Escolar Documentos
Profissional Documentos
Cultura Documentos
Distillation Tutorial 1
Enviado por
Richardt LootsDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Distillation Tutorial 1
Enviado por
Richardt LootsDireitos autorais:
Formatos disponíveis
CHEMICAL ENGINEERING TECHNOLOGY 3B
TUTORIAL 1: DISTILLATION
30 July 2018
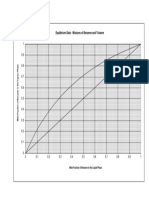
1. A fractionating column is required to separate 100 kg/h a liquid containing 25% benzene and 75%
toluene by mass, to give a distillate product of 98 mass% benzene. 90% of the benzene by mass
should be recovered in the distillate. A reflux ratio of 3.5 is to be used and the feed will enter at its
bubble point.
If the plates used are 50% efficient, calculate by McCabe-Thiele method, the composition of the
liquid on the third plate from the bottom of the column.
3. 100 kmol/h of a feed mixture, which is 50 mol% benzene and the rest toluene, is fed to a
continuous still. A top product of 95 mol% benzene and a bottom product of 95 mol% toluene is
required.
(a) What is the distillate flow rate, D and bottoms flow rate, B?
(b) If Vn is 235 kmol/h, what is the slope of the top operating line?
(c) Construct the top operating line on the equilibrium curve you have constructed.
(d) If the liquid flow rate leaving the bottom of the column, is 160 kmol/h, how many
theoretical plates are required in a distillation column to effect the above duty?
(e) What are the compositions of the streams entering and leaving ideal plate 3? Label each
composition with the appropriate notation.
(f) If it is needed to withdraw a small liquid stream of mole fraction 0,3 from the column, from
which ideal plate should it be taken?
3. A saturated vapour feed, which is 35 mol% benzene and the rest toluene is fed to a continuous
fractionating column. A top product of 95 mol% benzene and a bottom product of 90 mol%
toluene is required. The reflux ratio used is twice the minimum reflux ratio. How many ideal
plates are required and what is the position of the feed tray? Redo this question algebraically.
4. Redo Q3 for a saturated liquid instead of a saturated vapour feed. Comment on the number of
ideal plates required.
5. A liquid feed mixture of benzene and toluene is separated in a continuous fractionating column
operating at atmospheric pressure. The feed is 45 mole% benzene and is at a temperature of
40oC. The distillate product is 95 mol% benzene while the bottoms product is 10 mol% benzene.
The relevant physical constants are supplied.
(a) What is the minimum number of ideal stages that will accomplish the required duty?
(b) What is the minimum reflux ratio, Rmin, and how many ideal stages are required for the above duty
if R = 1,5 Rmin?
(c) What is the optimum position of the feed tray?
(d) If two real plates do the duty one ideal plate, how many real plates would be in the column?
Given for the feed:
Bubble point: 108oC
Heat capacity: 162 J/gmol.K
Molar heat of vaporisation: 40 500 J/gmol
6. Reflecting upon your answers to the above questions, fully discuss the effect of reflux ratio, R, on
the number of ideal plates required in a distillation column. What effect would the reflux ratio have
on the diameter of the column required for the distillation?
7. Starting with the slope of the q-line, q/(q-1), and the definition of q fully discuss the effect of the
condition of the feed, on the number of ideal plates required in a distillation column.
Você também pode gostar
- Distillation Problem Set PDFDocumento1 páginaDistillation Problem Set PDFEfraim AbuelAinda não há avaliações
- 400L Chemical Engr. Past Questions (2012/2013)Documento52 páginas400L Chemical Engr. Past Questions (2012/2013)ifiokAinda não há avaliações
- FALLSEM2015-16 CP3149 04-Aug-2015 RM01 Tutorial-1Documento2 páginasFALLSEM2015-16 CP3149 04-Aug-2015 RM01 Tutorial-1ShashwatAgarwalAinda não há avaliações
- Assignment 2Documento3 páginasAssignment 2deepika snehi100% (1)
- Sample Problems On Gas AbsorptionDocumento2 páginasSample Problems On Gas AbsorptionKevin Laganao67% (3)
- Individual Assignment 200412Documento2 páginasIndividual Assignment 200412Zaidi ZakariaAinda não há avaliações
- Assignment On Continuous Distillation - McCabe-Thiele Method (1) - 1442573024785Documento5 páginasAssignment On Continuous Distillation - McCabe-Thiele Method (1) - 1442573024785sri pragna0% (1)
- Separo Quiz No. 2Documento1 páginaSeparo Quiz No. 2Bench GuecoAinda não há avaliações
- Che 246 - Mass Transfer and Unit Operations Tutorial-Chapter 2 (Distillation)Documento5 páginasChe 246 - Mass Transfer and Unit Operations Tutorial-Chapter 2 (Distillation)fatien zakariaAinda não há avaliações
- Mass Transfer Operations (ENV 425) Problem Set 4: Zewail City of Science and TechnologyDocumento3 páginasMass Transfer Operations (ENV 425) Problem Set 4: Zewail City of Science and TechnologyMayar H. HaggagAinda não há avaliações
- Distillation Problem SetDocumento1 páginaDistillation Problem SetEfraim Abuel100% (1)
- TareaDocumento3 páginasTareaAydee GarciaAinda não há avaliações
- Separation Processes - I (CHE F244) Total Marks - 15 Due Date & Time: 01/07/2020, 5:00 PM AssignmentDocumento4 páginasSeparation Processes - I (CHE F244) Total Marks - 15 Due Date & Time: 01/07/2020, 5:00 PM AssignmentElliot AldersonAinda não há avaliações
- Set 4Documento3 páginasSet 4Ibtisam FarhaniAinda não há avaliações
- MSOCHA3 Tutorial 1 Multicomponent AbsorptionDocumento5 páginasMSOCHA3 Tutorial 1 Multicomponent AbsorptionTshwarelo MahlakoaneAinda não há avaliações
- Distillation Example 4 and 5Documento2 páginasDistillation Example 4 and 5DirkMyburghAinda não há avaliações
- Tutorial DistillationDocumento3 páginasTutorial DistillationManu Indivare Nundoolall100% (1)
- Solved Problem Question (Gas Ab)Documento2 páginasSolved Problem Question (Gas Ab)Seruzna IshxAinda não há avaliações
- Tutorial Leaching 2017Documento11 páginasTutorial Leaching 2017Victor M. Jaki100% (1)
- Tutorial 1Documento4 páginasTutorial 1Hanee Farzana HizaddinAinda não há avaliações
- 9A23401 Mass Transfer OperationsDocumento8 páginas9A23401 Mass Transfer OperationssivabharathamurthyAinda não há avaliações
- Absorption of Carbon Dioxide Into WaterDocumento11 páginasAbsorption of Carbon Dioxide Into WaterEstelle Jean CauilanAinda não há avaliações
- Problem Set 3Documento2 páginasProblem Set 3Nigel Kow0% (1)
- Distillation LabDocumento18 páginasDistillation LabWong XimeiAinda não há avaliações
- 6871981Documento2 páginas6871981honeylet tayactacAinda não há avaliações
- Cge642 Tutorial 3 PDFDocumento2 páginasCge642 Tutorial 3 PDFyatiAinda não há avaliações
- 7HC - Mt-Ii Oct-98,99 Apr-00Documento7 páginas7HC - Mt-Ii Oct-98,99 Apr-00Ahmed AliAinda não há avaliações
- CHE201ch12Documento25 páginasCHE201ch12chandro57Ainda não há avaliações
- Gas Absorption and Gas StrippingDocumento14 páginasGas Absorption and Gas StrippingEK63Ainda não há avaliações
- Practice Problems in Absorption and HumidificationDocumento4 páginasPractice Problems in Absorption and HumidificationJenna BraszAinda não há avaliações
- Mass Transfer Operations 1: List of Books Information of CrystallizationDocumento13 páginasMass Transfer Operations 1: List of Books Information of CrystallizationZaid MansuriAinda não há avaliações
- Problems For Distillation Column Sequencing - Tutorial - 3Documento4 páginasProblems For Distillation Column Sequencing - Tutorial - 3eelya93Ainda não há avaliações
- Ideal Reactors Part 2 Solved ProblemsDocumento15 páginasIdeal Reactors Part 2 Solved ProblemsWaldi SagalaAinda não há avaliações
- Halibut LeachingDocumento1 páginaHalibut LeachingClint Regondola Mohammed25% (4)
- Leaching TutoDocumento1 páginaLeaching TutoFikrie MuhdAinda não há avaliações
- Chemical Process CalculationsDocumento8 páginasChemical Process Calculationsbhaskar5377Ainda não há avaliações
- Al Duri Tutorial1 AbsorptionDocumento2 páginasAl Duri Tutorial1 AbsorptionJia YiAinda não há avaliações
- CH138P WS 1.2 Geromo HALDocumento11 páginasCH138P WS 1.2 Geromo HALLora Bell100% (1)
- Flash Distillation ProblemDocumento2 páginasFlash Distillation ProblemprudhvifireAinda não há avaliações
- Chapter 4Documento43 páginasChapter 4aliAinda não há avaliações
- Distillation - Written ReportDocumento17 páginasDistillation - Written ReportmichsantosAinda não há avaliações
- Tutorial Reactive SystemsDocumento33 páginasTutorial Reactive Systemssiti azilaAinda não há avaliações
- Distillation Column PDFDocumento74 páginasDistillation Column PDFAsad KhanAinda não há avaliações
- ME 346 Lab Final ExamDocumento9 páginasME 346 Lab Final ExamSaad RasheedAinda não há avaliações
- Liquid - Liquid Extraction in A Packed Bed: Experiment No: 2Documento23 páginasLiquid - Liquid Extraction in A Packed Bed: Experiment No: 2Sameep JainAinda não há avaliações
- Chee3004 Unit Operations Mccabe-Thiele Method & Multicomponent DistillationDocumento14 páginasChee3004 Unit Operations Mccabe-Thiele Method & Multicomponent Distillationking4lifeAinda não há avaliações
- 6645646Documento2 páginas6645646honeylet tayactacAinda não há avaliações
- Distillation TypesDocumento34 páginasDistillation TypesJoshua Johnson100% (1)
- Problems in Mass TransferDocumento3 páginasProblems in Mass TransferAngelica Joyce BenitoAinda não há avaliações
- Exp-9 - Liquid Liquid Extraction in A Packed ColumnDocumento5 páginasExp-9 - Liquid Liquid Extraction in A Packed ColumnSiddharth MohapatraAinda não há avaliações
- Mass Transfer - II 3350502: Parth Modi, LecturerDocumento39 páginasMass Transfer - II 3350502: Parth Modi, LecturerSMIT CHRISTIANAinda não há avaliações
- Liquid LiquidDocumento8 páginasLiquid LiquidAnonymous b9fcR5Ainda não há avaliações
- Isothermal CSTR PDFDocumento9 páginasIsothermal CSTR PDFprashant_cool_4_uAinda não há avaliações
- Ch8551 Mass Transfer-I Unit I - (Diffusion) C303.1: Department of Chemical Engineering, VSBECDocumento12 páginasCh8551 Mass Transfer-I Unit I - (Diffusion) C303.1: Department of Chemical Engineering, VSBECSaravanan SundaramAinda não há avaliações
- Liquid Membranes: Principles and Applications in Chemical Separations and Wastewater TreatmentNo EverandLiquid Membranes: Principles and Applications in Chemical Separations and Wastewater TreatmentAinda não há avaliações
- 2023 SPU260S Tutorial 3 QuestionsDocumento6 páginas2023 SPU260S Tutorial 3 QuestionsMABUKE NDINAINWI INNOCENTIAAinda não há avaliações
- DistillationDocumento4 páginasDistillationArvind SinghAinda não há avaliações
- MTO Assignment3Documento2 páginasMTO Assignment3Krishnaveni RAinda não há avaliações
- Distillation Aspen HysysDocumento66 páginasDistillation Aspen HysysCzarina MasicatAinda não há avaliações
- Tutorial-Chapter 2 (June - Oct 2013)Documento5 páginasTutorial-Chapter 2 (June - Oct 2013)paulineanakmawatAinda não há avaliações
- Cape Penisula University of Technology Bellville Campus Department of Chemical Engineering ND: Chemical EngineeringDocumento7 páginasCape Penisula University of Technology Bellville Campus Department of Chemical Engineering ND: Chemical EngineeringRichardt LootsAinda não há avaliações
- Benzene Toluene Equlibrium CurveDocumento1 páginaBenzene Toluene Equlibrium CurveRichardt LootsAinda não há avaliações
- E CH01 GOV1 A3 InstructionsDocumento2 páginasE CH01 GOV1 A3 InstructionsRichardt LootsAinda não há avaliações
- GO PCH01 A1 - Big Island 21 InstructionsDocumento2 páginasGO PCH01 A1 - Big Island 21 InstructionsRichardt LootsAinda não há avaliações
- Minitrim ST Minitrim Auto ST Minitrim Auto XT: Original InstructionsDocumento8 páginasMinitrim ST Minitrim Auto ST Minitrim Auto XT: Original InstructionsRichardt LootsAinda não há avaliações
- Reciprocating Air Compressors: Compression RatioDocumento25 páginasReciprocating Air Compressors: Compression RatioRichardt LootsAinda não há avaliações
- Refrigeration PracticalDocumento15 páginasRefrigeration PracticalRichardt Loots100% (2)
- Fluid Flow PracticalDocumento24 páginasFluid Flow PracticalRichardt Loots40% (5)
- Cooling Tower PracticalDocumento17 páginasCooling Tower PracticalRichardt LootsAinda não há avaliações
- III 3 HeatTransfer 1 3Documento3 páginasIII 3 HeatTransfer 1 3SannyBombeoJomocAinda não há avaliações
- ME-204 - Slides Set# 05 (1st Law of Thermo)Documento21 páginasME-204 - Slides Set# 05 (1st Law of Thermo)mamoona noreenAinda não há avaliações
- Dotech Sensing & ControlDocumento15 páginasDotech Sensing & ControlAnsari1918Ainda não há avaliações
- Written Report Thermo Group 10Documento8 páginasWritten Report Thermo Group 10Patrick SumalaAinda não há avaliações
- Evpd Air Con 1.2 PDFDocumento9 páginasEvpd Air Con 1.2 PDFdeboline mitra100% (1)
- 48 50P 7T Servicio Arranque Operacion y MantenimientoDocumento252 páginas48 50P 7T Servicio Arranque Operacion y MantenimientoRaymundo Rangel RdzAinda não há avaliações
- Boiler Circulation SystemDocumento32 páginasBoiler Circulation SystemRitik Dewangan100% (1)
- Steam Circulation SystemDocumento36 páginasSteam Circulation Systemnavdeeplakhera100% (1)
- ETA 00 32Km2.Ctl Bloc de NotasDocumento2 páginasETA 00 32Km2.Ctl Bloc de NotasAníbal López PeñaAinda não há avaliações
- Boiler Safety BanglaDocumento48 páginasBoiler Safety BanglaMazharul IslamAinda não há avaliações
- ThermodynamicsDocumento17 páginasThermodynamicsShahid MasoodAinda não há avaliações
- Instrument AirDocumento6 páginasInstrument Airasarobin1989Ainda não há avaliações
- PH, Ka, Pka and KW Exam Questions MarkschemeDocumento3 páginasPH, Ka, Pka and KW Exam Questions Markscheme장채윤Ainda não há avaliações
- Xii Chem CT 2Documento3 páginasXii Chem CT 2Kunal PANDYAAinda não há avaliações
- 4.1 Stagnation State For Ideal Gas Model: 4.1.1 General RelationshipDocumento34 páginas4.1 Stagnation State For Ideal Gas Model: 4.1.1 General Relationshipmori hartantoAinda não há avaliações
- 2015 Volvo S60 2.0L Eng VIN 26 T5Documento106 páginas2015 Volvo S60 2.0L Eng VIN 26 T5Data TécnicaAinda não há avaliações
- Air Separation Unit PDFDocumento2 páginasAir Separation Unit PDFbodhi_cheAinda não há avaliações
- MALIK Phy Kinetic TheoryDocumento34 páginasMALIK Phy Kinetic TheoryShadab HanafiAinda não há avaliações
- Worksheet On Kinetic ParticleDocumento3 páginasWorksheet On Kinetic ParticlePontuChowdhuryAinda não há avaliações
- Manual Termostato EspañolDocumento24 páginasManual Termostato EspañoljuancgranadoAinda não há avaliações
- q3t02 Sol PDFDocumento2 páginasq3t02 Sol PDFTrầnHạVyAinda não há avaliações
- 1 CompressorsDocumento25 páginas1 CompressorsCJ CerezoAinda não há avaliações
- CIPRIANI A5 Saldobrasati SCDocumento4 páginasCIPRIANI A5 Saldobrasati SCSebastian MirandaAinda não há avaliações
- Heat and Mass Transfer Module 1 Lesson 2Documento13 páginasHeat and Mass Transfer Module 1 Lesson 2cool kidAinda não há avaliações
- Hitachi Air-Cooled Chiller PDFDocumento46 páginasHitachi Air-Cooled Chiller PDFJeffrey Chua60% (5)
- Welcome Back To School New SemesterDocumento23 páginasWelcome Back To School New SemesterMohd Najmi FahmiAinda não há avaliações
- WeeDocumento34 páginasWeerozAinda não há avaliações
- CHAPTER 4-Energy Analysis of Closed SystemDocumento26 páginasCHAPTER 4-Energy Analysis of Closed SystemDenisse Torizo OlanAinda não há avaliações
- 01 Dynamic Modelling For Vapour Compression Systems - RasmussenDocumento23 páginas01 Dynamic Modelling For Vapour Compression Systems - RasmussenAllyson LuizAinda não há avaliações
- QuizBowl QuestionsDocumento76 páginasQuizBowl Questionsedmark icalina50% (4)