S. G. Proudfoot. 1971. Interactions between
phosphatidyl-ethanolamine-monolayers and phenols in relation to
antibacterial activity. J. Ph arm. Pharmacal. 23(SuppLl:223S.
43. Kim. K. S., H. M. Moon. V. Sapienza, R. I. Carp, and R.
Pullarkat. 1978. Inactivation of cytomegalovirus and Semliki Forest
Virus by butylated hydroxytoluene. J. Infect. Dis. 138:91-94.
44. Klarmann, E. G .. and V. A. Shternov. 1936. Bactericidal value
of coal-tar disinfectants. Limitation of the B. typhosus phenol
coefficient as a measure. Ind. Eng. Chern. 8:369-372.
45. Klindworth, K. J.. P. M. Davidson. C. J. Brekke, and A. L.
Branen. 1979. Inhibition of Clostridium perfringens by butylated
hydroxyanisole. J. Food Sci. 44:564-567.
46. Konowalchuk, J., and J. I. Speirs. 1976. Antiviral activity of fruit
extracts. J. Food Sci. 41:1013-1017.
47. Konowalchuk, J., and J. I. Speirs. 1976. Virus inactivation by
grapes and wines. Appl. Environ. Microbial. 32:757-763.
48. Konowalchuk, J .. and J. I. Speirs. 1978. Antiviral effect of
commercial juices and beverages. Appl. Environ. Microbial. 35:
1219-1220.
49. Konowalchuk, J., and J. I. Speirs. 1978. Antiviral effect of apple
beverages. Appl. Environ. Microbial. 36:798-801.
SO. Lamanna, C .. M. R. Mallette. and L. Zimmerman. 1973. Basic
bacteriology: its biological and chemical background, 2nd ed.
Williams and Wilkins, Baltimore.
51. Leifertova, I., N. Hejtmankova, H. Hlava, J. Kudrnacova, and
F. Santavy. 1975. Antifungal and antibacterial effects of phenolic
substances: A study of the relationship between the biological
activity and the constitution of the investigated compounds. Acta
Univ. Palacki. Olomuc., Fac. Med. 74:83-101.
52. Maga, J. A. 1978. Simple phenol and phenolic compounds in
food flavor. Crit. Rev. Food Sci. Nutr. 10:323-372.
53. Martin, J. H., K. S. S. Chung, and L. Ogrosky. 1972. Inhibition
of growth of Alcaligenes viscolactis by some common food preservatives. J. Dairy Sci. 55:1179-1181.
54. Masquelier, J. 1959. The bactericidal action of certain phenolics
of grapes and wine. pp. 123-131. In J. W. Fairbairn (ed.). The
pharmacology of plant phenolics. Academic Press Inc., New York.
55. Moustafa. H. H .. and E. B. Collins. 1969. Effects of selected
food additives on growth of Pseudomonas fragi. J. Dairy Sci.
52:335-340.
56. Pelczar, M. J .. and R. D. Reid. 1972. Microbiology, Jrd ed.
McGraw-Hill Book Co. New York. 948 p.
57. Pierson, M.D .. L.A. Smoot, and K. R. Vantassell. 1980. Inhibition of Salmonella typhimurium and Staphylococcus aureus by
butylated hydroxyanisole and the propyl ester of p-hydroxybenzoic
acid. J. Food Prot. 43:191-194.
58. Powers, J. J.. D. Somaatmadja, D. E. Pratt. and M. K. Hamdy.
1960. Anthocyanins. II. Action of anthocyanin pigments and
related compounds on the growth of certain microorganisms. Food
Techno!. 14:626-632.
59. Pratt, D. E .. J. J. Powers. and D. Somaatmadja. 1960. Antho
cyanins. I. The influence of strawberry and grape anthocyanins on
the growth of certain bacteria. Food Res. 25:26-32.
60. Prindle, R. F .. and E. S. Wright. 1977. Phenolic compounds. p.
219-251. In S. S. Block (ed.) Disinfection, sterilization. and preservation. Lea and Febiger. Philadelphia.
61. Robach. M. C. and C. L Stateler. 1980. Inhibition of Staphy
/ococcus aureus by potassium sorbate in combination with sodium
chloride, tertiary butyl hydroquinone, butylated hydroxyanisole
or ethylenediamine tetracetic acid. J. Food Prot. 43:208-211.
62. Robach. M. C .. LA. Smoot, and M. E. Pier>on. 1977. Inhibition
of Vibrio parahaemolyticus 04: Kll by butylated hydroxyanisole
J. Food Prot. 40:549-551.
63. Robach. M. C .. and M. D. Pierson. 1978. Int1uence of parahydroxybenzoic acid esters on the growth and toxin production of
Clostridium botulinum 10755A. J. Food Sci. 43:787-789.
64. Robach. M. C., and M.D. Pierson. 1979. Inhibition of Clostridium
botulinum Types A and B by phenolic antioxidants. J. Food Prot.
42:858-861.
65. Roherts. M. H .. and 0. Rahn. 1946. The amount of enzyme
inactivation at bacteriostatic and bactericidal concentrations of
disinfectants. J. BacteriaL 52:639-644.
66. Schanderl. H. 1962. Der Eint1uss von Polyphenolen und
Gerbstoffen auf die Physiologic der Weinhefe und der Wert des
pH
7 Testes fi.ir die Auswahl von Sektgrundweinen. Mitt.
(Klosterneuburg) l2A:265-274.
tl 7 Schraufstatter. E. 1948. Die bakteriostatische Wirkung von
Chalkon, Flavanon, Flavon und Flavonol. Experientia 4:484-485.
68. Sgaragli, G .. L Della Corte. M. Rizzotti Conti, and A. Giotti.
con 't. p. 64 7
JOURNAL OF FOOD PROTECTION. VOL. 44. AUGUST 1981
DESIGNING FOOD PRESERVATIVE SYSTEMS
Staphylococcus aureus, Clostridium peifringens, and C.
botulinum in cooked, uncured sausage. Appl. Microbiol.
28:262-264.
101. Trelease, R. D. 1976. Retardation of oxidation and microbial
growth in foods. U.S. Patent 3,955,005. May 4.
102. Vaughn, R. H., and L.O. Emard. 1951. Selectivity of sorbic acid
media for catalase negative lactic acid bacteria and obligate
sporulating anaerobes. Bacterial Abstr. p. 38.
103. Wanda, P .. I. Cupp, W. Snipes, A. Keith, T. Rucinsky, L. Polish,
and J. Sands. 1976. Inactivation ofthe enveloped bacteriophage
06 by butylated hydroxytoluene and butylated hydroxyanisole.
Antimicrobial Agents Chemother.10:96-101.
Sofas and Busta,
Davidson and Branen,
70.
71.
72.
7.3.
74.
75.
76.
77
'78.
79.
104.
Ward, M.S., and B. Q. Ward. 1967. Initial evaluation of the
effect of butylated hydroxytoluene upon Salmonella sentifenbert
775W. Poultry Sci. 46:1601-1606.
105. Werner, H. 1920. Neure Anschauungen auf dem Gebiete der
anorganischen Chemie, 4th ed. Friedrich and Sohn, Brunswick.
p.44.
106. Wyss, 0., B. J. Ludwig, and R. R. Joimer. 1945. The fungistatic
and fungicidal action of fatty acids and related compounds.
Arch. Biochem. 7:415-425.
107. York, G.K., and R. H. Vaughn. 1954. Use of sorbic acid
enrichment media for species of clostridia. I. Bacteriol.
68:739-744.
con't.fromp. 622
1975. The preservative effects of sorbic acid for fish sausage. J.
Food Hyg. Soc. Japan 17:95-100.
111. Warth, A. D. 1977. Mechanism of resistance of Saccharomyces
bailii to benzoic, sorbic and other weak acids used as food
preservatives. I. Appl. Bacteriol. 43:215-230.
112. Whitaker, I. R.1959. Inhibition of sulfhydryl enzymes with sorbic
acid. Food Res. 24:37-43.
113. York, G. K., and R. H. Vaughn. 1954. Use of sorbic acid
enrichment media for species of Clostridium. J. Bacterial. 68:
739-744.
69.
647
114. York, G. K., and R. H. Vaughn.1955. Resistance of Clostridium
parabotulinum to sorbic acid. Food Res. 20:60-65.
115. York, G. K., and R. H. Vaughn. 1955. Site of microbial inhibi
tion by sorbic acid. Bacterial. Proc. 20.
116. York, G. K., and R. H. Vaughn. 1960. Studies on microbial
inhibition by sorbic acid. Bacterial. Proc. pp. 4 7-48.
117. York, G. K., and R. H. Vaughn. 1964. Mechanisms in the inhibition of microorganisms by sorbic acid. J. Bacterial. 88:411-417.
con't.from P 632
1977. Effects of monocyclic compounds on biomembranes.
Biochem. Pharmacol. 26:2145-2149.
Shih, A. L., and N. D. Harris. 1977. Antimicrobial activity of
selected antioxidants. J. Food Prot. 40:520-522.
Sikovec, S. 1966. Der Einfluss einiger Polyphenole auf die
Physioiogie von Weinhefen. I. Der Einfluss von Polyphenolen auf
den Verlauf der alkoholischen Giirung insbesondere von
Emgarungen. Mitt. (Kosterneuberg). 16:127-138.
Sikovec, S. 1966. Der Einfluss von Polyphenolen auf die
Physiologic von Weinhefen. II. Der Einfluss von Polyphenolen
auf die Vermehrung und Atmung von Hefen. Mitt. {Klosterneuberg) 16:272-281.
Singer, M., and J. Wan. 1977. Interaction of butylated hydroxytoluene (BHT) with phospholipid bilayer membranes: EtTect on
22 Na permeability and membrane fluidity. Biochem. Pharmacal.
26:2259-2268.
Singleton, V. L., and P. Esau. 1969. Phenolic substances in grapes
and wine, and their significance. Academic Press. New York.
Snipes, W .. S. Person, A. Keith, and J. Cupp. 1975. Butylated
hydroxytoluene inactivates lipid-containing viruses. Science 188:
64-66.
Snipes. W .. and A. Keith. 1978. Hydrophobic alcohols and ditert-butyl phenols as antiviral agents. pp. 63-73. In J. J. Kabara
(ed.) Symposium on the pharmacological effect of lipids. Amer.
Oil Chern. Soc. Champaign, IL.
Sofos, J. N.. and F. F. Busta. 1980. Alternatives to the use of
nitrite as an amibotulinal agent. Food Techno!. 34:244-251.
Sokol. H. 1952. Recent developments in the preservation of
pharmaceuticals. Drug. Stand .. 20:89-106.
Sondheimer, E. 1958. On the distribution of caffeic acid and the
chlorogenic acid isomers in plants. Arch. Biochem. 74:131.
Stern. N.J., L.A. Smoot, and M.D. Pierson. 1979. Inhibition of
Staphylococcus aureus growth by combinations of butylated
hydroxy anisole, sodium chloride, and pH. J. Food Sci. 44:710 712.
80. Surak, J. G., R. L. Bradley, A. L. Branen, and E. Shrago. 1976.
Effects of butylated hydroxyanisole on Tetrahymena pynJormis.
Food Cosmet. Toxicol. 14:277-281.
81. Surak, J. G., R. L. Bradley, A. L. Branen, E. Shrago, and W. E.
Ribelin. 1976. Effect ofbutylated hydroxytoluene on Tetrahymena
pyriformis. Food Cosmet. Toxicol. 14:541.
82. Surak, J. G. 1977. Monotertiary butyl hydroquinone effects on
Tetrahymena pyriformis. Life Sci. 20:1735.
83. Suter, C. M. 1941. Relationships between the structures and
bactericidal properties of phenols. Chern. Rev. 28:269-299.
84. Trelease, R. D., and R. B. Tompkin. 1976. Retardation of
oxidation and microbial growth in foods. U.S. Patent 3,955,005.
85. Turbovsky, M. W., F. Filipello, W. V. Creuss, andP. Esau.l934.
Observations on the use of tannin in wine making. Fruit Prod. J.
14:106, 121, 123.
86. Valle, E. 1957. On anti-fungal factors in potato leaves. Acta Chern.
Scand. 11 :395.
87. Vardaman, T. H .. I. D. May, and J. H. Drott. 1978. Studies on
the effect of butylated hydroxytoluene on Mycoplasma synoviae.
Poultry Sci. 57:1526-1529.
88. Vas, K. 1953. Mechanism of antimicrobial action. Interference
with the cytoplasmic membrane. Agrokemia es Talajtan 2:1-16.
!Chern. Abstr. 48:794d, 1954.)
89. von Schelhorn, M. 1953. Efficacy and specificity of chemical food
preservatives. Food Techno!. 7:97-101.
90. Wanda, P., J. Cupp, W. Snipes. A. Keith, T. Rucinsky, L. Polish,
and J. Sands. 1976. Inactivation of the enveloped bacteriophage
06 by butylated hydroxytoluene and butylated hydroxyanisole.
Antimicrobial Agents Chemother. 10:96-101.
91. Ward, M. S., and B. Q. Ward. 1967. Initial evaluation of the
effect of butylated hydroxytoluene upon Salmonella senftenberg
775W, Poultry Sci. 46:1601.
92. Woldringh, C. L.l973. Effects oftoluene and phenethyl alcohol on
the ultrastructure of Escherichia coli. J. Bacteriol. 114:1359-1361.
JOURNAL OF FOOD PROIECTJON. VOL.44.AUGUST 1981
Você também pode gostar
- 2011 Hunyadi Et Al PLoS ONEDocumento10 páginas2011 Hunyadi Et Al PLoS ONENoemiAinda não há avaliações
- 2008 Tóth Et Al J.Nat - Prod. 26-OH SilvirDocumento3 páginas2008 Tóth Et Al J.Nat - Prod. 26-OH SilvirNoemiAinda não há avaliações
- Jurand 1973Documento10 páginasJurand 1973NoemiAinda não há avaliações
- Rosenkrantz 1988Documento12 páginasRosenkrantz 1988NoemiAinda não há avaliações
- 2023 Tóth Et Al Minor Ecdy BBB JNatProdDocumento7 páginas2023 Tóth Et Al Minor Ecdy BBB JNatProdNoemiAinda não há avaliações
- 2012 Martins Et Al J.Med - Chem.Documento10 páginas2012 Martins Et Al J.Med - Chem.NoemiAinda não há avaliações
- Lewis 2004Documento162 páginasLewis 2004NoemiAinda não há avaliações
- Geber 1975Documento9 páginasGeber 1975NoemiAinda não há avaliações
- Antibody Response To Sars-Cov-2 Infection in Humans: A Systematic ReviewDocumento27 páginasAntibody Response To Sars-Cov-2 Infection in Humans: A Systematic ReviewNoemiAinda não há avaliações
- Science of The Total Environment: Alfonso BalmoriDocumento5 páginasScience of The Total Environment: Alfonso BalmoriNoemiAinda não há avaliações
- Finnigan 1948Documento8 páginasFinnigan 1948NoemiAinda não há avaliações
- Electromagnetic Pollution Alert: Microwave Radiation and Absorption in Human Organs and TissuesDocumento19 páginasElectromagnetic Pollution Alert: Microwave Radiation and Absorption in Human Organs and TissuesNoemiAinda não há avaliações
- Ac and VaccineDocumento15 páginasAc and VaccineMayaLunaAinda não há avaliações
- Short CommunicationDocumento4 páginasShort CommunicationNoemiAinda não há avaliações
- Amberlite Irp64Documento5 páginasAmberlite Irp64NoemiAinda não há avaliações
- Hypoxia-Induced Coronary Flow Changes in The Perfused Rat Heart: Effects of High L-Carnitine ConcentrationsDocumento5 páginasHypoxia-Induced Coronary Flow Changes in The Perfused Rat Heart: Effects of High L-Carnitine ConcentrationsNoemiAinda não há avaliações
- Acetyl - Carnitine Treatment in Minimal Hepatic EncephalopathyDocumento8 páginasAcetyl - Carnitine Treatment in Minimal Hepatic EncephalopathyNoemiAinda não há avaliações
- L-Carnitine in Omnivorous Diets Induces An Atherogenic Gut Microbial Pathway in HumansDocumento16 páginasL-Carnitine in Omnivorous Diets Induces An Atherogenic Gut Microbial Pathway in HumansNoemiAinda não há avaliações
- Process Design Special EditionDocumento105 páginasProcess Design Special EditionNoemiAinda não há avaliações
- Effect of Dietary Carnitine Isomers and 7-Butyrobetaine On L-Carnitine Biosynthesis and Metabolism in The Rat1-2Documento8 páginasEffect of Dietary Carnitine Isomers and 7-Butyrobetaine On L-Carnitine Biosynthesis and Metabolism in The Rat1-2NoemiAinda não há avaliações
- Ivermectin ToxicityDocumento8 páginasIvermectin ToxicityNoemiAinda não há avaliações
- Dietary Supplement GMP.2012Documento314 páginasDietary Supplement GMP.2012333cubi333100% (3)
- Ivermectin GABA - Binding SiteDocumento13 páginasIvermectin GABA - Binding SiteNoemiAinda não há avaliações
- Acrivastine StabilityDocumento8 páginasAcrivastine StabilityNoemiAinda não há avaliações
- IVT Cleaning Validation IV 0Documento87 páginasIVT Cleaning Validation IV 0Noemi100% (7)
- Mrsatreatment GuideDocumento2 páginasMrsatreatment GuideNoemiAinda não há avaliações
- Continuing Process Verification - 1Documento106 páginasContinuing Process Verification - 1Noemi100% (1)
- Continuing Process Verification - 1Documento106 páginasContinuing Process Verification - 1Noemi100% (1)
- Stability of Cyanocobalamine in Sugar Coated TabletsDocumento8 páginasStability of Cyanocobalamine in Sugar Coated TabletsNoemiAinda não há avaliações
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (399)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (266)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (121)
- Food Microbiology Lab ManualDocumento48 páginasFood Microbiology Lab ManualRaja Craze75% (8)
- 2315 1 4634 1 10 20160108Documento8 páginas2315 1 4634 1 10 20160108ayuAinda não há avaliações
- A Seminar On Microgansims of Public Health Importance in WaterDocumento40 páginasA Seminar On Microgansims of Public Health Importance in Waterjoe topeAinda não há avaliações
- Microbial Enhanced Oil Recovery: Aliya Yernazarova Gulzhan KaiyrmanovaDocumento24 páginasMicrobial Enhanced Oil Recovery: Aliya Yernazarova Gulzhan KaiyrmanovaSayed Afg HashimiAinda não há avaliações
- AP Biology Outline - Chapter 1Documento4 páginasAP Biology Outline - Chapter 1Omar LopezAinda não há avaliações
- BACTERIAL MORPHOLOGY AND ULTRASTRUCTURE - Dr. GuintoDocumento4 páginasBACTERIAL MORPHOLOGY AND ULTRASTRUCTURE - Dr. GuintoMonicaAinda não há avaliações
- Class 11 Cell The Unit of Life - Practice MCQsDocumento8 páginasClass 11 Cell The Unit of Life - Practice MCQsMohammed ajaz AHMEDAinda não há avaliações
- Morgavi 2012Documento18 páginasMorgavi 2012uusAinda não há avaliações
- Amoeba PDFDocumento5 páginasAmoeba PDFmanoj_rkl_07100% (1)
- 7 MRSA and Controling-12Documento12 páginas7 MRSA and Controling-12RavinderenPichanAinda não há avaliações
- Bi0 310 Bacteria Lab ReportDocumento11 páginasBi0 310 Bacteria Lab ReportChiletso PhiriAinda não há avaliações
- Surgical InfectionsDocumento310 páginasSurgical InfectionsOmar Ed ChavezAinda não há avaliações
- Press ReleaseBolsa Chica BacteriaDocumento2 páginasPress ReleaseBolsa Chica Bacteriasurfdad67% (3)
- 15 Vegan Recipes For ImmunityDocumento53 páginas15 Vegan Recipes For ImmunitySanja BarabaAinda não há avaliações
- Instant Download Ebook PDF Fighting Multidrug Resistance With Herbal Extracts Essential Oils and Their Components PDF ScribdDocumento42 páginasInstant Download Ebook PDF Fighting Multidrug Resistance With Herbal Extracts Essential Oils and Their Components PDF Scribdannie.kahn136100% (41)
- Bachelor of PharmacyDocumento28 páginasBachelor of Pharmacyimtiaj-shovon-510Ainda não há avaliações
- Bharti 1927Documento19 páginasBharti 1927vikasAinda não há avaliações
- Cave 74 01 PDFDocumento136 páginasCave 74 01 PDFalegria1975Ainda não há avaliações
- Cooling Instruction HandoutDocumento2 páginasCooling Instruction HandoutJosephAinda não há avaliações
- TleDocumento8 páginasTleCarole GonzalesAinda não há avaliações
- Manual of Clinical Microbiology 1Documento10 páginasManual of Clinical Microbiology 1Drashua Ashua0% (2)
- Test For Absence of Clostridium SpeciesDocumento4 páginasTest For Absence of Clostridium SpeciesLuisSanabriaSaavedraAinda não há avaliações
- Activity # 7 Simple StainingDocumento3 páginasActivity # 7 Simple StainingClaire DuranteAinda não há avaliações
- Artikel Keperawatan Luka Dengan MaduDocumento9 páginasArtikel Keperawatan Luka Dengan MaduAntonius Handy KristiawanAinda não há avaliações
- Review HDocumento62 páginasReview HStephanie Louisse Gallega HisoleAinda não há avaliações
- Food Safety - BacteriaDocumento18 páginasFood Safety - Bacteriaapi-265125200Ainda não há avaliações
- Pharmaceutical Microbiology: PHAR443BDocumento98 páginasPharmaceutical Microbiology: PHAR443BAlfrida Putri PuspitaAinda não há avaliações
- ENMANUEL MC 3 BSN1 Assignment No. 2 Prokaryotic Eukaryotic First TrinalDocumento6 páginasENMANUEL MC 3 BSN1 Assignment No. 2 Prokaryotic Eukaryotic First TrinalJason Carbon EnmanuelAinda não há avaliações
- Ostemyelitis by Pseudomonas Aeruginosa I PDFDocumento1 páginaOstemyelitis by Pseudomonas Aeruginosa I PDFJúlio SimõesAinda não há avaliações
- Lecture 14Documento19 páginasLecture 14DIMPHO ZILWAAinda não há avaliações