Escolar Documentos
Profissional Documentos
Cultura Documentos
Boyleslawandcharlesslawwkst
Enviado por
api-325864985Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Boyleslawandcharlesslawwkst
Enviado por
api-325864985Direitos autorais:
Formatos disponíveis
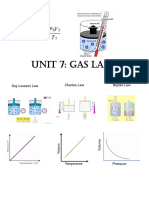
Worksheet: Boyle’s Law and Charles’s Law Name______________
1. Boyle’s Law: When ____________ is held constant, the pressure and volume
of a gas are ____________ proportional.
2. Mathematically, Boyle’s law is stated PV = ____________ or P1V1 =
____________.
3. At a pressure of 405 kPa, the volume of a gas is 6.00 cm3. Assuming the
temperature remains constant, at what pressure will the new volume be 4.00
cm3?
4. A volume of gas at 1.10 atm was measured at 326 cm3. What will be the volume
if the pressure is adjusted to 1.90 atm?
5. If 36.5 m3 of a gas are collected at a pressure of 755 mm Hg, what volume will
the gas occupy if the pressure is changed to 632 mm Hg?
CHEMISTRY: A Study of Matter
© 2004, GPB
9.13
6. Charles’s Law: When ____________ is held constant, the volume and
temperature of a gas are ____________ proportional.
V V
7. Mathematically, Charles’s Law is stated: = _____ or 1 =______.
T T1
8. The ____________ temperature scale must be used in all gas law problems.
9. At 189 K, a sample of gas has a volume of 32.0 cm3. What volume does the gas
occupy at 242 K?
10. The gas in a balloon occupies 2.25 L at 298 K. At what temperature will the
balloon expand to 3.50 L?
11. A sample of gas has a volume of 852 mL at 25°C. What Celsius temperature is
necessary for the gas to have a volume of 945 mL?
CHEMISTRY: A Study of Matter
© 2004, GPB
9.14
Você também pode gostar
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterNo EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterNota: 5 de 5 estrelas5/5 (1)
- Practice Makes Perfect in Chemistry: The Physical Behavior of Matter with AnswersNo EverandPractice Makes Perfect in Chemistry: The Physical Behavior of Matter with AnswersAinda não há avaliações
- Gas Laws Test ReviewDocumento2 páginasGas Laws Test ReviewXzyle1213Ainda não há avaliações
- Anchor Guide GasesDocumento6 páginasAnchor Guide GasesDaniel ZhangAinda não há avaliações
- Mixedgaslaws HonorsDocumento3 páginasMixedgaslaws HonorsJeromeAinda não há avaliações
- Combined Avogadros and Ideal Gas LawsDocumento25 páginasCombined Avogadros and Ideal Gas Lawslevi0417Ainda não há avaliações
- HWDocumento6 páginasHWapi-248733530Ainda não há avaliações
- Gas Laws I SP 1617 (PreAP)Documento3 páginasGas Laws I SP 1617 (PreAP)Nikhil Singh100% (1)
- Basic Gas Law Notes 2017Documento14 páginasBasic Gas Law Notes 2017Sreeja TipirneniAinda não há avaliações
- GLWS9Documento6 páginasGLWS9Vince HernándezAinda não há avaliações
- HW Packet Gas LawsDocumento3 páginasHW Packet Gas Lawsumaru chanAinda não há avaliações
- Class 9 Gas LawsDocumento1 páginaClass 9 Gas Lawssuranjana26Ainda não há avaliações
- Homework Packet: Gas Law: Boyle'S Law Problems: P V P V 1 Atm 760.0 MM HG 101.3 KpaDocumento6 páginasHomework Packet: Gas Law: Boyle'S Law Problems: P V P V 1 Atm 760.0 MM HG 101.3 Kpakikoy20Ainda não há avaliações
- Worksheet GasesDocumento6 páginasWorksheet GasesakladffjaAinda não há avaliações
- Gas Laws Worksheet Answer KeyDocumento4 páginasGas Laws Worksheet Answer KeyJovenil Bacatan50% (2)
- Unit 7 Gas Laws Packet 2021Documento40 páginasUnit 7 Gas Laws Packet 2021Alberto Laborte Jr.Ainda não há avaliações
- Gaslawworksheet 1Documento1 páginaGaslawworksheet 1ravichandra.d.dasariAinda não há avaliações
- ME 221 03 Ideal GasesDocumento17 páginasME 221 03 Ideal GasesAnn NavarroAinda não há avaliações
- Ideal Gas LawDocumento1 páginaIdeal Gas LawLarry BugaringAinda não há avaliações
- Ww1 Boyle, Charles, Gay Lussac, CombinedDocumento3 páginasWw1 Boyle, Charles, Gay Lussac, Combinedroland bautistaAinda não há avaliações
- Monthly Test GasesDocumento3 páginasMonthly Test GasesTristan PereyAinda não há avaliações
- Gas Laws Worksheet #1 - Boyle's, Charles', Gay-Lussac's, and Combined Gas LawDocumento8 páginasGas Laws Worksheet #1 - Boyle's, Charles', Gay-Lussac's, and Combined Gas Lawnina lykka calaraAinda não há avaliações
- Gas Laws Worksheet Answer KeyDocumento4 páginasGas Laws Worksheet Answer KeyHrishikesh Sumesh0% (1)
- Gay-Lussac's LawDocumento26 páginasGay-Lussac's LawJustin BorjaAinda não há avaliações
- LAS SCI10 4QTR WK1.2 RevisedDocumento3 páginasLAS SCI10 4QTR WK1.2 RevisedCurt Andrie T PotenciaAinda não há avaliações
- Mixedgaslaws Honors Make UpDocumento3 páginasMixedgaslaws Honors Make UpARVIN CONCHAAinda não há avaliações
- CH Gas Laws NotesDocumento6 páginasCH Gas Laws Notesapi-293306937Ainda não há avaliações
- Joseph Louis Gay-Lussac Absolute TemperatureDocumento4 páginasJoseph Louis Gay-Lussac Absolute TemperatureMira VeranoAinda não há avaliações
- 21 Ale 21 Ideal Gases KM f09Documento6 páginas21 Ale 21 Ideal Gases KM f09api-2933069370% (1)
- Science 10 - Week 28Documento4 páginasScience 10 - Week 28Mira VeranoAinda não há avaliações
- Gas LawsDocumento33 páginasGas LawspopiscanzAinda não há avaliações
- Gas Laws Practice Ideal Gas Law Worksheet PV NRT: R 0.0821 (L Atm) / (K Mol) or R 8.31 L Kpa / (K Mole)Documento5 páginasGas Laws Practice Ideal Gas Law Worksheet PV NRT: R 0.0821 (L Atm) / (K Mol) or R 8.31 L Kpa / (K Mole)Alyssa ColeAinda não há avaliações
- Gas Laws Worksheetsand SolutionsDocumento9 páginasGas Laws Worksheetsand SolutionskjgfsogkpsAinda não há avaliações
- Gay Lussac Combined Avogadro Ideal Gas Law ApplicationDocumento28 páginasGay Lussac Combined Avogadro Ideal Gas Law ApplicationAlexandra Venice Ann M. PerezAinda não há avaliações
- Complete Gas Laws PracticeDocumento4 páginasComplete Gas Laws PracticeJensen Ryan LimAinda não há avaliações
- Name: - Yr. and Section: - Date: - Exercise #10 Gas Laws Boyle's Law ProblemsDocumento2 páginasName: - Yr. and Section: - Date: - Exercise #10 Gas Laws Boyle's Law ProblemsKeannoAinda não há avaliações
- Gas Laws Worksheet 2 Boyles Charles and Combined - CompressDocumento2 páginasGas Laws Worksheet 2 Boyles Charles and Combined - CompressZar ArhAinda não há avaliações
- Intro To Gases and Gas LawsDocumento44 páginasIntro To Gases and Gas Lawsivy omongosAinda não há avaliações
- Chapter 14Documento42 páginasChapter 14Aubrey LanotAinda não há avaliações
- Gas LawDocumento35 páginasGas LawNonoy GuiribsAinda não há avaliações
- Charles LawDocumento3 páginasCharles Lawsarausos.zoe08Ainda não há avaliações
- Ch17 ISMDocumento60 páginasCh17 ISMshaniceniaAinda não há avaliações
- 9 - Unit 6 - WS - Gases Laws ReviewDocumento2 páginas9 - Unit 6 - WS - Gases Laws ReviewAdam BurnettAinda não há avaliações
- EAS 1600 Fall 2018 Lab 03: The Ideal Gas Law + Heat TransferDocumento22 páginasEAS 1600 Fall 2018 Lab 03: The Ideal Gas Law + Heat TransfersamAinda não há avaliações
- Ideal Gas Law PacketDocumento6 páginasIdeal Gas Law PacketFhaye PerezAinda não há avaliações
- Intro To Gases and Gas LawsDocumento61 páginasIntro To Gases and Gas LawsLuigie100% (1)
- The Ideal Gas LawDocumento6 páginasThe Ideal Gas Lawkoko blueAinda não há avaliações
- KMT ws2Documento10 páginasKMT ws2Troy MateoAinda não há avaliações
- Gas Laws Exam: Escribe Tu Nombre CompletoDocumento4 páginasGas Laws Exam: Escribe Tu Nombre CompletoEver MendezAinda não há avaliações
- Phat (Necro) Ho - 5-00 Gases Unit Pack - 2021Documento8 páginasPhat (Necro) Ho - 5-00 Gases Unit Pack - 2021Just an Abnormal SIMPAinda não há avaliações
- Module 5.2b: Gas Laws Part 2Documento26 páginasModule 5.2b: Gas Laws Part 2Ryan PazonAinda não há avaliações
- Boyles' and Charles' Law WorksheetDocumento2 páginasBoyles' and Charles' Law WorksheetHazel Cosep Rico100% (1)
- Physics II - EngineeringG PDFDocumento110 páginasPhysics II - EngineeringG PDFRami JarrarAinda não há avaliações
- Gas Laws ThermodynamicsDocumento27 páginasGas Laws Thermodynamicsasparomaxine2Ainda não há avaliações
- Unit 5 - Combined Gas Law WorksheetDocumento1 páginaUnit 5 - Combined Gas Law WorksheetFe Pakias Gullod0% (1)
- Science10 - Q4 - Week 2 Charles Law Volume and TemperatureDocumento14 páginasScience10 - Q4 - Week 2 Charles Law Volume and TemperatureJomelyn Arzaga100% (1)
- Intro To Gases and Gas LawsDocumento42 páginasIntro To Gases and Gas LawsArcee TogyAinda não há avaliações
- The Ideal Gas Law and Gas Stoichiometry Hon)Documento3 páginasThe Ideal Gas Law and Gas Stoichiometry Hon)Camilo RosasAinda não há avaliações
- General Chemistry 1 Week 5 6Documento10 páginasGeneral Chemistry 1 Week 5 6Emmanuel ValenzuelaAinda não há avaliações
- Cheat Sheet Simple MachinesDocumento1 páginaCheat Sheet Simple Machinesapi-325864985Ainda não há avaliações
- Forces and Motion Hs Study Guide KeyDocumento2 páginasForces and Motion Hs Study Guide Keyapi-325864985Ainda não há avaliações
- Simple Machines Calculations KeyDocumento9 páginasSimple Machines Calculations Keyapi-325864985Ainda não há avaliações
- Friday Sound MediumDocumento21 páginasFriday Sound Mediumapi-325864985Ainda não há avaliações
- Motion GraphingDocumento12 páginasMotion Graphingapi-325864985Ainda não há avaliações
- 8th Grade Energy Study Guide AnswersDocumento8 páginas8th Grade Energy Study Guide Answersapi-325864985Ainda não há avaliações
- Sound Waves IntroDocumento22 páginasSound Waves Introapi-325864985Ainda não há avaliações
- Specific HeatDocumento16 páginasSpecific Heatapi-325864985Ainda não há avaliações
- Nuclearchemistry 2014 BLDocumento17 páginasNuclearchemistry 2014 BLapi-325864985Ainda não há avaliações
- Sig FigsDocumento11 páginasSig Figsapi-325864985Ainda não há avaliações
- Unit 2 Study Guide AnswersDocumento5 páginasUnit 2 Study Guide Answersapi-325864985Ainda não há avaliações
- Heattempphases 1 HspsDocumento12 páginasHeattempphases 1 Hspsapi-325864985Ainda não há avaliações
- 8th Grade Study Guide AnswersDocumento10 páginas8th Grade Study Guide Answersapi-325864985100% (1)
- Physical Science - Waves ReviewDocumento8 páginasPhysical Science - Waves Reviewapi-325864985Ainda não há avaliações
- Study Guide Electricity Magnetism AnswersDocumento3 páginasStudy Guide Electricity Magnetism Answersapi-325864985Ainda não há avaliações
- Molecular Motion HspsDocumento15 páginasMolecular Motion Hspsapi-325864985Ainda não há avaliações
- Electromagnets and Ohms Law LabDocumento2 páginasElectromagnets and Ohms Law Labapi-3258649850% (1)
- Electricity Intro Powerpoint 2017Documento10 páginasElectricity Intro Powerpoint 2017api-325864985Ainda não há avaliações
- Eoct Prep Day 1Documento8 páginasEoct Prep Day 1api-325864985Ainda não há avaliações
- 7th Grade Week of 3Documento22 páginas7th Grade Week of 3api-325864985Ainda não há avaliações
- Classification Study Guide AnswersDocumento2 páginasClassification Study Guide Answersapi-325864985100% (1)
- Unit 5 Classification Study Guide Edited 1Documento3 páginasUnit 5 Classification Study Guide Edited 1api-325864985Ainda não há avaliações
- Unit 4 Evolution Study Guide Answers 1Documento3 páginasUnit 4 Evolution Study Guide Answers 1api-32586498550% (2)
- Genetics Unit Test Study GuideDocumento2 páginasGenetics Unit Test Study Guideapi-325864985Ainda não há avaliações
- PBL Coaster 2018Documento2 páginasPBL Coaster 2018api-325864985Ainda não há avaliações
- Hsps Midterm Study GuideDocumento6 páginasHsps Midterm Study Guideapi-325864985Ainda não há avaliações
- Genetics Study Guide With AnswersDocumento26 páginasGenetics Study Guide With Answersapi-325864985100% (2)