Escolar Documentos
Profissional Documentos
Cultura Documentos
Enviado por
Nikunj PatelDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Enviado por
Nikunj PatelDireitos autorais:
Formatos disponíveis
Chemistry & Biology Vol.
1 Issue 1
2015
Pyrolysis of waste tyres and future
Pundlik Shivaji Ware
In the world, India is the third largest producer, fourth largest consumer of natural
rubber and fifth largest consumer of synthetic rubber [1]. Indian Rubber Industry plays a
core sector role in the Indian national economy. Globally, it is estimated that 13.5 million
tonnes of tyres are scrapped every year; 40% of which come from emerging markets such as
China, India, South America, Southeast Asia, South Africa and Eastern Europe. In the US
alone, exports of waste tyres amounted to almost 140,000 tonnes/ year from 2002-2011.
Figure 1 shows that the latest estimates of the scrap tyre distribution in the world.
Fig.1. Scrap tyres per year [2]
It is found that annually, about a Million Tons ( about 10 Lakh Tons) of scrap tyres are
available in India, and this figure is increasing in leaps and bounds as the vehicle numbers in
the passenger, commercial and industrial sectors in India too are catching up to the Western
levels! The disposal of waste tyre has become a major environmental concern globally and
this can be attributed to the increase in automobile usage as well as population especially in
areas of large population and highly industrialized nations [4-8]. The problems caused by the
waste tyres is majorly because they are not biodegradable and can last for several decades if
no proper handling is carried out.
In the year 2011, in India, The Gujarat Pollution Control Board (GPCB) has issued closure
notices to 45 units in the city and district which manufactured oil from all types of waste
rubber due to complaints of air and odour pollution and cheaper version for the process of
pyrolysis [3].
According to the PPAC (Petroleum Planning & Analysis Cell), in financial year 2014 the India
produced ~37,800 TMT (thousand metric tons) of crude oil. The total consumption for the
1 | Pa g e
Chemistry & Biology Vol. 1 Issue 1
2015
year was 158,400 TMT. As a result, 77.6% of the requirement was fulfilled by imports. This
trend continues to date. In November 2014, Indias crude oil production only accounted
for 22% of its domestic consumption.
Fig.2. Indias domestic production and import demand (Source; USEnergy Information
Administration)
From figure 2 we can conclude that demand of crude oil increases on each year. Crude oil
Rate hike in international market indicate burden on Indian economy [figure 3].
Fig.3. World oil production (Source: Analysis, International eCHEM data, Boomberg, BP
energy review)
Can Waste Tyres will help Indian economy? Lets find the answer.
What are the Difficulties with the Waste Tyres?
Tyres are made of vulcanized rubbers (with styrene butadiene (SBR), natural rubber
(NR) and polybutadiene (BR), carbon black, steel, textile cord and little amount of
2 | Pa g e
Chemistry & Biology Vol. 1 Issue 1
2015
other additives [Table 1]. The toughness and resistance of the over material to
biological removal make their removal and reprocessing difficult.
Table 1: Components of truck Tyre [15]
Component
Proportion (%)
Natural Rubber
45
Synthetic Polymer
4
Carbon Black
22
Oil
6
Chemicals
4
Steel Wire
16.5
Others
2.5
Home to Mosquito breeders.
It cant burn or landfills due to pollution problems.
What are Market sectors for scrap tyres?
Fig.4. U.S. Scrap tyre Disposition (Source: Scrap Tire Markets in the United States 9th
Biennial Report)
Figure 4 shows that 52.8% tire derived fuel obtained from pyrolysis of scrap tyres. For the
India following opportunities can find.
Through pyrolysis:
3 | Pa g e
Chemistry & Biology Vol. 1 Issue 1
2015
Tyre-derived fuels (TFDs) have demanded as only end-market as fuels for
power cement kilns, paper mills and utility boilers.
High-grade char that can replace carbon blacks, a vital ingredient in rubber
products. The global demand for carbon blacks will grow to almost 4.3%
annually through 2013, according to a Freedonia report.
Tyres contain steel wires and the amount range of 10% to 15% of the total
tyre wastage. Valuable steel wires are pressed and sold to steel and scrap
dealers.
Non-Condensable gases arise during the pyrolysis process used as fuel.
The tyre-derived aggregate (TDA), according to a report by the US-based Rubber
Manufacturers Association (RMA), this sector occupied a market share of 10% during
its peak in the early 1990s. It currently still shows stability, especially with the
demand in civil engineering applications, such as road sub-grades and walls and
bridge backfills.
Recycling through grinding: Crumb is used in sports and play surfaces, brake linings,
landscaping mulch, carpet underlay, absorbents for wastes and shoe soles.
Recycling through de-vulcanization: Treating vulcanized rubber with heat or
chemicals can produce de-vulcanized rubber, which can be used to replace part of
the virgin material in automotive and cycle tyres, conveyor belts and footwear.
Recycling through microwave technology: Advance Molecular Agitation Technology
(AMAT) has developed a prototype using microwave technology. This breaks the
tyres into their original components. The steel is of grade a quality and can therefore
be sold for recovery; the carbon and oil are also reusable. The amount of emissions
produced is minimal.
Other uses of waste tyres:
Boat and dock fenders
Under road surfaces
Sports tracks
Weights on silage sheeting on farms
Crash barriers at motor racing circuits
Children's play surfaces and furniture
Protection for young plants and trees
Compost heap containers
Roof tiles
Noise control products
Structural support for earth walls
Motorway embankments
Artificial reefs and coastal defenses
4 | Pa g e
Chemistry & Biology Vol. 1 Issue 1
2015
What is Pyrolysis?
It is most widely used option for scrap tyre recycling in all countries (fig.4). Tyre pyrolysis is
the thermal breakdown in the absence of oxygen is presently getting renewed attention.
Various products are obtained during the tyre pyrolysis process such as: liquid residue (4550 wt %), steel; wire (10-15%), Solid char (30-35 wt %), and gases (5- 15 wt %) (fig.5). In the
pyrolysis process noncondensable organics like H2, H2S, CO, CO2, CH4, C2H4, C3H6 etc are
also present which gives the gaseous fraction. The little bit part of gas can be used as fuel in
the pyrolysis process.
Fig.5. Pyrolysis process
Types of Pyrolysis:
Based on Nature of Pyrolysis; C. Roy [16] et al have worked on vacuum pyrolysis of
automobile shredder residue. The process recovers around 27.7 wt % of the tyres as organic
liquids and 52.5 wt % as solid residue of which 14 wt.% are useful metals which can be
recovered and the rest can be used safely for land. W. J. Pelaez et al [20] have published
their results on flash vacuum pyrolysis. Flash pyrolysis of biomass particles has been carried
out and a report is published by Shurong Wang et al [17]. Fluidized bed pyrolysis have been
done by Shurong Wang et al[17] and P. T. Williams et al[21]. A review of fast pyrolysis of
biomass has been published by A. V. Bridgwater[18]. The process Based on Residence Type
are most commonly used vulcanized tire rubber is a styrene-butadiene-copolymer (SBR)
containing about 25 wt. % styrene [9]. A typical composition of tire rubber is: 60-65% SBR.
29-31% carbon black, 1.9-3.3% zinc oxide, 1.1-2.1 sulfur, - 2% extender oil, and - 0.7%
additives (wt.70. as received) [9-10].
5 | Pa g e
Chemistry & Biology Vol. 1 Issue 1
2015
In most cases, tire pyrolysis studies were performed under inert conditions [9, 10, 11].
Pyrolysis may also be carried out in mildly oxidizing atmospheres, such as steam and carbon
dioxide, to improve the quality of pyrolytic products [12-14].
Gas Analysis: - Gases produced from tire pyrolysis are mainly hydrogen, carbon dioxide,
carbon monoxide, Methane, ethane and butadiene, with lower concentrations of propane,
propene, butane and other hydrocarbon gases [9]. These gases have properties similar to
synthesis gas [22] as shown in table 2.
Table 2: Tyre pyrolysis gas composition [15]
0il Analysis: Physical properties of oil (Table 3) from tire pyrolysis has high density,
viscosity and carbon residue and gross calorific value is close to diesel; values. This indicates
pyrolysis oil is best option for diesel. The oils have high aromaticity and are considered
relatively good fuels [14]. The molecular weight range for the oils is up to 1600 with an
average molecular weight between 300 and 400 [9, 23].
Infrared analysis of the oils indicates the presence of alkanes, alkenes, ketones or
aldehydes, aromatic, polyaromatic and substituted aromatic groups [9, 23]. The derived oils
may also be, an important source of refined chemicals, because it has been reported that
they contain high concentrations of valuable chemical feedstocks, such as benzene, toluene
and xylene [9].
Carbon Residue Analysis:- The carbon residue could become a marketable product if its
properties were similar to those of manufactured carbons [24]. The simultaneous
production of valuable solid products and gaseous and/ or liquid fuels from what is currently
6 | Pa g e
Chemistry & Biology Vol. 1 Issue 1
2015
a waste material would make tire pyrolysis economical if a large supply is readily available.
This situation exists in many regions of the US.
Table 3: Physical properties of pyrolytic oil, gasoline and diesel [23]
Basically, there are two uses of tire chars: as reinforcing filler and as an adsorbent.
Commercial carbon black is usually used for filling polymers and vulcanizates. Use of tire
char as an end product for the tire and printing ink industries has been reported to be
unsatisfactory [9-11]. This is due to the high ash content of tire char. Chars from tire
pyrolysis contain as much as 15 wt. of ash, with the majority of this ash being zinc oxide.
A means of removing the ash from tire char is an important issue in the process of
producing useful carbon black from solid residue from waste tires [24]. An alternative
approach, which is advocated in the current study, is to use the solid residue to produce
activated carbon for which the ash content is less critical.
Conclusion:
Pyrolysis is only process for recycling the waste tyres. Use of 5th and 6th generation pyrolysis
plant pollution can be minimizing. Carbon black yield increases with decreasing pyrolysis
temperature and decreasing heating rate. High-quality carbon black can be made from the
liquid products, which are absolutely ash-free, and finely divided carbon can additionally be
obtained from the CO produced during char activation. Physical properties of tyre oil
indicate relatively good fuel. It is important to note that India can minimize load on crude oil
by using advance technology to produce the tyre oil.
Future scope and challenges:
7 | Pa g e
Chemistry & Biology Vol. 1 Issue 1
2015
Optimization of the process to achieve an economical and eco-friendly method.
Easy and more economical methods for removal of ash content from pyrolyzed
carbon black for their wide applications.
Different catalysts or modification of the catalysts can be used to obtain more yields
of the oil products.
Application of fractionating columns for separation of the oil fuel into different
fractions during pyrolysis.
Different kinetic methods and models can be used for designing a suitable reactor to
maximize the oil product.
References:
1. Indian Rubber Industry at a Glance. http:/ / allindiarubber.net
2. Recycling of tyres. www.tifac.org.in
3. The Times of India, Oct 12, 2011.
4. Mazloom G, Farhadi F, Khorasheh F.(2009) Kinetic modeling of pyrolysis of scrap
tires. J. Anal. Appl. Pyrolysis; 84:15764.
5. Cheung K-Y, Lee K-L, Lam K-L, Lee C-W, Hui C-W.(2011). Integrated kinetics and heat
flow modelling to optimise waste tyre pyrolysis at different heating rates. Fuel
Process. Technol.
6. Senneca O, Salatino P, Chirone R.(1999) Fast heating-rate thermogravimetric study
of the pyrolysis of scrap tyres. Fuel; 78:157581.
7. Leung DYC, Wang CL.(1998) Kinetic study of scrap tyre pyrolysis and combustion. J.
Anal. Appl. Pyrolysis; 45:15369.
8. Roy C, Unsworth J. Pyrolysis and gasication. In: Ferrero GL, Maniatis K, Buekens A,
Bridgewater A,(1989) editors. London: Elsevier Applied Science. p. 180 189.
9. Williams, P.T.. Besler, S., and Ta lor D T , W 69. 1474 (1 990).
10. Ogasawara. S.. Kuroda. M., and wakao, N., Ind. Eng. Chem, Res. 26,2552 (1987).
11. Petrich, M.A., "Conversion of Plastic Waste to Valuable Solid Carbons", Final Report
of a Project in the Innovative Concepts Program, US. DOE, Jan., 1991.
12. Torikai, N., Meguro, T., and Nakamura, Y. Nippon Kagaku Kaishi 11, 1604 (1 979).
13. Funazukuri, T., Takanashi, T., and W a k a o N ,J. of chem. Eng Japan : 20 ,23 (1987)
14. Merchant, A. and Torkelson, J.M., "Pyrolysis of Scrap Tires , Chemical Lngineering
Dept., Northwestern U., Spring, 1990.
15. Sharma, A., and Murugan, S., 2013. \ Investigation on the behaviour of a di diesel
engine fueled with jatropha methyl ester (jme) and tyre pyrolysis oil (tpo) blends".
Process Safety and Environmental Protection, 108, June, pp. 699-708.
16. Roy, C., and Chaala, A., 2001. \ Vacuum pyrolysis of automobile shredder residues".
Resources, Conservation and Recycling, 32(1), May, pp. 1-27.
17. Wang, S., Fang, M., Yu, C., Luo, Z., and Cen, K., 2005. \ Flash pyrolysis of biomass
particles in uidized bed for bio-oil production". China Particuology, 3(1-2), April, pp.
136-140.
8 | Pa g e
Chemistry & Biology Vol. 1 Issue 1
2015
18. Bridgwater, A. V., 2012. \ Review of fast pyrolysis of biomass and product upgrading".
Biomass and Bioenergy, 38, March, pp. 68-94.
19. Rodriguez, I. M., and Laresgoiti, M. F., 2001. \ Pyrolysis of scrap tyres". Fuel
Processing Technology, 72(1), August, pp. 9-22.
20. Pel_aez, W. J., Szakonyi, Z., Fulop, F., and Yranzo, G. I., 2008. \ Flash vacuum pyrolysis
(fvp) of some hexahydroquinazolin-4(1h)-ones". Tetrahedron, 64(6), February, pp.
1049-1057.
21. Williams, P. T., and Brindle, A. J., 2002. \ Fluidised bed catalytic pyrolysis of scrap
tyres: Inuence of catalyst: tyre ratio and catalyst temperature". Waste Management
and Research, 20(6), December, pp. 546-555.
22. M. Bajus, N. Olahova, Thermal Conversion of Scrap Tyres, Petroleum and Coal vol.
53, 98-105, 2011.
23. R.K. Singh, Debalaxmi Pradhan, Thesis entitle Recovery of value added fuels from
waste polyolefins/ bicycle tyre and tube, 2011.
24. Pundlik Ware et al. Int. J. Res. Chem. Environ. Vol.3 Issue 1 January 2013(208-212)
9 | Pa g e
Você também pode gostar
- StatusDocumento5 páginasStatusNikunj PatelAinda não há avaliações
- FNFDocumento3 páginasFNFNikunj PatelAinda não há avaliações
- Investor Behavior Towards Investment OptionsDocumento2 páginasInvestor Behavior Towards Investment OptionsNikunj PatelAinda não há avaliações
- Pyrolysis of Waste Tyres for Future FuelsDocumento1 páginaPyrolysis of Waste Tyres for Future FuelsNikunj PatelAinda não há avaliações
- Low Price-To-Book-Value Stocks: Finding The Winners AmongDocumento4 páginasLow Price-To-Book-Value Stocks: Finding The Winners AmongNikunj PatelAinda não há avaliações
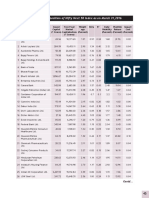
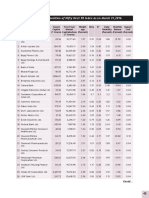
- Nifty 50 PDFDocumento2 páginasNifty 50 PDFNikunj PatelAinda não há avaliações
- Pyrolysis of Waste Tyres for Future FuelsDocumento1 páginaPyrolysis of Waste Tyres for Future FuelsNikunj PatelAinda não há avaliações
- Nifty 50Documento2 páginasNifty 50Nikunj PatelAinda não há avaliações
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (119)
- Type 2 Diabetes MellitusDocumento6 páginasType 2 Diabetes MellitusJoy NisoladaAinda não há avaliações
- Baby NamesDocumento9 páginasBaby Namesppremamca_617705407Ainda não há avaliações
- Service Manual: EQ1030T47D-820 Light Commercial TruckDocumento175 páginasService Manual: EQ1030T47D-820 Light Commercial TruckYonny ColqueAinda não há avaliações
- Great Gatsby Study NotesDocumento69 páginasGreat Gatsby Study NotesLara Westwood100% (2)
- Pre Test and Post TestDocumento27 páginasPre Test and Post TestMATALANG GRACEAinda não há avaliações
- PCS PADDLE SHIFTER INSTALL GUIDEDocumento21 páginasPCS PADDLE SHIFTER INSTALL GUIDEAndreas T P ManurungAinda não há avaliações
- S10 Electric Power PackDocumento12 páginasS10 Electric Power PackrolandAinda não há avaliações
- Classification of Placenta PDFDocumento5 páginasClassification of Placenta PDFAdarsh jainAinda não há avaliações
- Variants of NormalDocumento9 páginasVariants of NormalFaizah HannyAinda não há avaliações
- Mercedes ManDocumento7 páginasMercedes Manien yeyenAinda não há avaliações
- Nest Installation GuideDocumento8 páginasNest Installation GuideOzzyAinda não há avaliações
- Kelas 1 AlphabetTITLE Kelas 1 Numbers ConversationTITLE Kelas 2 Feelings Body PartsDocumento54 páginasKelas 1 AlphabetTITLE Kelas 1 Numbers ConversationTITLE Kelas 2 Feelings Body PartsArti Hikmatullah Perbawana Sakti BuanaAinda não há avaliações
- Narayana Sukta MeaningDocumento4 páginasNarayana Sukta Meaningvinai.20Ainda não há avaliações
- CIVL-365 Tutorial 8 SolutionDocumento3 páginasCIVL-365 Tutorial 8 SolutionIvsAinda não há avaliações
- Abbey Pain Scale assessment toolDocumento2 páginasAbbey Pain Scale assessment toolMuhammad RezgiaAinda não há avaliações
- Diesel HatchbackDocumento14 páginasDiesel HatchbackloganathprasannaAinda não há avaliações
- TICSA - Diesel Uno Petroleos Guatemala (13.01.23)Documento1 páginaTICSA - Diesel Uno Petroleos Guatemala (13.01.23)Luis M LópezAinda não há avaliações
- Australian 9 Grade Physics Lesson 1Documento32 páginasAustralian 9 Grade Physics Lesson 1binoyrajcrAinda não há avaliações
- 5.case Study: Effects of Homeopathic Medicines in AdultsDocumento2 páginas5.case Study: Effects of Homeopathic Medicines in AdultsAMEEN ARTSAinda não há avaliações
- Limbah PabrikDocumento2 páginasLimbah Pabrikindar dewaAinda não há avaliações
- Matrix Analysis of Group Structure Reveals Key InsightsDocumento22 páginasMatrix Analysis of Group Structure Reveals Key InsightsMahnooranjumAinda não há avaliações
- SI44M 60H 80H-DeN1730-V12web DownloadedDocumento4 páginasSI44M 60H 80H-DeN1730-V12web DownloadedtauraimukumbaAinda não há avaliações
- Fe in Black TeaDocumento6 páginasFe in Black TeaHerni Nur AeniAinda não há avaliações
- Clay ShonkwilerDocumento9 páginasClay ShonkwilerJeoff Libo-onAinda não há avaliações
- Soal ReadingDocumento3 páginasSoal ReadingSendi PuspaAinda não há avaliações
- Introducing Inspira's: Managed Noc & Itoc ServicesDocumento2 páginasIntroducing Inspira's: Managed Noc & Itoc ServicesmahimaAinda não há avaliações
- Unit 3 Assignment - CompletedDocumento7 páginasUnit 3 Assignment - CompletedSu GarrawayAinda não há avaliações
- 1 s2.0 S2210803316300781 MainDocumento8 páginas1 s2.0 S2210803316300781 MainGilang Aji P. EmonAinda não há avaliações
- Nokia N97 User Guide: 9221217 Issue 2.0Documento76 páginasNokia N97 User Guide: 9221217 Issue 2.0Boris CavarAinda não há avaliações
- Life Below WaterDocumento10 páginasLife Below Watertrisha sobito0% (1)