Escolar Documentos
Profissional Documentos
Cultura Documentos
CD11b Expression
Enviado por
Tisha Patricia OedoyDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
CD11b Expression
Enviado por
Tisha Patricia OedoyDireitos autorais:
Formatos disponíveis
Cytometry (Communications in Clinical Cytometry) 46:243246 (2000)
Relationship Between Oxidative Burst Activity and
CD11b Expression in Neutrophils and Monocytes From
Healthy Individuals: Effects of Race and Gender
Muhammad Siddiqi,1 Zenaida C. Garcia,2 Dana S. Stein,2 Thomas N. Denny,2 and
Zoltan Spolarics1*
1
Department of Anatomy Cell Biology and Injury Sciences, New Jersey Medical School, Newark, New Jersey
2
Department of Pediatrics, New Jersey Medical School, Newark, New Jersey
Oxidative burst activity and the expression of adhesion molecules have been used as indicators of
leukocyte activation status. The aim of the study was to delineate the relationship of oxidative burst activity
and the expression of adhesion molecules in neutrophils and monocytes from a pool of healthy volunteers
(n 96). We also tested the potential role of gender and a racial background in the individual response
differences. Basal and phorbol myristate acetate (PMA)-stimulated oxidative burst and CD11b expression
were determined using dihydrorhodamine 123 and phycoerythrin (PE)-conjugated anti-CD11b monoclonal
antibodies. PMA markedly increased CD11b expression and cellular oxidant content in neutrophils and
monocytes in all samples. However, the responses showed considerable variability among individuals. A
positive correlation was observed between the responsiveness of neutrophils and monocytes in their basal
or PMA-stimulated CD11b expressions and PMA-stimulated oxidative burst activities. In contrast, no
correlation was found between the level of adhesion molecule expression and cellular oxidant content in
monocytes or neutrophils either under basal or under PMA-stimulated conditions. The reactivity of oxidative
burst (i.e., PMA-stimulated over basal) was significantly lower in neutrophils from African American males
compared with cells from African American females, white females, or white males. In contrast, reactivity
of monocytes was significantly elevated in white males compared with all other groups. These findings
indicate that leukocytes with a relatively high degree of adhesion molecule expression may display an
average or decreased oxidative burst activity, and vice versa. Our findings also indicate that ethnic
background may influence the oxidative burst activity in neutrophils and monocytes. This needs consideration in clinical studies utilizing healthy volunteers with mixed gender and ethnic backgrounds. Cytometry
(Comm. Clin. Cytometry) 46:243246, 2000. 2000 Wiley-Liss, Inc.
Key terms: neutrophils; monocytes; cell activation; flow cytometry; human
Determination of oxidative burst and the expression of
adhesion molecules by flow cytometry technique have
been widely used in the assessment of leukocyte functional status (1,2). It has been accepted that oxidative
burst activity and the expression of cell adhesion molecules parallel the activation status of neutrophils and macrophages. Elevated oxidative burst activity or CD11b expression indicates cell activation whereas decreased
responses reflect downregulated cellular functions. The
value of these tests has been well established in a variety
of disease processes. Marked differences have been observed in leukocyte CD11b expression (3 6) and oxidative burst response (79) between patients and healthy
volunteers. However, empirical observations have suggested that considerable variability exists among individuals in the degree of oxidative burst and CD11b expression in leukocytes. It is not known whether decreased (or
2000 Wiley-Liss, Inc.
augmented) responsiveness of oxidative burst is also manifested in a parallel decrease (or augmentation) in cell
adhesion molecule expression. Furthermore, whereas ample evidence indicates that gender and age have major
influences on leukocyte responses and on the host response to injury and infection (10 12), the potential influence of ethnic background has not been investigated.
Therefore, the aims of the study were to delineate the
relationship between oxidative burst activity and expression of the CD11b adhesion molecule in neutrophils and
monocytes from a representative pool of young healthy
*Correspondence to: Zoltan Spolarics, M.D., Ph.D., Department of
Anatomy, Cell Biology and Injury Sciences, UMDNJ- New Jersey Medical
School, 185 South Orange Avenue, Newark, NJ 07103.
E-mail: spolaric@umdnj.edu
Received 2 November 2000; Accepted 27 March 2001
244
SIDDIQI ET AL.
volunteers. Furthermore, we also aimed to delineate the
potential influence of ethnic background on individual
response differences. Basal and phorbol 12-myristate 13acetate (PMA)-stimulated oxidative burst and CD11b expression were determined in the same blood sample from
each individual. PMA was selected as a stimulatory agent
in order to minimize the effect of potential differences on
the surface receptor status among individuals. It is assumed that the PMA-induced response reflects the overall
activity of oxidative burst and cell adhesion expression in
both neutrophils and monocytes as it acts through the
direct activation of protein kinase C.
MATERIALS AND METHODS
Flow cytometry analyses were carried out in a centralized laboratory using a FACScan flow cytometer equipped
with a 488-nm laser, 530/30 and 585/42-nm band pass
filters, and a 650-nm long pass filter (Becton Dickinson
[BD] Biosciences, San Jose, CA). Instrument calibration
was performed daily employing Calibrite Beads (BD Biosciences). For each of the assays, target channel values
were initially set using Sphero beads (Spherotech, Libertville, IL). The channel value used was the mean of the
third brightest peak on the logarithmic scale. These target
channel values were adhered to throughout the study.
This study was approved by the Institutional Review
Board of the New Jersey Medical School (Newark, NJ).
Informed consent was obtained from all individuals who
participated in the study. Healthy volunteers were
screened for glucose 6 phosphate dehydrogenase deficiency (G6PD) as described earlier (13). G6PD deficiency
is a common genetic polymorphism, with a particularly
high frequency in the African American and Hispanic
populations (14). Volunteers with G6PD deficiency were
excluded from the study due to the potential effects of the
deficiency on leukocyte responses.
Initiation of cell incubations and acquisitions were carried out in a time-scattered fashion and incubation times
were strictly adhered to throughout the study. Cell-associated oxidant content was measured by using dihydrorhodamine 123 (DHR; Molecular Probes, Eugene, OR) as
previously described (1). Blood was collected in tubes
containing EDTA as anticoagulant. Whole blood was
treated with prewarmed hypotonic ammonium chloride
solution. Cells were sedimented and washed in Hanks
balanced salt solution (HBSS) containing 5 mM glucose.
Isolated white blood cells were incubated in the presence
of 1 M DHR for 10 min, followed by incubation with
10 90 ng/ml PMA or vehicle. Cell-associated fluorescence
was measured 20 min later using the 530/30-nm filter.
Determination of CD11b expression on neutrophils and
monocytes was performed as follows (2): 0.1 ml whole
blood was incubated at 37C for 15 min in the presence or
absence of 90 ng/ml PMA. Cells were incubated for 15
min at room temperature in the dark in the presence of
phycoerythrin (PE)-conjugated monoclonal antibodies
against CD11b. PE-conjugated nonspecific IgG served as a
control in parallel incubations. After lysing the red blood
cells, white blood cells were suspended in 2 ml HBSS and
FIG. 1. CD11b expression and oxidative burst in healthy volunteers.
Cell-associated fluorescence was determined in neutrophils and monocytes using PE-conjugated CD11b antibodies or isotypic IgG (top panels). Cell-associated oxidants (lower panels) were determined by using
DHR as described in the Materials and Methods section. Mean RFI
SEM, n 96.
washed once. Cells were fixed with 0.3 ml 1% formaldehyde and cell-associated fluorescence was measured using
the 582/42-nm filter.
The mean of the relative fluorescence intensity (RFI)
from logarithmically amplified data expressed as linear
value was recorded and compared.
Statistical Analysis
Statistical calculations were performed using JMP software (SAS Institute, Cary, NC). Differences in multiple
comparisons were tested by analysis of variance (ANOVA)
followed by the Tukey-Kramer test. Statistically significant
differences were concluded at P 0.05.
RESULTS AND DISCUSSION
Figure 1 depicts the mean response of CD11b expression in neutrophils and monocytes from 96 individuals.
PMA stimulation increased CD11b expression by an average of 20 and 8.5-fold in neutrophils and monocytes,
respectively (top panels). PMA administration resulted in a
dose-dependent increase in cellular oxidant content in
these cells (lower panels). The employed maximally stimulating PMA dose (90 ng/ml) increased the basal oxidant
content by approximately 120-fold in neutrophils and
7-fold in monocytes.
Figure 2 indicates that basal or PMA-stimulated levels of
oxidants and CD11b expression show considerable individual variability in neutrophils as well as in monocytes.
Also, there was no correlation between the degree of
OXIDATIVE BURST AND CD11B EXPRESSION
245
FIG. 2. Correlation between oxidative metabolism and CD11b expression in monocytes and neutrophils. AD: Relationships between oxidant
content and CD11b expression in neutrophils (A,C) and monocytes (B,D)
under basal (A,B) or PMA-stimulated (C,D) conditions. EH: Relationship
between monocyte and neutrophil responses testing CD11b expression
(E,F) or cellular oxidant content (G,H) under basal (E,G) or PMA-stimulated (F,H) conditions (N 96). Statistically significant correlations are
shown when present.
CD11b expression and cellular oxidant content under
basal (Fig. 2A,B) or PMA-stimulated (Fig. 2C,D) conditions.
These observations indicate that, in a particular individual,
a relatively high responsiveness of cell adhesion molecule
expression may be accompanied by a relatively normal or
even decreased responsiveness of oxidative burst activity,
and vice versa.
Figure 2EH depict the relationship between the responsiveness of neutrophils and monocytes within an
individual in the investigated group. A positive correlation
was observed between neutrophil and monocyte responses testing basal (Fig. 2E) or PMA-stimulated (Fig. 2F)
CD11b expression or after PMA-stimulated oxidative burst
activity (Fig. 2H). These data indicate that an augmented
responsiveness of monocyte oxidative metabolism or cell
adhesion property is accompanied by a similarly elevated
responsiveness of neutrophils in a particular individual.
The same set of data was also analyzed to test the
potential role of gender together with a racial background
FIG. 3. Effect of ethnic background and gender on the oxidative burst
response in neutrophils and monocytes. Following PMA stimulation,
oxidant levels in neutrophils (top panels) or monocytes (bottom panels)
were compared in cells from AAM, AAF, WM, and WF. Insets depict
values calculated as PMA stimulated over basal levels of oxidants (i.e.,
reactivity or fold increase). Stars indicate statistically significant differences compared with all the other groups. & depicts statistically
significant difference compared with WF.
in the observed individual differences in neutrophil and
monocyte responses. The analyzed pool of individuals
comprised 19 African American females (AAF), 19 white
females (WF), 32 African American males (AAM), and 26
white males (WM). Further subdivision of ethnic groups
was not feasible due to the low number of observations.
PMA-stimulated oxidative burst was significantly lower
in neutrophils (Fig. 3A) and monocytes (Fig. 3B) from
AAM compared with AAF, WF, and WM. The reactivity of
oxidative burst (i.e., PMA-stimulated over basal) in neutro-
246
SIDDIQI ET AL.
phils from AAM individuals was also markedly decreased
compared with cells from all the other groups (inset, Fig.
3A). In contrast, the reactivity of monocytes was significantly greater in WM compared with cells from the other
groups (inset, Fig. 3B). No statistically significant differences were observed in basal or PMA-stimulated CD11b
expression among the investigated gender or racial
groups. The employed pool of healthy volunteers was
relatively young (mean age 38.4 0.8; range 2258) and
age showed no statistically significant correlation with
oxidative burst activity or cell adhesion molecule expression.
Interaction between leukocytes and endothelial cells
during neutrophil or monocyte adhesion and transmigration triggers oxidative burst. Oxidative burst has been
shown to play an important role in phagocyte-mediated
endothelial injury and subsequent tissue damage in a variety of disease processes (1518). Elevated expression of
cell adhesion molecules results in an augmented recruitment of leukocytes to injury sites or target organs. The
degree of phagocyte-mediated tissue damage is dependent
on a balance between phagocyte activation (e.g., degree
of cell recruitment, release of reactive oxidant or nitrogen
species or proteinases) and the activity of compensatory
defensive mechanisms in the target cells and organs (e.g.,
antioxidant status, presence of anti-proteinases; 15,19). A
divergence between oxidative burst activity and the responsiveness of cell adhesion expression in a particular
individual may represent a built in protective mechanism against phagocyte-mediated tissue injury under
pathological conditions. Namely, an elevated responsiveness of cell adhesion property will not necessarily be
prone to phagocyte-mediated tissue injury unless this condition is accompanied by an increased responsiveness of
oxidative burst activity, and vice versa.
Our observations also indicate that, in addition to the
known effects of gender and age (10 12,20,21), a racial
background may also have an important influence in determining the oxidative burst activity in neutrophils and
monocytes. It remains to be elucidated if the observed
individual characteristics or the gender and race-dependent differences in oxidative burst activity have an impact
on the inflammatory response under pathological conditions. Because the representation of minority patients or a
particular gender may be disproportionally high in certain
hospitals (trauma centers, inner city hospitals), our study
suggests the importance of race and gender-matched selection of healthy volunteer controls for clinical investigations utilizing these flow cytometry assays.
ACKNOWLEDGMENTS
This work was supported by NIH grants, NIGMS
GM55005 (Z.S.) and 1 S10 RR 14753 (T.M.D.)
LITERATURE CITED
1. Vowells SJ, Sekhsaria S, Malech HL, Shalit M, Fleisher TA. Flow
cytometric analysis of the granulocyte respiratory burst: a comparison study of fluorescent probes. J Immunol Methods 1995;178:
89 97.
2. OGorman MR, McNally AC, Anderson DC, Myones BL. A rapid whole
blood lysis technique for the diagnosis of moderate or severe leukocyte adhesion deficiency (LAD). Ann N Y Acad Sci 1993;677:427
430.
3. Kassirer M, Zeltser D, Prochorov V, Schoenman G, Frimerman A,
Keren G, Shapira I, Miller H, Roth A, Arber N, Eldor A, Berliner S.
Increased expression of the CD11b/CD18 antigen on the surface of
peripheral white blood cells in patients with ischemic heart disease:
further evidence for smoldering inflammation in patients with atherosclerosis. Am Heart J 1999;138:555559.
4. Cocks RA, Chan TY. Alteration in leukocyte adhesion molecule expression following minor, moderate and major trauma. Eur J Emerg
Med 1997;4:193195.
5. Simon DI, Rao NK, Xu H, Wei Y, Majdic O, Ronne E, Kobzik L,
Chapman HA. Mac-1 (CD11b/CD18) and the urokinase receptor
(CD87) form a functional unit on monocytic cells. Blood 1996;88:
31853194.
6. Hogevold HE, Lyberg T, Kahler H, Reikeras O. Expression of beta-2integrins and L-selectin by leukocytes and changes in acute-phase
reactants in total hip replacement surgery. Eur Surg Res 1996;28:
190 200.
7. Rhee P, Wang D, Ruff P, Austin B, DeBraux S, Wolcott K, Burris D,
Ling G, Sun L. Human neutrophil activation and increased adhesion
by various resuscitation. Crit Care Med 2000;28:74 78.
8. Hauser CJ, Desai N, Fekete Z, Livingston DH, Deitch EA. Priming of
neutrophil [Ca2]i signaling and oxidative burst by human fracture
fluids. J Trauma 1999;47:854 858.
9. Shih HC, Su CH, Lee CH. Superoxide production of neutrophils after
severe injury: impact of subsequent surgery and sepsis. Am J Emerg
Med 1999;17:1518.
10. Spitzer JA. Gender differences in some host defense mechanisms.
Lupus 1999;8:380 383.
11. Alvarez E, Santa MC. Influence of the age and sex on respiratory burst
of human monocytes. Mech Ageing Dev 1996;90:157161.
12. Mallery SR, Zeligs BJ, Ramwell PW, Bellanti JA. Gender-related variations and interaction of human neutrophil cyclooxygenase and oxidative burst metabolites. J Leukoc Biol 1986;40:133146.
13. Beutler E, Kuhl W, Vives-Corrons JL, Prchal JT. Molecular heterogeneity of glucose-6-phosphate dehydrogenase A. Blood 1989;74:2550
2555.
14. Luzzatto L, Mehta A. Glucose 6 phosphate dehydrogenase deficiency.
In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic
and molecular bases of inherited disease. New York: McGrawHill;
1995. p 33673398.
15. Jaeschke H, Smith CW, Clemens MG, Ganey PE, Roth RA. Mechanisms of inflammatory liver injury: adhesion molecules and cytotoxicity of neutrophils. Toxicol Appl Pharmacol 1996;139:213226.
16. Kokura S, Wolf RE, Yoshikawa T, Granger DN, Aw TY. Molecular
mechanisms of neutrophil-endothelial cell adhesion induced by redox imbalance. Circ Res 1999;84:516 524.
17. Lentsch AB, Ward PA. Regulation of inflammatory vascular damage.
J Pathol 2000;190:343348.
18. Murphy HS, Warner RL, Bakopoulos N, Dame MK, Varani J, Ward PA.
Endothelial cell determinants of susceptibility to neutrophil-mediated
killing. Shock 1999;12:111117.
19. Spolarics Z. Endotoxemia, pentose cycle, and the oxidant/antioxidant
balance in the hepatic sinusoid. J Leukoc Biol 1998;63:534 541.
20. Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and
sex hormones on immune responses following shock. Shock 2000;
14:8190.
21. Kahlke V, Angele MK, Ayala A, Schwacha MG, Cioffi WG, Bland KI,
Chaudry IH. Immune dysfunction following trauma-haemorrhage:
influence of gender and age. Cytokine 2000;12:69 77.
Você também pode gostar
- A Novel, Highly Sensitive and Specific Biomarker For Niemann-Pick Type C1 DiseaseDocumento8 páginasA Novel, Highly Sensitive and Specific Biomarker For Niemann-Pick Type C1 DiseaseTheodora TeddyAinda não há avaliações
- In Vivo in VitroDocumento9 páginasIn Vivo in VitroBintuHurairahAinda não há avaliações
- Citosol (Thiamylal Sodium) Triggers Apoptosis and Affects Gene Expressions of Murine Leukemia RAW 264.7 Cells - RS-C Wu, C-S Yu, 2Documento1 páginaCitosol (Thiamylal Sodium) Triggers Apoptosis and Affects Gene Expressions of Murine Leukemia RAW 264.7 Cells - RS-C Wu, C-S Yu, 2Alondra MaldonadoAinda não há avaliações
- CLL and ERs Manuscript.Documento15 páginasCLL and ERs Manuscript.Nadeem khanAinda não há avaliações
- 2019 - High-Resolution Metabolomic Profiling of Alzheimer's PDFDocumento10 páginas2019 - High-Resolution Metabolomic Profiling of Alzheimer's PDFEva Sánchez YébenesAinda não há avaliações
- Evaluation of NK Cells in HCV Patients on HemodialysisDocumento13 páginasEvaluation of NK Cells in HCV Patients on HemodialysisMaged SaadAinda não há avaliações
- Changes in Fluorescence Intensity of SelectedDocumento7 páginasChanges in Fluorescence Intensity of SelectedNatiAinda não há avaliações
- Out 5Documento11 páginasOut 5Kie Amelia SuwantoAinda não há avaliações
- Endothelial Cell NADPH Oxidase Mediates The Cerebral Microvascular Dysfunction in Sickle Cell Transgenic MiceDocumento19 páginasEndothelial Cell NADPH Oxidase Mediates The Cerebral Microvascular Dysfunction in Sickle Cell Transgenic MiceNovia MentariAinda não há avaliações
- Flow Cytometric Analysis of Normal and Reactive SpleenDocumento10 páginasFlow Cytometric Analysis of Normal and Reactive SpleenmisterxAinda não há avaliações
- Role of oxidative stress in AGE-induced mesangial cell signalingDocumento9 páginasRole of oxidative stress in AGE-induced mesangial cell signalingMutiaraAinda não há avaliações
- Journal CancerDocumento17 páginasJournal CancerbeklogAinda não há avaliações
- Chloremphenicol Leukemia LinkedDocumento17 páginasChloremphenicol Leukemia LinkedpadbidriAinda não há avaliações
- Wang 2021Documento7 páginasWang 2021Portgas D AceAinda não há avaliações
- Coagulation Changes in Individuals With Sickle Cell TraitDocumento6 páginasCoagulation Changes in Individuals With Sickle Cell TraitMohaAinda não há avaliações
- 901 3161 1 PBDocumento6 páginas901 3161 1 PBdaeron42Ainda não há avaliações
- Nitric Oxide Attenuates Normal and Sickle Red Blood Cell Adherence To Pulmonary EndotheliumDocumento5 páginasNitric Oxide Attenuates Normal and Sickle Red Blood Cell Adherence To Pulmonary EndotheliumpippoAinda não há avaliações
- Nejmoa 2211023Documento10 páginasNejmoa 2211023EvelynAinda não há avaliações
- Anti-C1q Antibodies: Association With Nephritis and Disease Activity in Systemic Lupus ErythematosusDocumento5 páginasAnti-C1q Antibodies: Association With Nephritis and Disease Activity in Systemic Lupus ErythematosusAlisAinda não há avaliações
- Citometria 2005Documento8 páginasCitometria 2005Leslie AraujoAinda não há avaliações
- IMH - 100 - 3MT02 - Journal Critiqu PDFDocumento2 páginasIMH - 100 - 3MT02 - Journal Critiqu PDFAxel ZeroAinda não há avaliações
- Fluoxtine InducedDocumento7 páginasFluoxtine InducedsufaAinda não há avaliações
- Clinicomolecular Identification of Conserved Features of Granulomatous Uveitis.Documento13 páginasClinicomolecular Identification of Conserved Features of Granulomatous Uveitis.morelaAinda não há avaliações
- Inter-Cellular Nanovesicle Mediated MicroRNA TransferDocumento19 páginasInter-Cellular Nanovesicle Mediated MicroRNA Transferanon_579968442Ainda não há avaliações
- Transplant International Special Issue: Abstracts of The 14th Congress of The European Society For Organ Transplantation Volume 22, Issue Supplement s2, Pages 95-222, August 2009Documento128 páginasTransplant International Special Issue: Abstracts of The 14th Congress of The European Society For Organ Transplantation Volume 22, Issue Supplement s2, Pages 95-222, August 2009Enrique Moreno GonzálezAinda não há avaliações
- Cam40002 0234Documento9 páginasCam40002 0234Domenico LombardiniAinda não há avaliações
- Utilizing Targeted Gene Therapy With Nano Particles Binding Alpha V Beta 3 For Imaging and Treating Choroidal NeovascularizationDocumento9 páginasUtilizing Targeted Gene Therapy With Nano Particles Binding Alpha V Beta 3 For Imaging and Treating Choroidal Neovascularizationtennisboy92Ainda não há avaliações
- Euromedlab 2013 Abstracts 211 and 219Documento103 páginasEuromedlab 2013 Abstracts 211 and 219Jagannadha Rao PeelaAinda não há avaliações
- Hodgkin LyphomaDocumento6 páginasHodgkin LyphomaJose Luis PittauAinda não há avaliações
- Small Interfering RNA To ReduceDocumento10 páginasSmall Interfering RNA To ReduceMahmoud Abu MayalehAinda não há avaliações
- Rapid Publication: Sickle CellDocumento5 páginasRapid Publication: Sickle CellVibhav SinghAinda não há avaliações
- 2253Documento6 páginas2253Rani JeyaramanAinda não há avaliações
- Tmp966e TMPDocumento3 páginasTmp966e TMPFrontiersAinda não há avaliações
- tmpD126 TMPDocumento9 páginastmpD126 TMPFrontiersAinda não há avaliações
- Effect of Low Dose Quercetin and CancerDocumento21 páginasEffect of Low Dose Quercetin and CancerMetcher Maa AkhiAinda não há avaliações
- 66 68 PBDocumento89 páginas66 68 PBAyesha FatimaAinda não há avaliações
- Endothelial Dysfunction, Biomarkers of Renal Function SCDDocumento7 páginasEndothelial Dysfunction, Biomarkers of Renal Function SCDAnonymous 9QxPDpAinda não há avaliações
- The Influence of Combination NAC with Vitamin C and E on Oxidative Stress Markers in HUVECs Exposed to Eclampsia PlasmaDocumento8 páginasThe Influence of Combination NAC with Vitamin C and E on Oxidative Stress Markers in HUVECs Exposed to Eclampsia PlasmaPantas Saroha SiburianAinda não há avaliações
- 1078 0432.CCR 10 0735.fullDocumento29 páginas1078 0432.CCR 10 0735.fullNurul Huda KowitaAinda não há avaliações
- GBS variants exhibit distinct phenotypes and virulenceDocumento24 páginasGBS variants exhibit distinct phenotypes and virulencenurcameliaAinda não há avaliações
- Preparation, Biological Activity and Endogenous Occurrence of N - BenzyladenosinesDocumento11 páginasPreparation, Biological Activity and Endogenous Occurrence of N - BenzyladenosinesvkrystofAinda não há avaliações
- 10 1016@j Fertnstert 2010 07 284Documento1 página10 1016@j Fertnstert 2010 07 284Makoto HanamiyaAinda não há avaliações
- Clinical: Circadian Variation Subpopulations: Study With MonoclonalDocumento3 páginasClinical: Circadian Variation Subpopulations: Study With MonoclonaldocumentosdescribdAinda não há avaliações
- CytometryDocumento11 páginasCytometrymitkazAinda não há avaliações
- Ol 23 5 13275 PDFDocumento9 páginasOl 23 5 13275 PDFSRIBDE STANLEYAinda não há avaliações
- 3407 FullDocumento5 páginas3407 FullCyprien YEAinda não há avaliações
- Evidence of Immediate in Systemic Lupus: Hypersensitivity ErythematosusDocumento6 páginasEvidence of Immediate in Systemic Lupus: Hypersensitivity ErythematosusJanti Tri HabsariAinda não há avaliações
- Reactive Oxygen and Nitrogen Species in Sepsis-Induced Hepatic Microvascular DysfunctionDocumento10 páginasReactive Oxygen and Nitrogen Species in Sepsis-Induced Hepatic Microvascular DysfunctionGiosué Reyes HernandezAinda não há avaliações
- Pathogenesis of Preeclampsia: Implications of Apoptotic Markers and Oxidative StressDocumento10 páginasPathogenesis of Preeclampsia: Implications of Apoptotic Markers and Oxidative StressAchmad Deza FaristaAinda não há avaliações
- Jurnal EnglishDocumento14 páginasJurnal EnglishKunia Ote TrieAinda não há avaliações
- VeselyDocumento7 páginasVeselyhitherehiAinda não há avaliações
- Cisplatin Cell Cicle ArrestDocumento7 páginasCisplatin Cell Cicle ArrestGabriele KrauseAinda não há avaliações
- INCLUIDOS-clusianone and Cancer - Search Results - PubMedDocumento6 páginasINCLUIDOS-clusianone and Cancer - Search Results - PubMedLidiaAmorimAinda não há avaliações
- Dna LoadingDocumento6 páginasDna LoadingSameer SachdevaAinda não há avaliações
- Celecoxib Combined With Salirasib Strongly Inhibits Pancreatic Cancer Cells in 2D and 3D CulturesDocumento8 páginasCelecoxib Combined With Salirasib Strongly Inhibits Pancreatic Cancer Cells in 2D and 3D CulturesRoyAinda não há avaliações
- Quinn Et Al-2016-British Journal of HaematologyDocumento9 páginasQuinn Et Al-2016-British Journal of HaematologyRagul BiotechAinda não há avaliações
- Han DKK., 2012 PDFDocumento14 páginasHan DKK., 2012 PDFChichi FauziyahAinda não há avaliações
- Analysis of Detergent-Free Lipid Rafts Isolated From CD4 T Cell Line: Interaction With Antigen Presenting Cells Promotes Coalescing of Lipid RaftsDocumento13 páginasAnalysis of Detergent-Free Lipid Rafts Isolated From CD4 T Cell Line: Interaction With Antigen Presenting Cells Promotes Coalescing of Lipid RaftsHeather BenjaminAinda não há avaliações
- Sloane1981 - Science - CathepsinsDocumento4 páginasSloane1981 - Science - CathepsinsPilar AufrastoAinda não há avaliações
- AACR 2022 Proceedings: Part A Online-Only and April 10No EverandAACR 2022 Proceedings: Part A Online-Only and April 10Ainda não há avaliações
- From: Clinical and Laboratory Diagnosis of Dengue Virus InfectionDocumento1 páginaFrom: Clinical and Laboratory Diagnosis of Dengue Virus InfectionTisha Patricia OedoyAinda não há avaliações
- Clostridium difficile Infection Prevention GuideDocumento80 páginasClostridium difficile Infection Prevention GuideTisha Patricia OedoyAinda não há avaliações
- Monocytic Expression of CD14 and CD18, Circulating AdhesionDocumento5 páginasMonocytic Expression of CD14 and CD18, Circulating AdhesionTisha Patricia OedoyAinda não há avaliações
- Calibration Fundamentals ExplainedDocumento10 páginasCalibration Fundamentals ExplainedCris RonaldAinda não há avaliações
- Urea TietzDocumento8 páginasUrea TietzTisha Patricia OedoyAinda não há avaliações
- Cardiac Enzymes and Biomarkers Review - Troponin, CK and Myoglobin - LearntheheartDocumento2 páginasCardiac Enzymes and Biomarkers Review - Troponin, CK and Myoglobin - LearntheheartTisha Patricia OedoyAinda não há avaliações
- Whats20your20type20abo20discrepancies2015 9-25-15Documento53 páginasWhats20your20type20abo20discrepancies2015 9-25-15Tisha Patricia OedoyAinda não há avaliações
- 9 13 06 Siebers Elevated LFTs and Weight LossDocumento34 páginas9 13 06 Siebers Elevated LFTs and Weight LossTisha Patricia OedoyAinda não há avaliações
- Stability of Common Biochemical Analytes in Serum Gel Tubes Subjected To Various Storage Temperatures and Times Pre-CentrifugationDocumento5 páginasStability of Common Biochemical Analytes in Serum Gel Tubes Subjected To Various Storage Temperatures and Times Pre-CentrifugationTisha Patricia OedoyAinda não há avaliações
- IFCCstd HbA1CDocumento4 páginasIFCCstd HbA1CTisha Patricia OedoyAinda não há avaliações
- Archem HbA1cDocumento5 páginasArchem HbA1cTisha Patricia OedoyAinda não há avaliações
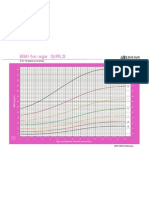
- Bmi For Age Z-ScoreDocumento1 páginaBmi For Age Z-ScoreTisha Patricia OedoyAinda não há avaliações
- Unpar Patofisiologi GatalDocumento14 páginasUnpar Patofisiologi GatalTisha Patricia OedoyAinda não há avaliações
- List of NGSP Certified MethodsDocumento17 páginasList of NGSP Certified MethodsTisha Patricia OedoyAinda não há avaliações
- Pediatric HistoryDocumento96 páginasPediatric Historyapi-3712326100% (2)
- Quantia HBA1C Insert KitDocumento1 páginaQuantia HBA1C Insert KitTisha Patricia OedoyAinda não há avaliações
- Diagnosing Foot Infection in DiabetesDocumento4 páginasDiagnosing Foot Infection in DiabetesTisha Patricia OedoyAinda não há avaliações
- Weight For Age Girls-Z ScoreDocumento1 páginaWeight For Age Girls-Z ScoreTisha Patricia OedoyAinda não há avaliações
- Bmi For Age Z-ScoreDocumento1 páginaBmi For Age Z-ScoreTisha Patricia OedoyAinda não há avaliações
- Z Score BMI 5-19yearsold GirlDocumento1 páginaZ Score BMI 5-19yearsold GirlVienny Widhyanti RosaryaAinda não há avaliações
- Management of Pulmonary EmbolismDocumento14 páginasManagement of Pulmonary EmbolismTisha Patricia OedoyAinda não há avaliações
- Introduction to Neural Network Applications in Image ProcessingDocumento12 páginasIntroduction to Neural Network Applications in Image ProcessingKornelius NdruruAinda não há avaliações
- Dwnload Full Medical Terminology For Health Professions 7th Edition Ehrlich Test Bank PDFDocumento36 páginasDwnload Full Medical Terminology For Health Professions 7th Edition Ehrlich Test Bank PDFmarchingpalmerbabg100% (9)
- 20-Advances in Cognitive TheoryDocumento24 páginas20-Advances in Cognitive TheoryAndrea ZeballosAinda não há avaliações
- Assignment AnswersDocumento2 páginasAssignment Answersxewoj59015Ainda não há avaliações
- Stress 2Documento4 páginasStress 2le thi lan anhAinda não há avaliações
- Prilabsa 2nd Workshop - MachalaDocumento67 páginasPrilabsa 2nd Workshop - MachalaJunior Esquivel TorresAinda não há avaliações
- Dna ExtracitionDocumento3 páginasDna Extracitionapi-374582387Ainda não há avaliações
- Nervous CoordinationDocumento36 páginasNervous CoordinationOlamide AyindeAinda não há avaliações
- Sterilization Validation GuideDocumento10 páginasSterilization Validation GuideVenkata Rama50% (2)
- Acanthostega ReconstructionDocumento14 páginasAcanthostega ReconstructionAndrew KirkAinda não há avaliações
- 28th July (Tuesday), 2015 Daily Global Rice E-Newsletter by Riceplus MagazineDocumento23 páginas28th July (Tuesday), 2015 Daily Global Rice E-Newsletter by Riceplus MagazineMujahid AliAinda não há avaliações
- Cepheids.: No. of Systematic Area Position Variables DeviationDocumento9 páginasCepheids.: No. of Systematic Area Position Variables DeviationLefrina GusrianiiAinda não há avaliações
- 12 Biology Notes Ch11 Biotechnology Principles and ProcessesDocumento9 páginas12 Biology Notes Ch11 Biotechnology Principles and ProcessesAnkit YadavAinda não há avaliações
- Mothership Character SheetDocumento2 páginasMothership Character SheetAlexander Dalton0% (1)
- Table of Specifications: Summative Test No. 1 in Science 10Documento1 páginaTable of Specifications: Summative Test No. 1 in Science 10Jannet FuentesAinda não há avaliações
- Prevention of Drowning: Visual Scanning and Attention Span in LifeguardsDocumento12 páginasPrevention of Drowning: Visual Scanning and Attention Span in LifeguardsQuique ParadaAinda não há avaliações
- Crossword Puzzles ScienceDocumento4 páginasCrossword Puzzles ScienceNorazah AhmadAinda não há avaliações
- Queen of Philippine Orchids - The Rare and Beautiful Waling-waling FlowerDocumento4 páginasQueen of Philippine Orchids - The Rare and Beautiful Waling-waling FlowerWendy BalaodAinda não há avaliações
- Thiemo Breyer (Eds.) - Epistemological Dimensions of Evolutionary Psychology-Springer-Verlag New York (2015)Documento248 páginasThiemo Breyer (Eds.) - Epistemological Dimensions of Evolutionary Psychology-Springer-Verlag New York (2015)LandoGuillénChávezAinda não há avaliações
- QTPPDocumento3 páginasQTPPRajesh Bhapkar100% (1)
- Fertigation For Vegetables: A Practical Guide For Small FieldsDocumento7 páginasFertigation For Vegetables: A Practical Guide For Small FieldsSophia DwiratnaAinda não há avaliações
- Cellular Memory and How It WorksDocumento8 páginasCellular Memory and How It Worksbiba 4life100% (1)
- MSU Campus Map and Building GuideDocumento3 páginasMSU Campus Map and Building GuidemindarsonAinda não há avaliações
- Misof Et Al 2014Documento6 páginasMisof Et Al 2014Grant Adams100% (1)
- Physical Basics of Informational InteractionDocumento45 páginasPhysical Basics of Informational Interactiondb1970100% (1)
- S5LT 11f 6Documento8 páginasS5LT 11f 6Kristine Joy PitaAinda não há avaliações
- Reading Explorer 4, Third Edition Additional Reading Practice Unit 1BDocumento29 páginasReading Explorer 4, Third Edition Additional Reading Practice Unit 1B응애Ainda não há avaliações
- 2019PSNLLB43642-648Syahdietal 1 PDFDocumento15 páginas2019PSNLLB43642-648Syahdietal 1 PDFAlina Vitali TabarceaAinda não há avaliações
- Klasifikasi NeoplasmaDocumento22 páginasKlasifikasi Neoplasmaoscar putraAinda não há avaliações
- Patty'S Industrial Hygiene: Fifth EditionDocumento11 páginasPatty'S Industrial Hygiene: Fifth EditionJorgeLucchesiAinda não há avaliações