Escolar Documentos
Profissional Documentos
Cultura Documentos
AOM IVIG Comparison Chart 0124 13
Enviado por
Heba_Al_KhozaeDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
AOM IVIG Comparison Chart 0124 13
Enviado por
Heba_Al_KhozaeDireitos autorais:
Formatos disponíveis
Your Choice for Home Infusion
IVIG Referral Fax Line: 800-528-9860
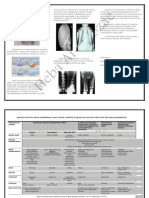
Gammaked
Gammaplex Bivigam Privigen Hizentra Flebogamma Gammagard Liquid Gammagard S/D Octagam
Product Specifics: Gamunex-C
Sizes 2.5g, 5g, 10g 5g, 10g 5g, 10g, 20g 1g, 2g, 4g 2.5g, 5g, 10g 1g, 2.5g, 5g, 10g, 20g 2.5g, 5g, 10g 1g, 2.5g, 5g, 10g, 20g 1g, 2.5g, 5g, 10g
Refridgerate between
Room temperature (25°C) (77°F) - Room temperature (25°C) (77°F) Room temperature (25°C) (77°F)
2 to 8° C (36 to 46°F) 2° to 25°C (36° to 77°F) 2° to 8°C (36°to 46°F) Not to exceed 25°C (77°F), 2° to 8°C (36°to 46°F). 2° to 8°C (36° to 46°F)
Storage 24 months Protect from light. Do not freeze. Do not freeze. Keep vials in
Do not freeze or heat. DO not Do not freeze. Do not shake. Do not freeze. Do not shake. Avoid freezing. Do not shake. Do not freeze. for 24 months.
Do not freeze. Do not shake. Do not shake. storage box until use.
shake.
Form Liquid Liquid Liquid Liquid Liquid Liquid Lyophilized Liquid Liquid
Reconstitution Fluid N/A N/A N/A N/A N/A N/A Sterile Water for Injection N/A N/A
Concentration Options 5% 10% 10% 20% 5% or 10% 10% 5% or 10% 10% 5%
PID + ITP + CLL +
Indications PID PI PID + ITP PID PID PID PID + ITP + CIDP PID
Kawasaki disease
History of anaphylactic or severe History of anaphylactic or severe
IgA deficiency with IgA deficiency with History of anaphylactic or severe
systemic reactions to human systemic reactions to human IgA deficiency with
antibodies to IgA. antibodies to IgA. systemic reactions to human Selective IgA deficiency where IgA
immunoglobulin. immunoglobulin. antibodies to IgA. IgA deficiency with IgA deficiency with
Contraindications Also contraindicated in Also contraindicated in immunoglobulin. deficiency is only abnormality of
IgA deficient patients with IgA deficient patients with Patient intolerant to sorbitol (ie. antibodies to IgA antibodies to IgA
hyperprolinemia patients due to hyperprolinemia patients due to IgA deficiency with concern.
antibodies against IgA and a antibodies against IgA and a Intolerant to fructose).
stabilizer: L-proline. stabilizer: L-proline. antibodies to IgA
history of hypersensitivity. history of hypersensitivity.
Subcutaneous only: Not for Intravenous (IV): Intravenous (IV):
intravenous administration. Initial: 0.8mg/kg/min Initial: 1mg/kg/min
Initial: 0.5mg/kg/min for the first
Injection sites: abdomen, thighs, Maximum: 8.9mg/kg/min Use a 5% solution at 0.5 Maximum: 8mg/kg/min
Initial: 0.5 mg/kg/min (0.01 10 minutes
upper arms, and/or lateral hip. mL/kg/hr. Patients who tolerate
Infusion Rates mL/kg/min) Maintenance Infusion Rate (if Initial:0.5mg/kg/min 0.01 mL/kg body weight/minute 0.01 mL/kg/minute
Initial Infusion: 15mL/hr/inj. site. Subcutaneous (SC): the 5% solution at up to 4 Subcutaneous (SC):
(Refer also to full prescribing Maximum: 4mg/kg/min tolerated): Increase every 20 Maximum: 4mg/kg/min (0.5 mg/kg body weight/minute) (0.5 mg/kg/minute)
Subsequent infusions may be Initial: 1.37 x current IV dose in mL/kg/hr can be infused with 10% Initial: 1.37 x current IV dose in
information). (0.08mL/kg/min) minutes by 0.8mg/kg/min up to
increased as tolerated to mg/kg per IV dose interval in solution starting at 0.5 mL/kg/hr. mg/kg per IV dose interval in
6mg/kg/min.
25/mL/site at a maximum rate of weeks weeks
50mL/hour for all sites combined. Maximum: 20 to 30mL/hr/site Maximum: 20mL/h/site
Warm to room temperature if If dilution is required, may be
Infuse product at approximately If dilution is required, may be
Other Allow refrigerated product to come If dilution is required, may be Warm to room temperature. refrigerated. Use pooled product diluted with 5% dextrose in water
room temperature. Infuse with set diluted with 5% dextrose in water If refrigerated, warm to room Warm to room temperature if
Administration to room temperature before diluted in 5% dextrose in water Discard unused product promptly (may transfer into empty (D5/W). May be pooled in either
preferable fitted with an in-line 15- (D5/W). Warm to room temperature prior to infusion. refrigerated prior to infusion.
Information infusing. (D5/W). immediately after use. sterile IV container using aseptic glass or plastic. Warm to room
20 micron filter. temperature before infusing.
technique). temperature prior to infusion.
Infuse product by separate line
Do not dilute. Do not dilute with other IV fluids. without mixing with other IV fluids
Infuse product by separate line Administer separately from other
Infuse using a serparate line by Infuse product by separate line Do not add any medications or IV or medications. Not compatible
Administer separately from other Should not be mixed with other without mixing other IV fluids or Administer separately from other drugs or medications.
Compatibility Issues itself, without mixing with other without mixing other medications fluids to product infusion with saline. If administered
drugs/medications. medicinal products. medications. drugs/medications. Flushing: any normal infusion
intravenous fluids or medications or fluids. container. through indwelling catheter, flush
Do not use NS 0.9% as a diluent. solution (i.e. D5W/saline).
the patient may be receiving. Infuse by separate IV line. with D5/W before and after
infusing product.
typically <4µg/ml
IgA Content ≤200 µg/mL ≤25 mcg/mL ≤50mcg/ml <50 µg/ml 37 µg/ml ≤2.2 µg/ml (5% concentration) 46 µg/ml <200 µg/mL
specification ≤10mg/ml
No sugar; stabilized with a
Sugar Content 5% D-Sorbitol No sugar; stabilized with glycine nonessential amino acid: None 5% D-Sorbitol No sugar; stabilized with glycine. 2% glucose (5% concentration) No sugar; stabilized with glycine. Maltose 100mg/mL
250 mmol/L L-proline
Approximately 8.5 mg/mL
Sodium Content 30-50mmol/L .100-0.140 M Sodium Chloride Trace amounts Trace Amounts <3.2 mEq/L (<0.02%) None detected Trace amounts ≤30 mmol/L
sodium chloride
320 m0smol/kg 636 m0smol/L (5%)
Osmolarity / Osmolality 460-500m0sm/kg 370-510 mOsm/kg 380 m0smol/kg 240-370 m0smol/kg 240-300 m0smol/kg 258 m0smol/kg 310-380 m0smol/kg
(240 to 440 range) 1250 m0smol/L (10%)
Latex in
Latex free Latex free Latex free Latex free Latex free Latex free Latex free Latex free Latex free
product stopper
Latex in
N/A N/A N/A N/A N/A N/A Contains Latex N/A N/A
diluent stopper
IMPORTANT NOTICE - The information provided herein is a summary of available information only. This summary is to be used as a general educational tool and is not intended for use as a guideline for clinical evaluations. Such evaluations (including, but not limited to, initial and/or subsequent dosing, conversion from specific product brands, etc) should utilize a thorough review of appropriate clinical data.
Product package inserts are the primary source of information. Please see full Prescribing Information before prescribing.
Você também pode gostar
- Disease Reprieve: Living into the Golden YearsNo EverandDisease Reprieve: Living into the Golden YearsNota: 5 de 5 estrelas5/5 (1)
- Generation XL: Raising Healthy, Intelligent Kids in a High-Tech, Junk-Food WorldNo EverandGeneration XL: Raising Healthy, Intelligent Kids in a High-Tech, Junk-Food WorldNota: 3.5 de 5 estrelas3.5/5 (2)
- Iodine Deficiency DisordersDocumento21 páginasIodine Deficiency Disorderslarrevity100% (4)
- Infectious MononucleosisDocumento35 páginasInfectious MononucleosisShv Naga100% (1)
- Ann Louise GittlemanDocumento22 páginasAnn Louise Gittlemanjose100% (1)
- Jack WolfsonDocumento14 páginasJack Wolfsonganadim9795100% (1)
- Gerard MullinDocumento21 páginasGerard Mullinchocoholic_soph91Ainda não há avaliações
- Life Extension Magazine August 2020Documento100 páginasLife Extension Magazine August 2020ScottAinda não há avaliações
- 7 Causes of Disease-Dr Sherry RogersDocumento10 páginas7 Causes of Disease-Dr Sherry RogersJose RodriguezAinda não há avaliações
- 7 Adaptogenic Herbs To Heal Adrenals Naturally - Dr. Jolene BrightenDocumento16 páginas7 Adaptogenic Herbs To Heal Adrenals Naturally - Dr. Jolene BrightenCarl MacCordAinda não há avaliações
- The Allergy-Fighting DietDocumento7 páginasThe Allergy-Fighting DietrooibergAinda não há avaliações
- Epstein-Barr Virus (Infectious Mononucleosis): Causes, Symptoms, DiagnosisDocumento36 páginasEpstein-Barr Virus (Infectious Mononucleosis): Causes, Symptoms, DiagnosisGîrbovanCristinaAinda não há avaliações
- The POTS (Postural Tachycardia Syndrome) Epidemic: Hydration and Nutrition IssuesDocumento10 páginasThe POTS (Postural Tachycardia Syndrome) Epidemic: Hydration and Nutrition IssuesPragyanAinda não há avaliações
- New guidelines for menopause managementDocumento10 páginasNew guidelines for menopause managementJuan FranciscoAinda não há avaliações
- Functional Diagnostics Hyman, MarkDocumento5 páginasFunctional Diagnostics Hyman, MarkAndrew LangeAinda não há avaliações
- Stop The Thyroid Madness Sanjay Dixit MDDocumento68 páginasStop The Thyroid Madness Sanjay Dixit MDdb50% (2)
- Vitamins, Supplements, Herbal Medicines, and ArrhythmiasDocumento12 páginasVitamins, Supplements, Herbal Medicines, and ArrhythmiashyntnenAinda não há avaliações
- 7 Natural Remedies For Epstein Barr VirusDocumento40 páginas7 Natural Remedies For Epstein Barr VirusDraganescu Violeta33% (3)
- 201 Reasons For LDNDocumento386 páginas201 Reasons For LDNbktango100% (2)
- Heartburn GERDDocumento34 páginasHeartburn GERDGummi100% (1)
- Day2 2 Jason Hawrelax Treatment Dysbiosis Small and Large BowelDocumento76 páginasDay2 2 Jason Hawrelax Treatment Dysbiosis Small and Large BowelNicoleta ButucAinda não há avaliações
- Essential home medicine cabinet items for surviving without antibioticsDocumento2 páginasEssential home medicine cabinet items for surviving without antibioticsPatrice PrattAinda não há avaliações
- Gut and Psychology Syndrome (GAPS) - Weston A Price Foundation"Documento13 páginasGut and Psychology Syndrome (GAPS) - Weston A Price Foundation"shadhilidarqawi75% (4)
- DrMercola SiimLandandJamesDiNicolantonio TheImmunityFixDocumento28 páginasDrMercola SiimLandandJamesDiNicolantonio TheImmunityFixRocco LamponeAinda não há avaliações
- Anti Fungal DietDocumento10 páginasAnti Fungal Dietn1gg8464100% (1)
- Alternative Lyme Disease Treatment ReviewDocumento2 páginasAlternative Lyme Disease Treatment Reviewkhnumdumandfullofcum100% (1)
- Low Salicylate Food Dictionary: The World’s Most Comprehensive Low Salicylate Diet Ingredient Dictionary: Food Heroes, #2No EverandLow Salicylate Food Dictionary: The World’s Most Comprehensive Low Salicylate Diet Ingredient Dictionary: Food Heroes, #2Ainda não há avaliações
- Izabella WentzDocumento20 páginasIzabella Wentzharmziie100% (3)
- The Calcium Connection: The Little-Known Enzyme at the Root of Your Cellular HealthNo EverandThe Calcium Connection: The Little-Known Enzyme at the Root of Your Cellular HealthAinda não há avaliações
- Berberine To Treat Insulin ResistanceDocumento5 páginasBerberine To Treat Insulin ResistanceRoxana FrincuAinda não há avaliações
- Why It Hurts: A Physician's Insights on The Purpose of PainNo EverandWhy It Hurts: A Physician's Insights on The Purpose of PainAinda não há avaliações
- Trigeminal Neuralgia: A Beginner's 3-Step Quick Start Guide to Managing TB Through Diet, With Sample RecipesNo EverandTrigeminal Neuralgia: A Beginner's 3-Step Quick Start Guide to Managing TB Through Diet, With Sample RecipesAinda não há avaliações
- Drugs Used in Bronchial Asthma & COPDDocumento71 páginasDrugs Used in Bronchial Asthma & COPDShabnam Binte AlamAinda não há avaliações
- A Diet To Treat AutismDocumento23 páginasA Diet To Treat Autismapi-273009135Ainda não há avaliações
- Low Histamine Diet: 40+Tart, Ice-Cream, and Pie recipes for a healthy and balanced Low Histamine dietNo EverandLow Histamine Diet: 40+Tart, Ice-Cream, and Pie recipes for a healthy and balanced Low Histamine dietAinda não há avaliações
- Dr. Cabral Detox Drop: 7-Day PlanDocumento21 páginasDr. Cabral Detox Drop: 7-Day Planharmziie100% (2)
- Blood Pressure: 10 Steps To Lower And Manage Your Blood Pressure NaturallyNo EverandBlood Pressure: 10 Steps To Lower And Manage Your Blood Pressure NaturallyAinda não há avaliações
- Amy MyersDocumento21 páginasAmy MyerssangroniscarlosAinda não há avaliações
- DR Daniel Pompa Smoothies To Heal Your Gut Ed 1115r3Documento20 páginasDR Daniel Pompa Smoothies To Heal Your Gut Ed 1115r3Rares Dinu100% (2)
- Beat Eczema: Skin Irritation can be a thing of your past! Natural Eczema Remedies PLUS Reduce Inflammation with BONUS Powerful Recipes and Food Tips for a Low Inflammation DietNo EverandBeat Eczema: Skin Irritation can be a thing of your past! Natural Eczema Remedies PLUS Reduce Inflammation with BONUS Powerful Recipes and Food Tips for a Low Inflammation DietAinda não há avaliações
- Colostrum, The Natural Prescription For Your Well BeingDocumento99 páginasColostrum, The Natural Prescription For Your Well BeingAnthony KleinsmithAinda não há avaliações
- Summary: "The Plant Paradox: The Hidden Dangers in "Healthy" Foods That Cause Disease and Weight Gain" by Steven R. Gundry | Discussion PromptsNo EverandSummary: "The Plant Paradox: The Hidden Dangers in "Healthy" Foods That Cause Disease and Weight Gain" by Steven R. Gundry | Discussion PromptsAinda não há avaliações
- Dirty Genes Course Copy 1Documento181 páginasDirty Genes Course Copy 1Rachel Bruce50% (4)
- A Case of Nonpharmacologic Conservative Management of Suspected Uncomplicated Subacute Appendicitis in An Adult MaleDocumento5 páginasA Case of Nonpharmacologic Conservative Management of Suspected Uncomplicated Subacute Appendicitis in An Adult MaleTrueNorth Health Center75% (4)
- Hair Elements 2013Documento5 páginasHair Elements 2013Meghanaram33Ainda não há avaliações
- Methylation Madness: Insight into the Biochemical and Personal Lives of HypermethylatorsNo EverandMethylation Madness: Insight into the Biochemical and Personal Lives of HypermethylatorsAinda não há avaliações
- 22 Little Known Dangers of Magnesium DeficiencyDocumento7 páginas22 Little Known Dangers of Magnesium DeficiencyDianne100% (4)
- Nursing: A Concept-Based Approach To Learning: Volume One, Third EditionDocumento40 páginasNursing: A Concept-Based Approach To Learning: Volume One, Third EditionSamip PatelAinda não há avaliações
- Jini's Healing Guide Natural Treatments for Gut InfectionNo EverandJini's Healing Guide Natural Treatments for Gut InfectionNota: 1 de 5 estrelas1/5 (1)
- Lecture 20 Hormones and Infertility LectureDocumento48 páginasLecture 20 Hormones and Infertility LectureJoseph Stans KasiryeAinda não há avaliações
- Gaps Diet FaqDocumento121 páginasGaps Diet FaqScott FreemanAinda não há avaliações
- Benefits of Raw EggsDocumento2 páginasBenefits of Raw EggsSinner100% (1)
- The Autoimmune Disease MythDocumento20 páginasThe Autoimmune Disease MythMark Sloan100% (4)
- Irritable Bowel Syndrome: Heal Your Gut Naturally in 90 Days!No EverandIrritable Bowel Syndrome: Heal Your Gut Naturally in 90 Days!Ainda não há avaliações
- Digoxin slows heart rate and improves heart functionDocumento6 páginasDigoxin slows heart rate and improves heart functionZiedTrikiAinda não há avaliações
- Dirty Girl: Ditch the Toxins, Look Great and Feel FREAKING AMAZING!No EverandDirty Girl: Ditch the Toxins, Look Great and Feel FREAKING AMAZING!Ainda não há avaliações
- Cost AccountingDocumento4 páginasCost AccountingHeba_Al_KhozaeAinda não há avaliações
- Digital Disruptor Honor Raises Another $50 Million - Leading Home CareDocumento3 páginasDigital Disruptor Honor Raises Another $50 Million - Leading Home CareHeba_Al_KhozaeAinda não há avaliações
- Another Disruption at A Digital Disruptor - Leading Home CareDocumento3 páginasAnother Disruption at A Digital Disruptor - Leading Home CareHeba_Al_KhozaeAinda não há avaliações
- Another Digital Disruptor Bites The Dust in Private Pay Home Care - Leading Home CareDocumento2 páginasAnother Digital Disruptor Bites The Dust in Private Pay Home Care - Leading Home CareHeba_Al_KhozaeAinda não há avaliações
- Another Chapter in The Book of Digital Disuptors - Leading Home CareDocumento3 páginasAnother Chapter in The Book of Digital Disuptors - Leading Home CareHeba_Al_KhozaeAinda não há avaliações
- Tuition Fees-New Students 2018/2017 For Master ProgramsDocumento1 páginaTuition Fees-New Students 2018/2017 For Master ProgramsHeba_Al_KhozaeAinda não há avaliações
- Paper8 SolutionDocumento25 páginasPaper8 SolutionHeba_Al_KhozaeAinda não há avaliações
- BMT RN Testimonials PDFDocumento2 páginasBMT RN Testimonials PDFHeba_Al_KhozaeAinda não há avaliações
- Updated Ch3Documento31 páginasUpdated Ch3Heba_Al_KhozaeAinda não há avaliações
- BleomycinDocumento1 páginaBleomycinHeba_Al_KhozaeAinda não há avaliações
- Managing Global DiversityDocumento2 páginasManaging Global DiversityHeba_Al_KhozaeAinda não há avaliações
- Our Tool English (Final)Documento5 páginasOur Tool English (Final)Heba_Al_KhozaeAinda não há avaliações
- Our Tool English (Final)Documento5 páginasOur Tool English (Final)Heba_Al_KhozaeAinda não há avaliações
- Relevant QuizDocumento4 páginasRelevant QuizHeba_Al_KhozaeAinda não há avaliações
- Rituxan PrescribingDocumento39 páginasRituxan PrescribingHeba_Al_KhozaeAinda não há avaliações
- Data Sheet Mabthera: Pharmaceutical FormDocumento42 páginasData Sheet Mabthera: Pharmaceutical FormHeba_Al_KhozaeAinda não há avaliações
- Avastin PrescribingDocumento37 páginasAvastin PrescribingHeba_Al_KhozaeAinda não há avaliações
- Mucositis 11Documento1 páginaMucositis 11Heba_Al_KhozaeAinda não há avaliações
- 15 NG - PPH Algorithm-Aug08Documento8 páginas15 NG - PPH Algorithm-Aug08Nurkholis AminAinda não há avaliações
- Thrombocytopenia TreatmentDocumento6 páginasThrombocytopenia TreatmentHeba_Al_KhozaeAinda não há avaliações
- SAA HaploDocumento10 páginasSAA HaploHeba_Al_KhozaeAinda não há avaliações
- AugmentinDocumento7 páginasAugmentinHeba_Al_Khozae0% (1)
- Ostepetrosis Table 1Documento1 páginaOstepetrosis Table 1Heba_Al_KhozaeAinda não há avaliações
- 2403 FullDocumento13 páginas2403 FullChristian HartonoAinda não há avaliações
- Bioethical Issues in HealthcareDocumento5 páginasBioethical Issues in HealthcareHeba_Al_KhozaeAinda não há avaliações
- Mucositis 11Documento1 páginaMucositis 11Heba_Al_KhozaeAinda não há avaliações
- OsteopetrosisDocumento3 páginasOsteopetrosisHeba_Al_KhozaeAinda não há avaliações
- Eating Disorders 1Documento17 páginasEating Disorders 1Heba_Al_Khozae100% (1)
- Eating Disorders 1Documento46 páginasEating Disorders 1Heba_Al_KhozaeAinda não há avaliações
- Autism - Kopie PDFDocumento214 páginasAutism - Kopie PDFMohrscribdAinda não há avaliações
- Structure and Classes of Immunoglobulins (IgDocumento5 páginasStructure and Classes of Immunoglobulins (IgsajjadAinda não há avaliações
- Composicion y Bioactividad Andreas2015Documento7 páginasComposicion y Bioactividad Andreas2015Nohely Reyes CachiqueAinda não há avaliações
- Antibodies - Immuno AssignmentDocumento30 páginasAntibodies - Immuno AssignmentShubham PandeyAinda não há avaliações
- Nutrition and Exercise ImmunologyDocumento204 páginasNutrition and Exercise ImmunologytodayisnovemberAinda não há avaliações
- Hematology & Immune SystemDocumento81 páginasHematology & Immune SystemAmanuel Maru100% (1)
- Immunologic Disorders ExplainedDocumento73 páginasImmunologic Disorders ExplainedDjayAinda não há avaliações
- Our Bodies are Made of Water: The Role of Lymphatic Circulation in HealthDocumento26 páginasOur Bodies are Made of Water: The Role of Lymphatic Circulation in HealthplantwisdomAinda não há avaliações
- Materi 5 - DR. Dr. Naomi Esthernita Preterm Infant FeedingDocumento39 páginasMateri 5 - DR. Dr. Naomi Esthernita Preterm Infant FeedingandinaAinda não há avaliações
- 3776011564Documento120 páginas3776011564chaido1100% (4)
- UNIT 4 and UNIT 5 ISDocumento9 páginasUNIT 4 and UNIT 5 ISMarinelle TumanguilAinda não há avaliações
- Amharic ConversationDocumento94 páginasAmharic Conversationtsehay asratAinda não há avaliações
- Saliva / Orthodontic Courses by Indian Dental AcademyDocumento191 páginasSaliva / Orthodontic Courses by Indian Dental Academyindian dental academyAinda não há avaliações
- Immunology - Chapter 3 - AntibodiesDocumento56 páginasImmunology - Chapter 3 - AntibodiesMajed IaalyAinda não há avaliações
- IMMUNITYDocumento58 páginasIMMUNITYkamala 123Ainda não há avaliações
- Principles of Immunodetection 2Documento59 páginasPrinciples of Immunodetection 2Martha RetnaningtyasAinda não há avaliações
- Oral Tolerance:: The Response of The Intestinal Mucosa To Dietary AntigensDocumento218 páginasOral Tolerance:: The Response of The Intestinal Mucosa To Dietary AntigensammarkochiAinda não há avaliações
- Dental Caries FinalDocumento40 páginasDental Caries FinalSimran KathuriaAinda não há avaliações
- Immunoglobulins: Prof - Dr.Gülden Burçak 2020-2021Documento25 páginasImmunoglobulins: Prof - Dr.Gülden Burçak 2020-2021Marwa AliAinda não há avaliações
- Understanding the Rare Lipschütz UlcerDocumento12 páginasUnderstanding the Rare Lipschütz UlcerChyntia Giska0% (1)
- Immunology of Dental Caries Journal ArticleDocumento4 páginasImmunology of Dental Caries Journal ArticleMutiara Maliha ZahraAinda não há avaliações
- (José Das Neves, Bruno Sarmento (Eds.) ) Mucosal DDocumento603 páginas(José Das Neves, Bruno Sarmento (Eds.) ) Mucosal Dfaysal100% (1)
- Immunology - 1200Documento2.640 páginasImmunology - 1200tcabanilAinda não há avaliações
- Endometritis: New Time, New ConceptsDocumento7 páginasEndometritis: New Time, New Conceptsrumaisyah alkatiriAinda não há avaliações
- Alergy Immunology ReviewDocumento574 páginasAlergy Immunology ReviewAriani Setyaningsih100% (1)
- PemphigusDocumento32 páginasPemphigusAlondra CastilloAinda não há avaliações
- ORE MCQsDocumento268 páginasORE MCQspawi18Ainda não há avaliações
- Therapy of Acute Respiratory Infections in Children: NoveltiesDocumento11 páginasTherapy of Acute Respiratory Infections in Children: NoveltiesrizkaAinda não há avaliações
- ImmunologyDocumento80 páginasImmunologyMaged HusseinAinda não há avaliações
- Immunology Flash CardsDocumento46 páginasImmunology Flash CardsRickyNoviantoAinda não há avaliações