Escolar Documentos
Profissional Documentos
Cultura Documentos
Previews: Krebs Cycle Moonlights in Caspase Regulation
Enviado por
Wanabud abudDescrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Previews: Krebs Cycle Moonlights in Caspase Regulation
Enviado por
Wanabud abudDireitos autorais:
Formatos disponíveis
Developmental Cell
Previews
Krebs Cycle Moonlights in Caspase Regulation
Adi Minis1 and Hermann Steller1,*
1Strang Laboratory of Apoptosis and Cancer Biology, Howard Hughes Medical Institute, The Rockefeller University, New York,
NY 10065, USA
*Correspondence: steller@mail.rockefeller.edu
http://dx.doi.org/10.1016/j.devcel.2016.03.016
In this issue of Developmental Cell, Aram et al. (2016) identify a mechanism that uses a Krebs cycle protein to
control local activation of a ubiquitin ligase complex at the mitochondrial outer membrane for temporally and
spatially restricted caspase activation during Drosophila sperm differentiation.
Caspases, a conserved family of cysteine and depends on apoptotic proteins, cells, it is not sufficient for caspase activa-
proteases, have been intensely studied as including caspase-3-type effector cas- tion in spermatids. Furthermore, although
key executioners of apoptosis, the most pases (Arama et al., 2003). Earlier studies the authors suggest that the binding of
common form of natural cell death. In had already revealed the critical impor- A-Sb to the CRL3 complex is important
addition, caspases also play important tance of the caspase inhibitor dBruce, the for its activation by neddylation, their
non-apoptotic roles in development and apoptosome activator Cytochrome C, RNAi experiments show a more promi-
differentiation (Yi and Yuan, 2009). A strik- and a Cullin-3-based ubiquitin ligase com- nent effect on the localization to the
ing example is the non-lethal use of plex (CRL3) for regulating caspase activity mitochondria than on the levels of neddy-
apoptotic effector caspases for cellular in a non-lethal manner (Arama et al., lation. Collectively, these results imply
remodeling (Fuchs and Steller, 2011; 2007). Furthermore, the Arama lab previ- that Cul3-neddylation and caspase acti-
Schuldiner and Yaron, 2015). Apoptotic ously identified a pseudosubstrate inhibi- vation are uncoupled in spermatids and
proteins are required for the regulated tor for CRL3, termed Soti, which inhibits that another layer of regulation operates
destruction of axons and dendrites to the activity of this complex in a spatially at the MOM. This could be mediated
sculpt the nervous system, the degrading graded manner and thereby restricts cas- through an interaction with other mito-
of the nucleus and membrane-bound or- pase activity in time and space (Kaplan chondrial proteins or lipids, post-transla-
ganelles during lens fiber differentiation, et al., 2010). This fine-tuning of caspase tional modification, or the proximity to
and terminal differentiation of spermatids. levels is crucial for male fertility, as either other apoptosis-related proteins that are
This raises the intriguing question of how too low or too high caspase activity has necessary for caspase activation, such
the activation of apoptotic caspases can disastrous consequences. as Cytochrome C.
be restricted in time and space to avoid In the current study, Aram et al. (2016) Irrespective of the exact underlying
death of the entire cell. In this issue of used a yeast two-hybrid approach fol- biochemical mechanism, the current
Developmental Cell, Aram et al. (2016) lowed by co-immunoprecipitation experi- work by Aram et al. (2016) reveals an
report a major advance that sheds new ments to identify an ATP-specific form of unexpected ‘‘moonlighting’’ function of
light onto this question. Using Drosophila the Succinyl-CoA synthetase b subunit A-Sb for the spatio-temporal regulation
melanogaster spermatogenesis as a (A-Sb) as another key regulator of CRL3 of a Cullin-3-based ubiquitin ligase com-
model system, the authors uncover an un- activity. Typically, A-Sb is found in the plex and caspase activity. At the same
expected role for a Krebs cycle protein, mitochondrial matrix and functions within time, these findings raise many new
Succinyl-CoA synthetase b (A-Sb), in pro- the Krebs cycle. However, in Drosophila and intriguing questions. Foremost, why
moting temporally and spatially restricted spermatids, a significant portion of A-Sb would a Krebs cycle protein involve a
caspase activation at the mitochondrial is localized on the MOM where it can function at the surface of mitochondria
outer membrane (MOM) (Figure 1). bind to CRL3. In a series of elegant exper- to regulate protein degradation? The
During terminal sperm differentiation in iments that combine cell fractionation, im- important role of mitochondria in the regu-
Drosophila, the vast majority of proteins muno-EM, biochemical analyses, domain lation of apoptosis has long been recog-
are degraded to generate highly special- swapping experiments, and genetic and nized, as a source of regulatory proteins,
ized and minimalistic cells devoid of major structure-function studies, Aram et al. but also as a platform for the sequestra-
organelles. This process occurs in cysts (2016) now demonstrate that A-Sb pro- tion of apoptotic proteins (Fuchs and
containing 64 haploid cells that remain motes caspase activation by antago- Steller, 2011). Furthermore, sperm differ-
interconnected due to incomplete cytoki- nizing Soti to activate the CRL3 complex entiation in insects involves very dramatic
nesis (Fuller, 1993). The terminal differen- at the MOM. They also show that the changes in the structure of mitochondria.
tiation of spermatids involves dramatic localization and function of A-Sb in devel- Therefore, it is possible that A-Sb helps
cell elongation and separation into indi- oping sperm depends on its N-terminal to sense the appropriate metabolic state
vidual spermatids; hence, this process is domain. This is interesting because for the initiation of caspase activity.
termed ‘‘individualization.’’ Importantly, although the CRL3-binding domain of Intriguingly, two additional non-canonical
this process involves the degradation of A-Sb, located at its C terminus, is suffi- functions of A-Sb outside mitochondria
the majority of proteins and organelles cient for caspase activation in S2 culture have also been suggested, namely in
Developmental Cell 37, April 4, 2016 ª2016 Elsevier Inc. 1
Developmental Cell
Previews
plexes. In addition, although the CRL3
complex is clearly important for caspase
activation during sperm differentiation,
there may be other substrates with rele-
vant biological functions. Another impor-
tant unresolved question is how exactly
A-Sb overcomes the inhibition of CRL3
by Soti. Furthermore, it will be interesting
to investigate the precise mechanism
that determines when and how A-Sb be-
comes located to the MOM, since this
appears to be the rate-limiting step in acti-
vating CRL3 in this system. Finally, it will
be important to explore whether these
findings are restricted to Drosophila
sperm differentiation or represent a more
general regulatory mechanism. Given
the tremendous importance of under-
standing both mechanisms that restrict
caspase activity and the regulation of
CRL3 complexes, it is likely that the
answers to many of these questions will
emerge soon.
REFERENCES
Aram, L., Braun, T., Braverman, C., Kaplan, Y.,
Ravid, L., Levin-Zaidman, S., and Arama, E.
(2016). Dev. Cell 37, this issue, 15–33.
Arama, E., Agapite, J., and Steller, H. (2003). Dev.
Cell 4, 687–697.
Arama, E., Bader, M., Rieckhof, G.E., and Steller,
H. (2007). PLoS Biol. 5, e251.
Fuchs, Y., and Steller, H. (2011). Cell 147, 742–758.
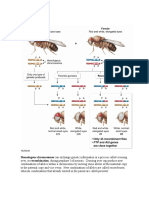
Figure 1. Spatially Restricted Caspase Activation at the Mitochondrial Membrane during
Sperm Differentiation Fuller, M.T. (1993). In The Development of
(A) The non-lethal activity apoptotic effector caspases is regulated by multiple pathways at the mito- Drosophila melanogaster, M. Bate and A.M. Arias,
chondrial surface. First, Cytochrome C is necessary to promote caspase activation. Second, the pseu- eds. (Cold Spring Harbor Laboratory Press),
dosubstrate Soti and A-Sbt, a testis-specific isoform of the Krebs cycle enzyme Succinyl-CoA synthase b, pp. 71–147.
have antagonistic effects on the activity of a Culin-3-based ubiquitin ligase (CRL3) complex. Activation of
CRL3 promotes degradation of the caspase-inhibitor dBruce, which in turn restricts the potentially lethal Gao, L., Fei, H., Connors, N.C., Zhang, J., and Lev-
activity of caspases in time and space. The interplay between Soti and A-Sbt achieves spatial and tem- itan, I.B. (2008). J. Neurophysiol. 99, 2736–2740.
poral restriction of caspase activity that is necessary for the selective destruction of subcellular structures
during sperm terminal differentiation. Hughes, J.R., Meireles, A.M., Fisher, K.H., Garcia,
(B) Soti and A-Sbt regulate CRL3 to promote caspase activation. Soti inhibits CRL3 by blocking substrate A., Antrobus, P.R., Wainman, A., Zitzmann, N.,
binding. Upon the onset of sperm differentiation, A-Sbt levels increase on the mitochondrial outer Deane, C., Ohkura, H., and Wakefield, J.G.
membrane (MOM) and compete with Soti for binding to CRL3. This allows for spatially restricted activation (2008). PLoS Biol. 6, e98.
of CRL3 at the MOM, which in turn promotes degradation of dBruce and caspase activation.
Kaplan, Y., Gibbs-Bar, L., Kalifa, Y., Feinstein-Rot-
kopf, Y., and Arama, E. (2010). Dev. Cell 19,
160–173.
modulating the activity of a voltage-gated possible that A-Sb has multiple moonlight
potassium channel and Skp-1-mediated jobs, or perhaps all these observations Schuldiner, O., and Yaron, A. (2015). Cell. Mol. Life
Sci. 72, 101–119.
centrosome regulation (Gao et al., 2008; reflect a more general role of this protein
Hughes et al., 2008). Therefore, it is in Cullin-based ubiquitin ligase com- Yi, C.H., and Yuan, J. (2009). Dev. Cell 16, 21–34.
2 Developmental Cell 37, April 4, 2016
Você também pode gostar
- Cddis 2013250 ADocumento7 páginasCddis 2013250 AgheaastridgayatriAinda não há avaliações
- Ansa SubclaviaDocumento18 páginasAnsa SubclaviaHalatiu DanielAinda não há avaliações
- Apoptosis 4Documento16 páginasApoptosis 4Kamila Środa-PomianekAinda não há avaliações
- Celllll Term PaperrrDocumento12 páginasCelllll Term PaperrrNarveer SinghAinda não há avaliações
- Nihms96320 Slo3 SpermDocumento12 páginasNihms96320 Slo3 SpermBiodiesel NegAceAinda não há avaliações
- Apoptosis in Cellular Society: Communication Between Apoptotic Cells and Their NeighborsDocumento15 páginasApoptosis in Cellular Society: Communication Between Apoptotic Cells and Their NeighborscarlAinda não há avaliações
- Slob, A Novel Protein That Interacts With The Slowpoke Calcium-Dependent Potassium ChannelDocumento9 páginasSlob, A Novel Protein That Interacts With The Slowpoke Calcium-Dependent Potassium ChannelDark playerAinda não há avaliações
- Major Project Proposal - BIODocumento10 páginasMajor Project Proposal - BIOsappy490Ainda não há avaliações
- Comparative and Functional Analysis of The Archaeal Cell Cycle PDFDocumento13 páginasComparative and Functional Analysis of The Archaeal Cell Cycle PDFnyxcAinda não há avaliações
- Gould y Simanis. Formación Del Septo en LevadurasDocumento14 páginasGould y Simanis. Formación Del Septo en LevadurasSaila Viridiana CazaresAinda não há avaliações
- Intrinsically Unstructured Proteins: Re-Assessing The Protein Structure-Function ParadigmDocumento11 páginasIntrinsically Unstructured Proteins: Re-Assessing The Protein Structure-Function ParadigmJose DanielAinda não há avaliações
- Human Chromosomes: An Illustrated Introduction to Human CytogeneticsNo EverandHuman Chromosomes: An Illustrated Introduction to Human CytogeneticsNota: 5 de 5 estrelas5/5 (1)
- Neurobiology of Circadian Rhythm RegulationDocumento10 páginasNeurobiology of Circadian Rhythm RegulationAgus AgueroAinda não há avaliações
- Re-Creating The RNA World: in VitroDocumento6 páginasRe-Creating The RNA World: in VitroMile ArdilaAinda não há avaliações
- Cell Cycle (Apoptosis)Documento11 páginasCell Cycle (Apoptosis)Siyad AdanAinda não há avaliações
- Sufficient Prespore-Specific Dictyostelium DiscoideumDocumento10 páginasSufficient Prespore-Specific Dictyostelium DiscoideummukiAinda não há avaliações
- 1 s2.0 S000634951403762X MainDocumento1 página1 s2.0 S000634951403762X MainDiego TulcanAinda não há avaliações
- Cho2016 Article RolesOfCross-MembraneTransportDocumento13 páginasCho2016 Article RolesOfCross-MembraneTransportTaurus PearsonAinda não há avaliações
- Aurora & MitosisDocumento11 páginasAurora & MitosisJubairAinda não há avaliações
- The Cycle 3Documento4 páginasThe Cycle 3MIKASAAinda não há avaliações
- Mitocondria ApoptosisDocumento16 páginasMitocondria ApoptosisAlexisEaglerAinda não há avaliações
- Pi Is 0006349518321933Documento1 páginaPi Is 0006349518321933ASP ValenciaAinda não há avaliações
- 1 s2.0 S104620231300193X MainDocumento3 páginas1 s2.0 S104620231300193X MainLucas PolizzeliAinda não há avaliações
- Structural Studies of A Calmodulin Mutant With Defective Regulation of Muscle Contraction John Emmons and Adina KilpatrickDocumento10 páginasStructural Studies of A Calmodulin Mutant With Defective Regulation of Muscle Contraction John Emmons and Adina KilpatrickzaxAinda não há avaliações
- Thansa Et Al 2016Documento9 páginasThansa Et Al 2016KwantaAinda não há avaliações
- Aurora Kinases-Spindle Assembly-Chromosome Segregation04Documento8 páginasAurora Kinases-Spindle Assembly-Chromosome Segregation04almaAinda não há avaliações
- European Journal of Biochemistry - 2001 - Kalies - Protein Translocation Into The Endoplasmic Reticulum ERDocumento5 páginasEuropean Journal of Biochemistry - 2001 - Kalies - Protein Translocation Into The Endoplasmic Reticulum ER12 yashika palAinda não há avaliações
- Bio Notes 1Documento180 páginasBio Notes 1JyNadarilAinda não há avaliações
- StudyingaprionDocumento5 páginasStudyingaprionapi-299087606Ainda não há avaliações
- Cause of GlioblastomaDocumento6 páginasCause of GlioblastomaJubairAinda não há avaliações
- Asymmetrical Cell: Submitted byDocumento5 páginasAsymmetrical Cell: Submitted byJeffrey VictoryAinda não há avaliações
- Broederz ExtrusionDocumento12 páginasBroederz Extrusionapratim.chatterjiAinda não há avaliações
- Tau Protein in Normal and Alzheimerís DiseaseDocumento23 páginasTau Protein in Normal and Alzheimerís DiseaseyusufAinda não há avaliações
- PIIS1550413108003264Documento10 páginasPIIS1550413108003264jendralaliciaAinda não há avaliações
- Protein TargetingDocumento7 páginasProtein TargetingRaven JohnsonAinda não há avaliações
- Biological Basis For Human Capacitation: Christopher de JongeDocumento10 páginasBiological Basis For Human Capacitation: Christopher de JongeVicAinda não há avaliações
- Biological Basis For Human Capacitation: Christopher de JongeDocumento10 páginasBiological Basis For Human Capacitation: Christopher de JongeLara RamundoAinda não há avaliações
- Methods: Guest Editor's IntroductionDocumento3 páginasMethods: Guest Editor's Introductionfsdf6554Ainda não há avaliações
- The Plant Journal - 2010 - J Rgens - A Role For ABIL3 in Plant Cell MorphogenesisDocumento11 páginasThe Plant Journal - 2010 - J Rgens - A Role For ABIL3 in Plant Cell MorphogenesisUhrigAinda não há avaliações
- 2009, Replication Timing-Finding From FibersDocumento6 páginas2009, Replication Timing-Finding From FibersErnesto RojoAinda não há avaliações
- Natural and Unnatural Answers To Evolutionary Questions: CommentaryDocumento3 páginasNatural and Unnatural Answers To Evolutionary Questions: CommentaryJorge AmadorAinda não há avaliações
- Edrv 0747Documento60 páginasEdrv 074705-OB-HU-DINA MERCEDES CAMPOSANO SALAZARAinda não há avaliações
- Chapter 19 LodishDocumento52 páginasChapter 19 LodishablucAinda não há avaliações
- The Nucleus - Express YourselfDocumento2 páginasThe Nucleus - Express YourselfbresojiarAinda não há avaliações
- Tracking Rna Structures As Rnas Transit Through The Cell: News & ViewsDocumento2 páginasTracking Rna Structures As Rnas Transit Through The Cell: News & ViewsThị Sô PhiaAinda não há avaliações
- Intestinal Dysmotility in A Zebrafish PresentationDocumento18 páginasIntestinal Dysmotility in A Zebrafish PresentationCamron DaviesAinda não há avaliações
- CholesterolDocumento12 páginasCholesterolankit sharmaAinda não há avaliações
- JZBiophysicsDocumento10 páginasJZBiophysicsZteban GarciaAinda não há avaliações
- Review Polarity Proteins in Axon Specification and SynaptogenesisDocumento14 páginasReview Polarity Proteins in Axon Specification and Synaptogenesisrocambolescas perthAinda não há avaliações
- Chapter 1: General Principles of Cellular OrganizationDocumento9 páginasChapter 1: General Principles of Cellular OrganizationWissam MoslehAinda não há avaliações
- Science Project RecommendationsDocumento9 páginasScience Project Recommendations柯泰德 (Ted Knoy)Ainda não há avaliações
- Human Sperm Capacitation and The Acrosome Reaction : L.J.D.Zaneveld, C.J.De Jonge, R.A.Anderson and S.R.MackDocumento10 páginasHuman Sperm Capacitation and The Acrosome Reaction : L.J.D.Zaneveld, C.J.De Jonge, R.A.Anderson and S.R.MackDaniel LunaAinda não há avaliações
- Banta (1988) JCB 107, 1369-Vacuole Morphology of Vps MutantsDocumento15 páginasBanta (1988) JCB 107, 1369-Vacuole Morphology of Vps MutantsDario FernándezAinda não há avaliações
- Proteasome Phase Separation A Novel Layer of Quality ControlDocumento2 páginasProteasome Phase Separation A Novel Layer of Quality ControlDoniAinda não há avaliações
- RyanDocumento9 páginasRyanthehardbait12Ainda não há avaliações
- The Chromosomal Passenger Complex (CPC) : From Easy Rider To The Godfather of MitosisDocumento15 páginasThe Chromosomal Passenger Complex (CPC) : From Easy Rider To The Godfather of MitosisRK VermaAinda não há avaliações
- Porating Chol PDFDocumento9 páginasPorating Chol PDFSoumava PalitAinda não há avaliações
- Inflammation and Neoplasia ApoptosisDocumento6 páginasInflammation and Neoplasia ApoptosisTapitAinda não há avaliações
- Aqa 74011 SQPDocumento24 páginasAqa 74011 SQPRobert EdwardsAinda não há avaliações
- Biology Exam #1 Study GuideDocumento12 páginasBiology Exam #1 Study GuideKevin TranAinda não há avaliações
- Bacterial Cell To Cell CommunicationDocumento338 páginasBacterial Cell To Cell CommunicationAllbiol100% (1)
- Academic Press Encyclopedia of Physical Science and Technology 3rd PolymerDocumento339 páginasAcademic Press Encyclopedia of Physical Science and Technology 3rd PolymerBLUECOMANCHE100% (1)
- Cell Cycle CheckpointsDocumento6 páginasCell Cycle CheckpointsjayweinxAinda não há avaliações
- Bio 308 Lecture NotesDocumento7 páginasBio 308 Lecture NotesPeter Sin-KeoAinda não há avaliações
- Cell Communication Ap Study GuideDocumento5 páginasCell Communication Ap Study GuideJulia HartwegerAinda não há avaliações
- Class 12 Biotechnology Principles and Processes Mind MapDocumento1 páginaClass 12 Biotechnology Principles and Processes Mind Mapupgratesleet704Ainda não há avaliações
- Protein and Peptide Drug Delivery SystemsDocumento55 páginasProtein and Peptide Drug Delivery Systemsanupnakat86% (7)
- Precision-Engineering The Pseudomonas Aeruginosa Genome With Two-Step Allelic ExchangeDocumento22 páginasPrecision-Engineering The Pseudomonas Aeruginosa Genome With Two-Step Allelic ExchangeMariaAinda não há avaliações
- Ap Biology Exam Review Questions OnlyDocumento20 páginasAp Biology Exam Review Questions Onlyapi-57781915100% (1)
- BIO F4 Chapter 4 Obj QuesttionDocumento3 páginasBIO F4 Chapter 4 Obj QuesttionIda KamalAinda não há avaliações
- Life Sciences Grade 12 Term 1 Week 1 - 2021Documento4 páginasLife Sciences Grade 12 Term 1 Week 1 - 2021Lucky Jr MonobeAinda não há avaliações
- Have Your Dna and Eat It TooDocumento4 páginasHave Your Dna and Eat It Tooapi-32133818Ainda não há avaliações
- A Level Biology: Nervous CoordinationDocumento39 páginasA Level Biology: Nervous CoordinationAhmedAinda não há avaliações
- เลคเชอร์รวมพันธุศาสตร์Documento16 páginasเลคเชอร์รวมพันธุศาสตร์ณัฐภัทร วิศิษฎ์วรณัฐAinda não há avaliações
- Cell Membrane TransportDocumento75 páginasCell Membrane Transportvinay chaudhary100% (1)
- Genetic DisordersDocumento2 páginasGenetic DisordersEzekiel Arteta100% (2)
- Science 8 Q4 Module 2 Cellular-ReproductionDocumento30 páginasScience 8 Q4 Module 2 Cellular-Reproductionsheron2218Ainda não há avaliações
- 18 01 10SequenceformatsanddatabasesinbioinformaticsDGL1Documento41 páginas18 01 10SequenceformatsanddatabasesinbioinformaticsDGL1sukanyas111Ainda não há avaliações
- BTE101 Course Outline DMH BRACU Physicalclass Section1 20-1-2023Documento4 páginasBTE101 Course Outline DMH BRACU Physicalclass Section1 20-1-2023M.H. RafidAinda não há avaliações
- Data RetrievalDocumento17 páginasData RetrievalAyesha Khan50% (2)
- Ch11 Test File-1Documento14 páginasCh11 Test File-1Jenny SmithAinda não há avaliações
- Cell Biology MCQDocumento17 páginasCell Biology MCQVineet Mehta78% (9)
- Latihan Bab 3Documento12 páginasLatihan Bab 3Hasnah GhaniAinda não há avaliações
- J. Biol. Chem.-1989-Boggaram-11421-7Documento7 páginasJ. Biol. Chem.-1989-Boggaram-11421-7Patrick RamosAinda não há avaliações
- Clinical Chemistry ImmunoassaysDocumento23 páginasClinical Chemistry ImmunoassaysVincent ReyesAinda não há avaliações
- EnzymesDocumento80 páginasEnzymesThisha MohanAinda não há avaliações
- BOOK Nei Kumar MolecularEvolutionAndPhylogeneticsDocumento350 páginasBOOK Nei Kumar MolecularEvolutionAndPhylogeneticswinegar1111Ainda não há avaliações
- Synthesis of Nucleic AcidsDocumento33 páginasSynthesis of Nucleic AcidsbijuarAinda não há avaliações