Escolar Documentos
Profissional Documentos
Cultura Documentos
Chapter 2. Expt Iron
Enviado por
Louie Shaolin LungaoDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Chapter 2. Expt Iron
Enviado por
Louie Shaolin LungaoDireitos autorais:
Formatos disponíveis
CHAPTER 2
DESIGN AND METHODOLOGY
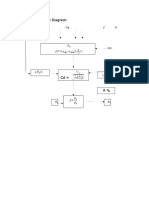
This experiment determined the iron content of our water sample, three trials were performed
and 15 ml of water sample for each trial were used for the experiment. Three 15 ml of water
sample
is then poured into clean and dry Erlenmeyer flask for titration. Sulfuric acid added to the three
Erlenmeyer flask with water sample with 3 molar concentration at exactly 8 ml of it and act as
indicator. Sulfuric acid and water sample were mixed. After mixing the mixture is then titrated
with a 0.1 molar concentration of potassium permanganate (KMnO4) solution. Light pink
obtained
which indicate that end point was achieved. We were able to compute the concentration of iron
content in the water sample by recording the potassium permanganate used.
Você também pode gostar
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- The Laplace TransformsDocumento61 páginasThe Laplace TransformsLouie Shaolin Lungao100% (1)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5795)
- Information Flow DiagramDocumento1 páginaInformation Flow DiagramLouie Shaolin LungaoAinda não há avaliações
- Chapter IV and V 2Documento2 páginasChapter IV and V 2Louie Shaolin LungaoAinda não há avaliações
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- Ilocanos: "Kuripot O Matipid?" #Ilocandia: Submitted ToDocumento21 páginasIlocanos: "Kuripot O Matipid?" #Ilocandia: Submitted ToLouie Shaolin LungaoAinda não há avaliações
- Case Study ChernobylDocumento4 páginasCase Study ChernobylLouie Shaolin LungaoAinda não há avaliações
- Information Flow DiagramDocumento1 páginaInformation Flow DiagramLouie Shaolin LungaoAinda não há avaliações
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Experiment 1 REPORTDocumento18 páginasExperiment 1 REPORTLouie Shaolin LungaoAinda não há avaliações
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (895)
- Mr. Dranreb D. Gayadon Ilubuagan Barako Coffee Mr. Dranreb D. Gayadon Ilubuagan Barako CoffeeDocumento2 páginasMr. Dranreb D. Gayadon Ilubuagan Barako Coffee Mr. Dranreb D. Gayadon Ilubuagan Barako CoffeeLouie Shaolin LungaoAinda não há avaliações
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- Maryellen Nerz-Stormes, PH.D.: (Study Aids)Documento2 páginasMaryellen Nerz-Stormes, PH.D.: (Study Aids)Louie Shaolin LungaoAinda não há avaliações
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (400)
- Marketing Department Pag-Asa SteelDocumento1 páginaMarketing Department Pag-Asa SteelLouie Shaolin LungaoAinda não há avaliações
- Biodegradable Materials 1Documento26 páginasBiodegradable Materials 1Louie Shaolin LungaoAinda não há avaliações
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (345)
- Table of Content: Vapour Liquid Equilibrium Lab ReportDocumento37 páginasTable of Content: Vapour Liquid Equilibrium Lab ReportLouie Shaolin Lungao0% (1)
- Ocw Chapter 4Documento48 páginasOcw Chapter 4Louie Shaolin LungaoAinda não há avaliações
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- Molar Mass of Polymers: X: Degree of PolymerizationDocumento9 páginasMolar Mass of Polymers: X: Degree of PolymerizationLouie Shaolin LungaoAinda não há avaliações
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (121)