Escolar Documentos
Profissional Documentos
Cultura Documentos
Subarachnoid Hemorrhage: Beyond Aneurysms: Carrie P. Marder Vinod Narla James R. Fink Kathleen R. Tozer Fink
Enviado por
Claire FowlTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Subarachnoid Hemorrhage: Beyond Aneurysms: Carrie P. Marder Vinod Narla James R. Fink Kathleen R. Tozer Fink
Enviado por
Claire FowlDireitos autorais:
Formatos disponíveis
N e u r o r a d i o l o g y / H e a d a n d N e c k I m a g i n g • R ev i ew
Marder et al.
Subarachnoid Hemorrhage
Neuroradiology/Head and Neck Imaging
Review
Downloaded from www.ajronline.org by 36.73.174.239 on 01/11/18 from IP address 36.73.174.239. Copyright ARRS. For personal use only; all rights reserved
FOCUS ON:
Subarachnoid Hemorrhage:
Beyond Aneurysms
Carrie P. Marder 1 OBJECTIVE. Spontaneous subarachnoid hemorrhage (SAH) typically prompts a search
Vinod Narla for an underlying ruptured saccular aneurysm, which is the most common nontraumatic
James R. Fink cause. Depending on the clinical presentation and pattern of SAH, the differential diagnosis
Kathleen R. Tozer Fink may include a diverse group of causes other than aneurysm rupture.
CONCLUSION. For the purposes of this review, we classify SAH into three main pat-
Marder CP, Narla V, Fink JR, Tozer Fink KR terns, defined by the distribution of blood on unenhanced CT: diffuse, perimesencephalic, and
convexal. The epicenter of the hemorrhage further refines the differential diagnosis and guides
subsequent imaging. Additionally, we review multiple clinical conditions that can simulate the
appearance of SAH on CT or MRI, an imaging artifact known as pseudo-SAH.
A
lthough trauma is the most com- tumor, vascular malformation, or acute arte-
mon cause of SAH, ruptured sac- rial dissection. In the third pattern, SAH is
cular aneurysms are the most com- localized to the cerebral convexities alone.
mon cause of nontraumatic SAH, This pattern is infrequent, and the differen-
accounting for approximately 85% of cases tial diagnosis encompasses a heterogeneous
of spontaneous SAH [1, 2]. Of the remaining group of diseases, including reversible cere-
15% of cases, two thirds are due to idiopathic bral vasoconstriction syndrome, cerebral am-
perimesencephalic hemorrhage, a benign non- yloid angiopathy (CAA), posterior reversible
aneurysmal form of SAH that is likely venous encephalopathy syndrome (PRES), cerebral
in origin [3, 4]. The remaining cases result venous thrombosis (CVT), and other less
from a wide variety of causes. common causes. Rarely, a fourth scenario
SAH can be classified into at least three is encountered, in which no blood is visible
distinct patterns by location on initial unen- on unenhanced CT and SAH is diagnosed by
hanced CT. Recognizing these patterns facili- lumbar puncture.
tates the differential diagnosis and refines fur-
ther imaging evaluation. Proper classification Distinct Presentations of
depends on unenhanced CT evaluation within Subarachnoid Hemorrhage as
Keywords: convexal subarachnoid hemorrhage,
3 days of symptom onset, because substantial Reflected by Anatomic Location
perimesencephalic hemorrhage, pseudo–subarachnoid
hemorrhage, subarachnoid hemorrhage redistribution occurs thereafter, fundamental- Suprasellar Central Cisterns With Diffuse
ly altering the pattern [5]. In the first pattern, Peripheral Extension
DOI:10.2214/AJR.12.9749 SAH is centered in the suprasellar or central Characteristically, saccular aneurysms arise
basal cisterns and extends peripherally in a from branch points in the circle of Willis and
Received August 4, 2012; accepted after revision
October 1, 2012.
diffuse manner. This pattern is characteristic produce a large volume of SAH when they
of saccular aneurysm rupture but may occur rupture. Accordingly, aneurysmal SAH often
1
All authors: Department of Radiology, University of with other entities, such as rupture of a non- fills the suprasellar, central, anterior, lateral,
Washington, Box 357115, 1959 NE Pacific St, NW011, saccular aneurysm or vascular malformation. posterior, and lower basal cisterns and may
Seattle, WA 98195-7115. Address correspondence to
C. P. Marder (cmarder@uw.edu).
In the second pattern, SAH is centered in the extend to the cerebral sulci. Associated in-
perimesencephalic or low basal cisterns and traventricular hemorrhage sometimes occurs,
This article is available for credit. does not extend peripherally. This pattern is such as with anterior communicating artery
characteristic of idiopathic perimesencephal- aneurysms that rupture into the third ventricle
AJR 2014; 202:25–37
ic hemorrhage but results from vertebrobasilar through the lamina terminalis. The epicenter
0361–803X/14/2021–25 aneurysm rupture in approximately 5% of cas- of SAH occasionally suggests the location of
es. Other rare causes of the perimesencephal- an underlying ruptured saccular aneurysm. For
© American Roentgen Ray Society ic pattern include a cervicomedullary junction example, SAH in the interhemispheric fissure
AJR:202, January 2014 25
Marder et al.
suggests an anterior communicating artery an- nous injury at the tentorial margin may also accompanied by neurologic deficits, hem-
eurysm, whereas sylvian fissure SAH suggests produce this pattern. orrhage, or ischemia. The entity classically
a middle cerebral artery aneurysm. Although affects young to middle-aged women after
saccular aneurysm rupture is, by far, the most Peripheral Convexal Subarachnoid exposure to a trigger, such as vasoactive or
frequent nontraumatic cause of the diffuse an- Hemorrhage Alone sympathomimetic agents, including migraine
eurysmal pattern, trauma remains the most fre- SAH may be localized to a few sulci of the medications, stimulants such as caffeine or
Downloaded from www.ajronline.org by 36.73.174.239 on 01/11/18 from IP address 36.73.174.239. Copyright ARRS. For personal use only; all rights reserved
quent overall cause of SAH and may produce cerebral convexities or to one of the sylvian amphetamines, serotonergic antidepressants,
a diffuse pattern resembling aneurysmal SAH fissures, without basal cistern or ventricular and smoking tobacco or marijuana. Other
in the setting of severe skull base fractures involvement. In the absence of trauma, iso- triggers include strenuous activity, such as
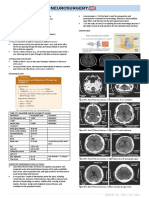
(Fig. 1) or acute arterial injuries. The differ- lated peripheral convexal SAH is rare. The sexual intercourse or exercise, or bathing in
ential diagnosis of diffuse SAH (Table 1) also condition may be underrecognized and has hot or cold water.
includes ruptured nonsaccular aneurysm, arte- only recently been described as a distinct Imaging may initially be normal or may
riovenous malformation (AVM), or dural arte- category of disease [9–16], with an estimat- show isolated convexal SAH (Fig. 5A), pa-
riovenous fistula (DAVF) (Fig. 2). ed incidence of 7% of all cases of spontane- renchymal or subdural hemorrhages, or in-
ous SAH [14]. farcts (ischemic or hemorrhagic) [23–25].
Perimesencephalic and Low Basal In the largest reported series of 29 patients Data from large prospective series suggest
Cisterns Only with nontraumatic isolated convexal SAH that convexal SAH is the most common early
Ten percent of spontaneous SAH cases are [14], the leading diagnoses were reversible ce- complication of reversible cerebral vasocon-
due to benign venous bleeding known as non- rebral vasoconstriction syndrome (Fig. 5) and striction syndrome, found in approximately
aneurysmal perimesencephalic hemorrhage CAA (Fig. 6). Other reported causes (Table 20–25% of cases [23]. The hallmark find-
[3] (Fig. 3). In 1985, van Gijn et al. [3] de- 1) include PRES (Fig. 7), CVT (Fig. 8), in- ing on vascular imaging is segmental vaso-
scribed a series of patients with SAH and fectious causes (Figs. 9 and 10), coagulation constriction (Fig. 5B) that resolves spontane-
normal angiograms who had a predominance disorders (Fig. 11), and moyamoya disease ously or with supportive treatment, including
of SAH confined to the cisterns surround- [9–11, 13, 14] (Fig. 12). Rare causes include removal of triggers and short-term use of cal-
ing the midbrain. This pattern occurred in 13 ruptured superficial vascular malformations, cium channel blockers.
of 28 patients with SAH and normal angio- tumors, and cerebral vasculitis [13, 17–21]. Cerebral amyloid angiopathy—CAA is de-
grams but in only 1 of 92 patients with SAH No cause is identified in 14–35% of cases [9, fined histopathologically by the deposition of
and ruptured aneurysms. Subsequent stud- 11, 14]. Ruptured saccular aneurysms of the amyloid protein in cortical and leptomeninge-
ies [5, 6] refined the definition of perimesen- circle of Willis are not reported to cause iso- al vessels. Because establishing a tissue diag-
cephalic hemorrhage as follows: first, blood lated convexal SAH. nosis is invasive, the Boston criteria have been
is centered immediately anterior to the mid- There are two main clinical presentations developed that allow the noninvasive desig-
brain or pons and may variably involve the of convexal SAH, roughly stratified by patient nations of “possible” or “probable” CAA for
interpeduncular, crural, ambient, quadrigem- age [14]. Younger patients (≤ 60 years old) patients older than 50 years who have lobar
inal, prepontine, or carotid cisterns; second, are more likely to have reversible cerebral va- hemorrhages or cortical or subcortical micro-
blood may thinly extend into the suprasellar soconstriction syndrome and to present with hemorrhages on imaging [26]. Convexal SAH
cistern and the basal portions of the sylvian sudden severe headache sometimes accom- has been increasingly recognized in associa-
and interhemispheric fissures, but may not panied by neurologic deficits or stroke. Head- tion with CAA, usually in elderly patients pre-
extend into the distal portions of the sylvian ache characteristics may be indistinguish- senting with transient ischemic attack–like
or interhemispheric fissures; and third, small able from those of aneurysmal SAH. Older symptoms [14, 16, 27–32]. Acutely, convexal
amounts of blood may sediment in the occipi- patients (> 60 years old) are more likely to SAH is best visualized on unenhanced CT or
tal horns of the lateral ventricles, but there is have CAA and to present with transient sen- FLAIR images and may not be visible on gra-
no frank intraventricular hemorrhage. sory or motor symptoms, such as numbness, dient-recalled echo or susceptibility-weighted
These criteria apply only to unenhanced tingling, or weakness. These symptoms oc- images. Gradient-recalled echo and suscepti-
CT obtained within 3 days of symptom on- cur with convexal SAH due to CAA, as well bility-weighted images are highly sensitive to
set, because redistribution of SAH may con- as other causes [22], and are probably relat- detect prior episodes of convexal SAH, show-
siderably alter the initial pattern. In approxi- ed to cortical irritation by blood products in ing low-signal-intensity hemosiderin filling a
mately 95% of cases that meet these criteria, the subarachnoid space. Headache is another sulcus (subarachnoid siderosis) or staining the
no aneurysm is found and the clinical course common clinical feature in this patient group, underlying cortex (superficial cortical sidero-
is favorable, with little to no risk of rebleed- but the onset is usually more gradual and sis) [29] (Fig. 6). Accordingly, a modification
ing, vasospasm, or symptomatic hydroceph- the intensity less severe compared with the to the Boston criteria has been proposed that
alus [3, 7, 8]. The presumed cause is venous “thunderclap” headache characteristic of an- would include these findings [30].
rupture [3, 4]. Only 5% of cases meeting eurysmal SAH. Other clinical presentations Posterior reversible encephalopathy syn-
these criteria are due to ruptured vertebro- of convexal SAH include altered mental sta- drome—PRES is a clinical-radiologic entity
basilar aneurysms [5, 6]. Other rare causes tus, lethargy, confusion, or seizures. most commonly associated with pregnancy-
include posterior fossa and cervical spinal Reversible cerebral vasoconstriction syn- induced hypertension, eclampsia, severe hy-
AVMs, DAVFs, and vascular tumors such drome—Reversible cerebral vasoconstriction pertension of any cause, and the use of the im-
as hemangioblastomas (Fig. 4 and Table 1). syndrome is characterized clinically by the munosuppressive medications cyclosporine
Trauma leading to arterial dissection or ve- sudden onset of severe headache, sometimes and tacrolimus after solid-organ or allogeneic
26 AJR:202, January 2014
Subarachnoid Hemorrhage
TABLE 1: Differential Diagnosis of Subarachnoid Hemorrhage by Pattern of Hemorrhage
Diffuse Perimesencephalic Convexal
Trauma Trauma Trauma
Saccular aneurysm Nonaneurysmal perimesencephalic hemorrhage Reversible cerebral vasoconstriction syndrome
Nonsaccular aneurysm Saccular aneurysm Cerebral amyloid angiopathy
Downloaded from www.ajronline.org by 36.73.174.239 on 01/11/18 from IP address 36.73.174.239. Copyright ARRS. For personal use only; all rights reserved
Arterial dissection Nonsaccular aneurysm Posterior reversible encephalopathy syndrome
Vascular malformation Arterial dissection Cerebral venous thrombosis
Tumor Vascular malformation (consider spinal) Septic emboli, septic aneurysm
Vasculitis Tumor (consider spinal) Coagulopathy
Moyamoya disease
Vascular malformation (superficial)
Tumor
Vasculitis
bone-marrow transplantation. PRES may oc- Infectious causes—SAH complicates ap- ciated with moyamoya disease include sickle
cur in a wide variety of other clinical condi- proximately 1–2% of cases of infectious en- cell disease, neurofibromatosis type 1, Down
tions and is sometimes idiopathic. Other com- docarditis. The distribution of SAH is usual- syndrome, and connective tissue diseases.
mon terms referring to the same entity include ly convexal (Fig. 9A) or localized within one Patients present with headaches, hypoperfu-
“reversible posterior leukoencephalopathy” sylvian fissure, but may be diffuse [41] or sion symptoms, transient ischemic attacks, or
and “hypertensive encephalopathy.” Patients may be occult on CT [42]. Patients may have strokes and may have infarcts or hemorrhag-
present with seizures, headache, visual loss, a history of injection drug use and present es. With disease progression, transdural and
or altered mental status. PRES is an impor- with fever, headache, mental status changes, transosseous pial collaterals from external ca-
tant cause of isolated convexal SAH that has and physical examination signs of systemic rotid artery branches may develop, and these
been recognized in multiple small series and emboli. Associated imaging findings include abnormal fragile collateral vessels may rup-
case reports [9–11, 14, 33–35] (Fig. 7A). embolic infarcts (Fig. 9B), microhemorrhag- ture, leading to SAH [45, 46] (Fig. 12). Sac-
Vasogenic edema involving the subcortical es (Figs. 9C and 9D), or microabscesses. Al- cular aneurysms may also form, so associated
white matter of the parietal, occipital, or pos- though some cases are secondary to rupture aneurysm rupture should be actively excluded
terior frontal lobes bilaterally, and sometimes of septic (mycotic) aneurysms (Fig. 10), an- in patients with moyamoya disease and SAH.
involving the cerebellum, basal ganglia, thala- giography may be normal. In this case, SAH
mus, or brainstem, is characteristic of PRES may result from focal endarteritis, vessel rup- Special Cases
(Fig. 7B). Occasionally, PRES may manifest ture at the site of embolic occlusion, or occult There are several rare causes of SAH that
as restricted diffusion, enhancement, and hem- septic aneurysm. Brain abscesses in the early lack specific imaging features on unenhanced
orrhages, including convexal SAH, lobar he- cerebritis stage may also rarely present with CT and could produce SAH in any anatomic
matomas, and microhemorrhages [36, 37]. convexal SAH [43]. distribution. These include both vascular and
Cerebral venous thrombosis—Venous throm- Coagulopathies—Anticoagulation and co- nonvascular causes. Vascular causes include
bosis involving either a cortical vein or dural agulation disorders such as idiopathic throm- vascular malformations and intracranial vas-
venous sinus is an important cause of convexal bocytopenia can also cause isolated convexal culitis. Vascular malformations, such as cav-
SAH [38–40] (Fig. 8). Patients present clinical- SAH [11, 14]. Complex disorders such as dis- ernous malformations, AVMs, and DAVFs,
ly with headache, seizures, altered mental sta- seminated intravascular coagulation, usually usually cause hemorrhage into the brain pa-
tus, and sequelae of increased intracranial pres- occurring as a complication of systemic sep- renchyma or ventricles, but may occasionally
sure, such as papilledema. Hallmark findings sis, may also lead to convexal SAH [44] (Fig. cause isolated SAH in a pattern indistinguish-
include the cord sign (i.e., a hyperdense cor- 11). SAH in this setting may result from a able from aneurysm rupture, confined to the
tical vein on unenhanced CT) and the empty systemic cascade of microclot formation, con- cerebral convexities, or within a perimesen-
delta sign (i.e., nonfilling of the superior sag- sumption of platelets and clotting factors, small cephalic distribution. Rarely, a spinal vascu-
ittal sinus on CT venography). Commonly de- vessel occlusions, and bleeding manifestations. lar malformation causes SAH [19]. Intracra-
scribed parenchymal findings result from ve- Moyamoya disease—Moyamoya (“puff of nial vasculitis can be due to primary angiitis
nous hypertension and include cerebral edema, smoke” in Japanese) refers to the distinctive of the CNS or secondary to systemic vasculi-
parenchymal hemorrhages, and ischemic and angiographic appearance of collateral lentic- tis or connective tissue diseases, and typical-
hemorrhagic infarcts. SAH is a relatively un- ulostriate vessels that develop after chronic ly presents with infarcts, parenchymal hem-
common complication, likely resulting from stenosis of the intracranial internal carotid ar- orrhages, nonspecific white matter lesions,
rupture of thin-walled cortical veins under el- teries and proximal branches of the circle of or enhancing areas. SAH in a convexal or
evated pressure. In this setting, SAH is usually Willis. The condition may be congenital (id- diffuse distribution is a rare presentation of
found within the cerebral convexities or sylvi- iopathic) or acquired after progressive vascu- intracranial vasculitis [17, 18]. Nonvascular
an fissures, sparing the basal cisterns [40]. lar occlusion from any cause. Conditions asso- causes include intraaxial and extraaxial tumors.
AJR:202, January 2014 27
Marder et al.
There are rare reports of meningiomas [21], in- Spontaneous intracranial hypotension is re- ic SAH. The artifact is caused by the inflow
filtrating gliomas [47], and angiolipomas [20] ported to produce pseudo-SAH on CT, occur- of nonnulled CSF in areas of high CSF flow
presenting with SAH. Pituitary apoplexy is an- ring in 10% of 40 consecutive patients in one rate, typically in the cervical or thoracic spi-
other rarely reported cause of SAH [2]. series [54]. The characteristic CT findings in- nal canal, the third or fourth ventricles, cere-
clude increased attenuation of the tentorium, bral aqueduct, and just superior to the fora-
Pathologic Entities and Imaging basal cisterns, and sylvian fissures, corre- men of Monro. Occasionally, CSF pulsation
Downloaded from www.ajronline.org by 36.73.174.239 on 01/11/18 from IP address 36.73.174.239. Copyright ARRS. For personal use only; all rights reserved
Artifacts That Mimic Subarachnoid sponding to MRI findings of pachymeningeal artifact interferes with evaluation of the basal
Hemorrhage tentorial enhancement and obliteration of the cisterns in the posterior fossa. Cross-referenc-
Several conditions can produce the false basal cisterns and sylvian fissures due to brain ing with coronal and sagittal imaging planes
appearance of SAH on CT or MRI, known as sagging. Subdural fluid collections were pres- or with faster sequences, such as gradient-re-
pseudo-SAH (Table 2). The causes of pseu- ent in each case, and the authors speculate that called echo or true fast imaging with steady-
do-SAH include nonhemorrhagic subarach- two previously published reports that describe state precession, is helpful [60].
noid space pathologic abnormalities, such as subdural hematomas as a cause of pseudo- An even more troublesome artifact relates
meningitis and leptomeningeal carcinomato- SAH were likely unrecognized cases of spon- to patient motion on single-shot fast spin-
sis, and imaging artifacts occurring in the ab- taneous intracranial hypotension. echo FLAIR images, a sequence reserved for
sence of subarachnoid space abnormality. Iatrogenic causes of pseudo-SAH on CT in- moving patients. In this scenario, head dis-
clude recently administered intrathecal or IV placement between the inversion and acqui-
Pathologic Abnormalities Affecting the contrast material. Hyperdense subarachnoid sition pulses may result in areas of nonnulled
Subarachnoid Space material over the convexities resembling CSF in regions not associated with high CSF
Acute bacterial leptomeningitis results in convexal SAH is sometimes found on unen- pulsatile flow, such as in the sulci overlying
disruption of the blood-brain barrier (BBB) hanced CT obtained after endovascular proce- the frontal convexities [61]. The artifactual
and leakage of proteins into the CSF. In severe dures, including aneurysm coiling (Fig. 14) nature of the hyperintense CSF FLAIR sig-
cases, the increase in CSF protein concentra- and stroke intervention [55, 56]. The presumed nal may be difficult to appreciate, because
tion may be sufficient to cause increased CSF mechanism is contrast material extravasation the images may otherwise appear free of mo-
attenuation on CT, giving the false appearance in areas of BBB breakdown related to hyper- tion artifact. Increasing the inversion pulse
of SAH [48]. Elevated CSF protein concentra- perfusion injury [56] or subclinical ischemia section width or using a non–section-selec-
tion also decreases the T1 relaxation time of resulting from temporary vessel occlusion by tive initial inversion pulse can help to reduce
CSF and creates hyperintense CSF on FLAIR microcatheters or balloon inflation [55]. Dif- the artifact [61].
images, mimicking SAH [49, 50]. A similar ferentiating postprocedural contrast extrava- Pseudo-SAH on MRI has been increasingly
increase in CSF signal intensity on FLAIR sation from SAH is important because it may reported in conditions associated with altered
images has been described in leptomeningeal affect patient treatment. Extravasated contrast perfusion and BBB disruption, including acute
carcinomatosis [50, 51], also likely related to material typically clears within several hours ischemic stroke [62] and after carotid artery
elevated CSF protein concentration. [56], helping to confirm the diagnosis. stenting [63] or balloon test occlusion [64]. The
hyperintense acute reperfusion marker refers to
Imaging Artifacts on CT Imaging Artifacts on MRI hyperintense CSF signal on FLAIR imaging,
Patients with anoxic encephalopathy (Fig. FLAIR imaging is sensitive to both acute found in association with compromised perfu-
13) sometimes exhibit diffuse hyperdensi- and subacute SAH, making it a viable alter- sion or hyperperfusion syndromes and caused
ty within the basal cisterns and subarachnoid native when CT is equivocal. Several clini- by leakage of small amounts of gadolinium-
spaces on CT, without SAH detected by lum- cal conditions, however, can mimic SAH based contrast material through a compro-
bar puncture or autopsy [52, 53]. Pseudo-SAH on FLAIR imaging. Patients receiving gen- mised BBB [65]. In one study, small quantities
in this situation results from a combination of eral anesthesia for MRI may have diffuse of gadolinium-based contrast material were
diffuse cerebral edema, resulting in decreased FLAIR signal hyperintensity in the basal cis- isolated from the CSF of patients with hyper-
attenuation of brain parenchyma, effacement terns and cerebral sulci, a finding originally intense acute reperfusion marker, and phantom
of the subarachnoid spaces, and engorgement thought to be an effect of inhaled anesthetic studies showed that the detected gadolinium-
of venous structures on the pial surfaces. The agents, but later shown to result from the T1- based contrast material concentrations pro-
apparent high attenuation of the subarachnoid shortening effects of the administration of duced enhancement on FLAIR images, where-
spaces is largely perceptual, and the actual CT 100% oxygen [57–59] (Fig. 15). as 10-fold higher concentrations were needed
attenuation values within the basal cisterns CSF pulsation artifact can also produce for enhancement on T1-weighted images [65].
measure lower than expected for blood prod- FLAIR signal hyperintensity in the ventri- Leakage of gadolinium-based contrast material
ucts [53]. cles or subarachnoid spaces that may mim- has also been associated with pseudo-SAH on
TABLE 2: Differential Diagnosis of Pseudo–Subarachnoid Hemorrhage
Subarachnoid Space Pathology CT Artifacts MRI Artifacts on FLAIR
Meningitis Anoxic encephalopathy Supplemental oxygen
Leptomeningeal carcinomatosis Spontaneous intracranial hypotension CSF pulsation
Iatrogenic Patient motion on single-shot technique
Gadolinium-based contrast material leak (particularly with renal failure or
disrupted blood-brain barrier)
28 AJR:202, January 2014
Subarachnoid Hemorrhage
FLAIR images in patients with renal failure, in Convexal Subarachnoid Hemorrhage Alone tends peripherally to the cerebral convexi-
patients receiving high concentrations of gad- MRI may be an appropriate test in some ties. However, saccular aneurysm rupture is
olinium-based contrast material, and in seem- patients presenting with isolated convexal an uncommon cause of SAH confined to the
ingly healthy patients with intact renal function SAH [10, 13], particularly to confirm a diag- perimesencephalic cisterns and is not a typ-
and an intact BBB [66]. nosis of PRES or CAA. CVT may be seen on ical cause of SAH confined to the cerebral
MRI but is typically confirmed by CT or MR convexities. Accordingly, the imaging find-
Downloaded from www.ajronline.org by 36.73.174.239 on 01/11/18 from IP address 36.73.174.239. Copyright ARRS. For personal use only; all rights reserved
Imaging Protocols venography. Peripheral SAH involving the ings on initial unenhanced CT can help focus
Unenhanced CT is the best initial test for sylvian fissures should probably be evaluat- the differential diagnosis and guide the sub-
patients clinically suspected to have SAH. ed similarly to diffuse SAH, so as to exclude sequent imaging evaluation.
When unenhanced CT findings are positive, middle cerebral artery bifurcation aneu-
or when clinical suspicion for aneurysm rup- rysms or septic aneurysms, which typically References
ture is high, CT angiography (CTA) or digital arise more distally than saccular aneurysms. 1. Rinkel GJ, van Gijn J, Wijdicks EF. Subarachnoid
subtraction angiography (DSA) is performed Patients with signs of infection and convex- hemorrhage without detectable aneurysm: a re-
to exclude underlying saccular aneurysm. In al SAH on unenhanced CT may have septic view of the causes. Stroke 1993; 24:1403–1409
some institutions, DSA may be repeated if emboli on MRI, but vascular imaging with 2. van Gijn J, Rinkel GJE. Subarachnoid hemor-
findings are negative. The imaging evaluation CTA, MR angiography, or DSA may help ex- rhage: diagnosis, causes and management. Brain
of diffuse SAH is shown schematically in Fig- clude septic aneurysms. Most other patients 2001; 124:249–278
ure 16A. Nonaneurysmal causes of SAH are with convexal SAH should undergo vascular 3. van Gijn J, van Dongen KJ, Vermeulen M, Hijdra
also usually diagnosed by this standard work- imaging to evaluate for reversible cerebral A. Perimesencephalic hemorrhage: a nonaneurys-
up for aneurysmal SAH, but MRI may be vasoconstriction syndrome, vasculitis or oth- mal and benign form of subarachnoid hemor-
needed in select cases to exclude rare causes er vasculopathies, small peripheral vascular rhage. Neurology 1985; 35:493–497
such as tumors. malformations, or thrombosed veins [12]. 4. van der Schaaf IC, Velthuis BK, Gouw A, Rinkel
Particular attention on these studies to high- GJE. Venous drainage in perimesencephalic hem-
Perimesencephalic Pattern of er order branches of the vascular tree is war- orrhage. Stroke 2004; 35:1614–1618
Subarachnoid Hemorrhage ranted to exclude such entities. The imaging 5. Velthuis BK, Rinkel GJE, Ramos LMP, Witkamp
Velthuis et al. [5] showed that if unen- evaluation of convexal SAH is shown sche- TD, van Leeuwen MS. Perimesencephalic hemor-
hanced CT was performed within 3 days of matically in Figure 16C. rhage: exclusion of vertebrobasilar aneurysms
symptom onset and strict criteria were fol- with CT angiography. Stroke 1999; 30:1103–1109
lowed for defining a perimesencephalic dis- No Visible Blood on CT 6. Rinkel GJE, Wijdicks EFM, Vermeulen M, et al.
tribution of blood, a high-quality CTA was Patients presenting with sudden severe Nonaneurysmal perimesencephalic subarachnoid
sufficient to accurately identify the 5% of pa- headache who are clinically suspected to hemorrhage: CT and MR patterns that differ from
tients harboring a vertebrobasilar aneurysm, have a saccular aneurysm but who have nor- aneurysmal rupture. AJNR 1991; 12:829–834
without the need for DSA and its inherent mal findings on unenhanced CT at the time 7. Rinkel GJE, Wijdicks EFM. Outcome in patients
risks. These findings have been reproduced of presentation should undergo delayed lum- with subarachnoid haemorrhage and negative angi-
in several studies [12, 67–69] and are sup- bar puncture to analyze for the presence of ography according to pattern of haemorrhage on
ported by a theoretic decision analytic model blood degradation products in the CSF [2, computed tomography. Lancet 1991; 338:964–968
that concluded that, in order for DSA to be 72]. A delay of 12 hours from symptom onset 8. Herrmann LL, Zabramski JM. Nonaneurysmal
the preferred imaging strategy, the complica- is required to distinguish true xanthochromia subarachnoid hemorrhage: a review of clinical
tion rate of catheter angiography would need from a traumatic tap. Patients in whom xan- course and outcome in two hemorrhage patterns. J
to be less than 0.2% [70]. Nonetheless, insti- thochromia is detected should undergo CTA Neurosci Nurs 2007; 39:135–142
tutional practices vary widely regarding im- to evaluate for a saccular aneurysm. Just as 9. Spitzer C, Mull M, Rohde V, Kosinski CM. Non-
aging protocols for evaluating SAH, which for the perimesencephalic hemorrhage pat- traumatic cortical subarachnoid haemorrhage:
may be partially informed by differences in tern, multiple studies have reported that CTA diagnostic work-up and aetiological background.
image postprocessing techniques used for is sufficient to exclude an aneurysm in cases Neuroradiology 2005; 47:525–531
CTA reconstruction. The imaging evaluation where no blood is visible on CT, without ex- 10. Patel KC, Finelli PF. Nonaneurysmal convexity
of perimesencephalic hemorrhage is shown posing the patient to the risks of DSA [12, subarachnoid hemorrhage. Neurocrit Care 2006;
schematically in Figure 16B. 67–69]. Before the advent of high-quality 4:229–233
An additional issue is whether radiologists CTA, it was argued that repeat DSA was not 11. Refai D, Botros JA, Strom RG, Derdeyn CP, Shar-
can accurately identify patients with a perimes- needed in these cases [3]. At many institu- ma A, Zipfel GJ. Spontaneous isolated convexity
encephalic hemorrhage pattern on unenhanced tions, however, DSA is still performed after subarachnoid hemorrhage: presentation, radio-
CT in whom aneurysms might be safely ex- negative CTA on clinical grounds. The imag- logical findings, differential diagnosis, and clini-
cluded by CTA alone. Although earlier studies ing evaluation of SAH with no visible blood cal course. J Neurosurg 2008; 109:1034–1041
[5, 6] reported excellent interobserver agree- on unenhanced CT is shown schematically in 12. Agid R, Andersson T, Almqvist H, et al. Negative
ment, a recent study found only good inter- and Figure 16D. CT angiography findings in patients with sponta-
intraobserver agreement in characterizing the neous subarachnoid hemorrhage: when is digital
perimesencephalic hemorrhage pattern [71]. Conclusion subtraction angiography still needed? AJNR
The authors suggest that sufficient disagree- Saccular aneurysm rupture is the most 2010; 31:696–705
ment may exist to warrant caution in deciding common cause of SAH that diffusely fills the 13. Cuvinciuc V, Viguier A, Calviere L, et al. Isolated
to withhold angiography in these patients. suprasellar and central basal cisterns and ex- acute nontraumatic cortical subarachnoid hemor-
AJR:202, January 2014 29
Marder et al.
rhage. AJNR 2010; 31:1355–1362 symptomatic or occult subarachnoid hemorrhage. 43. Rhode V, van Oosterhout A, Mull M, Gilsbach
14. Kumar S, Goddeau RP, Selim MH, et al. Atrau- Eur Neurol 2007; 57:103–105 JM. Subarachnoid haemorrhage as initial symp-
matic convexal subarachnoid hemorrhage. Neu- 28. Kleinig TJ, Kiley M, Thompson PD. Acute con- tom of multiple brain abscesses. Acta Neurochir
rology 2010; 74:893–899 vexity subarachnoid haemorrhage: a cause of au- (Wien) 2000; 142:205–208
15. Beitzke M, Gattringer T, Enzinger C, Wagner G, ra-like symptoms in the elderly. Cephalalgia 44. Schwartzman RJ, Hill JB. Neurologic complica-
Niederkorn K, Fazekas F. Clinical presentation, 2008; 28:658–663 tions of disseminated intravascular coagulation.
Downloaded from www.ajronline.org by 36.73.174.239 on 01/11/18 from IP address 36.73.174.239. Copyright ARRS. For personal use only; all rights reserved
etiology, and long-term prognosis in patients with 29. Linn J, Herms J, Dichgans M, et al. Subarachnoid Neurology 1982; 32:791–797
nontraumatic convexal subarachnoid hemorrhage. hemosiderosis and superficial cortical hemosider- 45. Marushima A, Yanaka K, Matsuki T, Kojima H,
Stroke 2011; 42:3055–3060 osis in cerebral amyloid angiopathy. AJNR 2008; Nose T. Subarachnoid hemorrhage not due to rup-
16. Raposo N, Viguier A, Cuvinciuc V, et al. Cortical 29:184–186 tured aneurysm in moyamoya disease. J Clin Neu-
subarachnoid haemorrhage in the elderly: a recur- 30. Linn J, Halpin A, Demaerel P, et al. Prevalence of rosci 2006; 13:146–149
rent event probably related to cerebral amyloid superficial siderosis in patients with cerebral amy- 46. Osanai T, Kuroda S, Nakayama N, Yamauchi T,
angiopathy. EurJ Neurol 2011; 18:597–603 loid angiopathy. Neurology 2010; 74:1346–1350 Houkin K, Iwasaki Y. Moyamoya disease present-
17. Fukumoto S, Kinjo M, Hokamura K, Tanaka K. 31. Brunot S, Osseby GV, Rouaud O, et al. Transient ing with subarachnoid hemorrhage localized over
Subarachnoid hemorrhage and granulomatous an- ischaemic attack mimics revealing focal sub- the frontal cortex: case report. Surg Neurol 2008;
giitis of the basilar artery: demonstration of the arachnoid hemorrhage. Cerebrovasc Dis 2010; 69:197–200
varicella-zoster-virus in the basilar artery lesions. 30:597–601 47. Hentschel S, Toyota B. Intracranial malignant
Stroke 1986; 17:1024–1028 32. Linn J. Central sulcus focal subarachnoid hemor- glioma presenting as subarachnoid hemorrhage.
18. Kumar R, Wijdicks EFM, Brown RD, Parisi JE, rhage in the elderly: cerebral amyloid angiopathy Can J Neurol Sci 2003; 30:63–66
Hammond CA. Isolated angiitis of the CNS pre- is the most frequent cause. AJNR 2011; 32:E161 48. Mendelsohn DB, Moss ML, Chason DP, Muphree
senting as subarachnoid hemorrhage. J Neurol 33. Teksam M, Casey SO, Michel E, Truwit CL. Sub- S, Casey S. Acute purulent leptomeningitis mim-
Neurosurg Psychiatry 1997; 62:649–651 arachnoid hemorrhage associated with cyclospo- icking subarachnoid hemorrhage on CT. J Com-
19. Kim CH, Kim HJ. Cervical subarachnoid floating rine A neurotoxicity in a bone-marrow transplant put Assist Tomogr 1994; 18:126–128
cavernous malformation presenting with recur- recipient. Neuroradiology 2001; 43:242–245 49. Melhem ER, Jara H, Eustace S. Fluid-attenuated
rent subarachnoid hemorrhage. J Neurol Neuro- 34. Servillo G, Striano P, Striano S, et al. Posterior inversion recovery MR imaging: identification of
surg Psychiatry 2002; 72:668 reversible encephalopathy syndrome (PRES) in protein concentration thresholds for CSF hyperin-
20. Vilela P, Saraiva P, Goulao A. Intracranial angio- critically ill obstetric patients. Intensive Care Med tensity. AJR 1997; 169:859–862
lipoma as cause of subarachnoid hemorrhage: 2003; 29:2323–2326 50. Maeda M, Yagishita A, Yamamoto T, Sakuma H,
case report and review of the literature. Neurora- 35. Shah AK. Non-aneurysmal primary subarachnoid Takeda K. Abnormal hyperintensity within the
diology 2005; 47:91–96 hemorrhage in pregnancy-induced hypertension subarachnoid space evaluated by fluid-attenuated
21. Rim N-J, Kim HS, Kim SYA. “Benign” sphenoid and eclampsia. Neurology 2003; 61:117–120 inversion-recovery MR imaging: a spectrum of
ridge meningioma manifesting as a subarachnoid 36. McKinney AM, Short J, Truwit CL, et al. Poste- central nervous system diseases. Eur Radiol
hemorrhage associated with tumor invasion into rior reversible encephalopathy syndrome: inci- 2003; 13:L192–L201
the middle cerebral artery. Korean J Radiol 2008; dence of atypical regions of involvement and im- 51. Tsuchiya K, Katase S, Yoshino A, Hachiya J. FLAIR
9:S10–S13 aging findings. AJR 2007; 189:904–912 MR imaging for diagnosing intracranial meningeal
22. Field DK, Kleinig TJ. Aura attacks from acute 37. Hefzy HM, Bartynski WS, Boardman JF, Laco- carcinomatosis. AJR 2001; 176:1585–1588
convexity subarachnoid hemorrhage not due to mis D. Hemorrhage in posterior reversible en- 52. al-Yamany M, Deck J, Bernstein M. Pseudo-sub-
cerebral amyloid angiopathy. Cephalalgia 2010; cephalopathy syndrome: imaging and clinical arachnoid hemorrhage: a rare neuroimaging pit-
31:368–371 features. AJNR 2009; 30:1371–1379 fall. Can J Neurol Sci 1999; 26:57–59
23. Ducros A, Boukobza M, Porcher R, Sarov M, Va- 38. Chang R, Friedman DP. Isolated cortical venous 53. Given CA, Burdette JH, Elster AD, Williams DW.
lade D, Bousser MG. The clinical and radiologic thrombosis presenting as subarachnoid hemorrhage: Pseudo-subarachnoid hemorrhage: a potential im-
spectrum of reversible cerebral vasoconstriction a report of 3 cases. AJNR 2004; 25:1676–1679 aging pitfall associated with diffuse cerebral ede-
syndrome: a prospective series of 67 patients. 39. Oppenheim C, Domigo V, Gauvrit J-Y. Subarach- ma. AJNR 2003; 24:254–256
Brain 2007; 130:3091–3101 noid hemorrhage as the initial presentation of du- 54. Schievink WI, Maya MM, Tourje J, Moser FG.
24. Moustafa RR, Allen CMC, Baron JC. Call-Flem- ral sinus thrombosis. AJNR 2005; 26:614–617 Pseudo-subarachnoid hemorrhage: a CT finding
ing syndrome associated with subarachnoid 40. Benabu Y, Mark L, Daniel S, Glikstein R. Cere- in spontaneous intracranial hypotension. Neurol-
haemorrhage: three new cases. J Neurol Neuro- bral venous thrombosis presenting with subarach- ogy 2005; 65:135–137
surg Psychiatry 2008; 79:602–605 noid hemorrhage: case report and review. Am J 55. Ozturk A, Saatci I, Pamuk AG, et al. Focal in-
25. Marder CP, Donohue M, Weinstein J, Fink KR. Emerg Med 2009; 27:96–106 creased cortical density in immediate postemboli-
Multimodal imaging of reversible cerebral vaso- 41. Chukwudelunzu FE, Brown RD, Wijdicks EFM, zation CT scans of patients with intracranial an-
constriction syndrome: a series of six cases. AJNR Steckelberg JM. Subarachnoid haemorrhage asso- eurysms. AJNR 2006; 27:1866–1875
2012; 33:1403–1410 ciated with infectious endocarditis: case report and 56. Kumar G, Soni CR, Sahota PK. Transient CT hy-
26. Knudsen KA, Rosand J, Karluk D, Greenberg literature review. Eur J Neurol 2002; 9:423–427 perattenuation after Merci clot retrieval and intra-
SM. Clinical diagnosis of cerebral amyloid angi- 42. Vincent FM, Zimmerman JE, Auer TC, Martin arterial thrombolysis in acute stroke mimicking
opathy: validation of the Boston criteria. Neurol- DB. Subarachnoid hemorrhage: the initial mani- subarachnoid hemorrhage. J Vasc Interv Radiol
ogy 2001; 56:537–539 festation of bacterial endocarditis—report of a 2010; 21:281–284
27. Karabatsou K, Lecky BRJ, Rainov RG, Broome case with negative arteriography and computed 57. Deliganis AV. Cerebrospinal fluid signal intensity
JC, White RP. Cerebral amyloid angiopathy with tomography. Neurosurgery 1980; 7:488–490 increase on FLAIR MR images in patients under
30 AJR:202, January 2014
Subarachnoid Hemorrhage
general anesthesia: the role of supplemental O2. FLAIR following acute stroke and intravenous con- subarachnoid hemorrhage. Neurosurgery 2006;
Radiology 2001; 218:152–156 trast medium. Neuroradiology 2000; 42:608–611 59:798–801; discussion, 801–802
58. Braga FT, Rocha AJ, Filho GH, Arikawa RK, Ri- 63. Martin AJ, Saloner DA, Roberts TPL, et al. Ca- 68. Westerlaan HE, Gravendeel J, Fiore D, et al. Mul-
beiro IM, Fonseca RB. Relationship between the rotid stent delivery in an XMR suite: immediate tislice CT angiography in the selection of patients
concentration of supplemental oxygen and signal assessment of the physiologic impact of extracra- with ruptured intracranial aneurysms suitable for
intensity of CSF depicted by fluid-attenuated inver- nial revascularization. AJNR 2005; 26:531–537 clipping or coiling. Neuroradiology 2007; 49:
Downloaded from www.ajronline.org by 36.73.174.239 on 01/11/18 from IP address 36.73.174.239. Copyright ARRS. For personal use only; all rights reserved
sion recovery imaging. AJNR 2003; 24:1863–1868 64. Michel E, Liu H, Remley KB, et al. Perfusion MR 997–1007
59. Frigon C, Shaw DW, Heckbert SR, Weinberger E, neuroimaging in patients undergoing balloon test 69. Kelliny M, Maeder P, Binaghi S, Levivier M, Reg-
Jardine D. Supplemental oxygen causes increased occlusion of the internal carotid artery. AJNR li L, Meuli R. Cerebral aneurysm exclusion by CT
signal intensity in subarachnoid cerebrospinal 2001; 22:1590–1596 angiography based subarachnoid hemorrhage pat-
fluid on brain FLAIR MR images obtained in 65. Köhrmann M, Struffert T, Frenzel T, Schwab S, tern: a retrospective study. BMC Neurol 2011; 11:8
children during general anesthesia. Radiology Doerfler A. The hyperintense acute reperfusion 70. Ruigrok YM, Rinkel GJE, Buskens E, Velthuis
2004; 233:51–55 marker on fluid-attenuated inversion recovery BK, van Gijn J. Perimesencephalic hemorrhage
60. Lisanti C, Carlin C, Banks K, Wang D. Normal magnetic resonance imaging is caused by gado- and CT angiography: a decision analysis. Stroke
MRI appearance and motion-related phenomena linium in the cerebrospinal fluid. Stroke 2012; 2000; 31:2976–2983
of CSF. AJR 2007; 188:716–725 43:259–261 71. Brinjikji W, Kallmes DF, White JB, Lanzino G,
61. Cianfoni A, Martin MGM, Du J, et al. Artifact 66. Morris JM, Miller GM. Increased signal in the Morris JM, Cloft HJ. Inter- and intraobserver
simulating subarachnoid hemorrhage and intra- subarachnoid space on fluid-attenuated inversion agreement in CT characterization of nonaneurys-
ventricular hemorrhage on single-shot, fast spin- recovery imaging associated with the clearance mal perimesencephalic subarachnoid hemorrhage.
echo fluid-attenuated inversion recovery images dynamics of gadolinium chelate: a potential diag- AJNR 2010; 31:1103–1105
caused by head movement: a trap for the unwary. nostic pitfall. AJNR 2007; 28:1964–1967 72. van der Wee N, Rinkel GJE, Hasan D, van Gijn J.
AJNR 2006; 27:843–849 67. Kershenovich A, Rappaport ZH, Maimon S. Detection of subarachnoid hemorrhage on early
62. Dechambre SD, Duprez T, Grandin CB, Lecouvet Brain computed tomography angiographic scans CT: is lumbar puncture still needed after a nega-
FE, Peeters A, Cosnard G. High signal in cerebrospi- as the sole diagnostic examination for excluding tive scan? J Neurol Neurosurg Psychiatry 1995;
nal fluid mimicking subarachnoid haemorrhage on aneurysms in patients with perimesencephalic 58:357–359
A B
Fig. 1—43-year-old man with severe trauma.
Unenhanced CT shows extensive subarachnoid Fig. 2—51-year-old man with history of hypertension and tobacco use who awoke from sleep with worst
hemorrhage in central pattern, filling suprasellar headache of his life.
(ss) and central cisterns, sylvian (Sy) and A and B, Unenhanced CT (A) shows subarachnoid hemorrhage in interhemispheric fissure (i), bilateral
interhemispheric (i) fissures, and cerebral sulci, with sylvian fissures (Sy), and bilateral cerebral convexities. Rounded density in right inferior frontal lobe (arrow,
associated hydrocephalus. Maxillofacial CT (not A) corresponds to dilated draining veins visible on digital subtraction angiography (DSA) (arrow, B) and CT
shown) showed fractures of sphenoid bone. angiography (not shown). DSA (left external carotid artery injection, lateral projection) (B) shows tangle of
vessels near cribriform plate supplied by internal maxillary and middle meningeal arteries and drained by
parasagittal cortical veins, consistent with dural arteriovenous fistula.
AJR:202, January 2014 31
Marder et al.
Downloaded from www.ajronline.org by 36.73.174.239 on 01/11/18 from IP address 36.73.174.239. Copyright ARRS. For personal use only; all rights reserved
A B
Fig. 3—58-year-old man with headache. Unenhanced
CT axial image obtained on day of presentation Fig. 4—55-year-old man with worst headache of his life, nausea, vomiting, and neck stiffness.
shows subarachnoid hemorrhage concentrated A, Unenhanced CT shows subarachnoid hemorrhage (SAH) filling cerebellomedullary cistern (cm). There was
in interpeduncular cistern (ip), characteristic of no SAH in central or suprasellar cisterns, but fourth ventricle was distended with blood (not shown), indicating
nonaneurysmal perimesencephalic hemorrhage. cause other than benign idiopathic perimesencephalic hemorrhage.
There was no extension to anterior or lateral cisterns, B, Digital subtraction angiography (left vertebral artery injection, transfacial projection) shows faint abnormal
convexities, or ventricular system. CT angiography, blush of contrast at C1–C2 junction (arrow). This corresponded to homogeneously enhancing mass at
digital subtraction angiography (DSA), and delayed cervicomedullary junction on MRI (not shown), proven to be hemangioblastoma.
repeat DSA (not shown) were normal with no
evidence of aneurysm.
A B
Fig. 6—60-year-old man found unconscious in his
Fig. 5—56-year-old woman with thunderclap headache accompanied by nausea and vomiting that began while home. Gradient-recalled echo MRI shows marked
swimming in hot springs. diffuse cortical superficial siderosis (thick solid
A, Unenhanced CT shows bilateral frontal convexal subarachnoid hemorrhage. CT angiography and digital arrow), subarachnoid siderosis (thin solid arrow), and
subtraction angiography (DSA) were initially normal (data not shown), but 5 days later DSA showed diffuse microhemorrhages (dashed arrow). Lobar hematomas
segmental vasoconstriction involving multiple vascular territories. were also present (not shown). Probable cerebral
B, DSA at day 5 (left vertebral artery injection) shows segmental vasoconstriction in posterior cerebral artery amyloid angiopathy with supporting pathology (from
branches bilaterally, consistent with reversible cerebral vasoconstriction syndrome. evacuated hematoma) was diagnosed using Boston
criteria (see text).
32 AJR:202, January 2014
Subarachnoid Hemorrhage
Fig. 7—69-year-old woman with history of migraine
headaches who presented with severe headache
for 1 day, accompanied by seizure and hypertensive
emergency.
A, Unenhanced CT shows left parietal convexal
subarachnoid hemorrhage (arrow).
B, FLAIR MRI shows bilateral occipital subcortical
white matter areas of signal hyperintensity (arrows),
Downloaded from www.ajronline.org by 36.73.174.239 on 01/11/18 from IP address 36.73.174.239. Copyright ARRS. For personal use only; all rights reserved
consistent with posterior reversible encephalopathy
syndrome. There was no associated diffusion
restriction (not shown).
A B
A B C
Fig. 8—68-year-old man with headache for several days and abrupt onset of left-sided weakness and seizure.
A, Unenhanced CT shows moderate subarachnoid hemorrhage filling right sylvian fissure (arrow) and scattered within sulci of right cerebral convexity.
B, Unenhanced CT at more superior level shows hyperdense cord sign (arrow).
C, CT venogram shows filling defects in corresponding right cortical veins (arrow) and superior sagittal sinus, consistent with cortical venous and dural sinus thrombosis.
AJR:202, January 2014 33
Marder et al.
Downloaded from www.ajronline.org by 36.73.174.239 on 01/11/18 from IP address 36.73.174.239. Copyright ARRS. For personal use only; all rights reserved
A B
C D
Fig. 9—44-year-old woman with history of injection drug use, now with fevers and altered mental status.
A, Unenhanced CT shows multifocal convexal subarachnoid hemorrhage (arrow); additional foci are not shown.
B, Four days later, she developed expressive aphasia, and repeat unenhanced CT shows new left frontoparietal
acute infarct (arrow). Transesophageal echocardiogram revealed vegetations on mitral valve.
C and D, Gradient-recalled echo MRI shows foci of low signal intensity and blooming artifact (arrows) at
locations of convexal subarachnoid hemorrhage and acute infarct, consistent with septic emboli secondary to
infectious endocarditis.
34 AJR:202, January 2014
Subarachnoid Hemorrhage
Downloaded from www.ajronline.org by 36.73.174.239 on 01/11/18 from IP address 36.73.174.239. Copyright ARRS. For personal use only; all rights reserved
A B
Fig. 11—56-year-old woman with history of acute
Fig. 10—54-year-old man with history of aortic valve replacement for bicuspid aortic valve, now taking myelogenous leukemia admitted for neutropenic
warfarin (Coumadin, Bristol-Myers Squibb), who presented with worsening headache and blurry vision. One fever and pneumonia, now unresponsive.
month earlier he had spontaneous intra-parenchymal hemorrhage (IPH) with negative workup. Unenhanced CT shows multifocal convexal
A, Unenhanced CT shows right frontal convexal subarachnoid hemorrhage (arrow) and encephalomalacia at subarachnoid hemorrhage (arrow); additional foci
location of prior left occipital IPH. are not shown. Patient died soon after scan because
B, CT angiography (not shown) and digital subtraction angiography, which was normal 1 month earlier (not of multiorgan failure secondary to disseminated
shown), show bilobed pseudoaneurysm (arrow) arising from frontopolar branch of right middle cerebral artery, intravascular coagulation.
consistent with septic (mycotic) aneurysm.
A B
Fig. 13—50-year-old woman who was strangled by
Fig. 12—35-year-old woman with severe bilateral headache and history of stroke. her husband. Unenhanced CT shows diffuse low
A, Unenhanced CT shows convexal subarachnoid hemorrhage in parasagittal convexities bilaterally. attenuation with loss of gray-white differentiation,
B, Digital subtraction angiography (right internal carotid artery [ICA] injection, anteroposterior projection) effacement of cerebral sulci, and hyperdense
shows high-grade stenosis of right supraclinoid ICA, near-complete occlusion of M1 and A1 segments, appearance of subarachnoid spaces (arrows),
and collateral filling of distal middle cerebral artery and anterior cerebral artery segments via pial surface consistent with pseudo–subarachnoid hemorrhage
collaterals and superficial temporal artery, consistent with moyamoya disease. due to anoxic encephalopathy. Diagnosis was
supported by focal lenticular low attenuation in basal
ganglia bilaterally (not shown).
AJR:202, January 2014 35
Marder et al.
Fig. 14—70-year-old Fig. 15—58-year-old
woman immediately man with history of
after stenting and lung cancer and new-
occlusion of right onset lower extremity
internal carotid artery weakness. FLAIR MRI
aneurysm. Unenhanced shows sulcal areas of
CT obtained routinely signal hyperintensity in
shows hyperdensity parietal and occipital
Downloaded from www.ajronline.org by 36.73.174.239 on 01/11/18 from IP address 36.73.174.239. Copyright ARRS. For personal use only; all rights reserved
in subarachnoid lobes, more on left side
space overlying right (arrow). Corresponding
frontal convexity, areas on unenhanced
with associated sulcal CT same day (not
effacement. Patient shown) revealed no
complained only subarachnoid
of transient finger hemorrhage (SAH).
numbness. These Findings on MRI were
findings persisted at attributed to high-
4 hours but resolved flow oxygen therapy
completely at 24 hours mimicking SAH.
(not shown) and likely
represent extravasated
iodinated contrast
material.
Problem-solving
Diffuse or Central Cisterns
in select cases
(e.g., recurrent SAH)
DSA
Exclude aneurysm,
other vascular Brain MRI
causes Repeat (up to 3×)
to detect occult
aneurysm
Head CTA STOP
Problem-solving
Low Basal Cisterns
in select cases
(e.g., recurrent SAH)
DSA
Exclude aneurysm, Brain or
other vascular spine MRI Fig. 16—Imaging approach to nontraumatic
Practices vary—
causes
if performed, subarachnoid hemorrhage (SAH) by pattern of
usually 1× hemorrhage on unenhanced CT.
Head CTA A–D, Schematic diagrams illustrate stepwise
STOP approach to diagnostic testing. Arrows denote
transitions to next imaging steps after negative
test. Dark gray boxes indicate standard steps,
B whereas light gray boxes indicate optional steps.
Diffuse or central cistern pattern (A) is classic
for aneurysmal SAH, and workup is directed at
Yes Follow algorithm for diffuse SAH excluding saccular aneurysm rupture. Algorithm
Convexal for low basal cisterns (B) applies to cases in which
strict criteria for perimesencephalic hemorrhage
Sylvian fissure RCVS, vasculitis, other have been met (see text). Otherwise, algorithm
S
SAH? vascular causes DSA for diffuse SAH applies. With convexal SAH (C),
Disease may be compared with other patterns, brain MRI has more
Head CTA/MRA
subtle or initially fundamental role and may be performed as initial
occult test. CAA = cerebral amyloid angiopathy, CTA = CT
No Helpful in PRES, CAA, angiography, CVT = cerebral venous thrombosis,
CVT, complications of DSA = digital subtraction angiography, MRA =
vasculitis or vasculopathy MR angiography, PRES = posterior reversible
encephalopathy syndrome, RCVS = reversible
Brain MRI
cerebral vasoconstriction syndrome.
(Fig. 16 continues on next page)
C
36 AJR:202, January 2014
Subarachnoid Hemorrhage
Fig. 16 (continued)—Imaging approach to
No Blood Visible nontraumatic subarachnoid hemorrhage (SAH) by
pattern of hemorrhage on unenhanced CT.
A–D, In cases of no visible blood (D), vascular imaging
DSA
Xanthochromia on is performed only if xanthochromia is found on
lumbar puncture? Practices vary— delayed lumbar puncture. CAA = cerebral amyloid
if performed, angiopathy, CTA = CT angiography, CVT = cerebral
Exclude aneurysm, other
usually only 1× venous thrombosis, DSA = digital subtraction
Downloaded from www.ajronline.org by 36.73.174.239 on 01/11/18 from IP address 36.73.174.239. Copyright ARRS. For personal use only; all rights reserved
No angiography, MRA = MR angiography, PRES =
vascular causes
posterior reversible encephalopathy syndrome,
Head CTA STOP RCVS = reversible cerebral vasoconstriction
STOP
syndrome.
F O R YO U R I N F O R M AT I O N
This article is available for CME and Self-Assessment (SA-CME) credit that satisfies Part II requirements for
maintenance of certification (MOC). To access the examination for this article, follow the prompts associated with
the online version of the article.
AJR:202, January 2014 37
Você também pode gostar
- Cavernomas of the CNS: Basic Science to Clinical PracticeNo EverandCavernomas of the CNS: Basic Science to Clinical PracticeOndřej BradáčAinda não há avaliações
- Subarachnoid Hemorrhage: Beyond Aneurysms: Carrie P. Marder Vinod Narla James R. Fink Kathleen R. Tozer FinkDocumento13 páginasSubarachnoid Hemorrhage: Beyond Aneurysms: Carrie P. Marder Vinod Narla James R. Fink Kathleen R. Tozer FinkDaylamiAinda não há avaliações
- Cerebral Circulation in Moyamoya Disease: A Clinical Study Using Transcranial Doppler SonographyDocumento8 páginasCerebral Circulation in Moyamoya Disease: A Clinical Study Using Transcranial Doppler SonographyDewi SartikaAinda não há avaliações
- Cirsoid Aneurysm OF THE Uterus: Specific Arteriographic: Read As I8o/oo Mm. HG But Fellto Io/8o MMDocumento7 páginasCirsoid Aneurysm OF THE Uterus: Specific Arteriographic: Read As I8o/oo Mm. HG But Fellto Io/8o MMLouis HadiyantoAinda não há avaliações
- Chapter 2 Introduction To Brain ImagingDocumento3 páginasChapter 2 Introduction To Brain Imagingsybico.xray.abadclinicAinda não há avaliações
- Cardiac MRI 1Documento8 páginasCardiac MRI 1drpankajsAinda não há avaliações
- Subarachnoid-Hemorrhage FinalDocumento12 páginasSubarachnoid-Hemorrhage FinalAshwini KatareAinda não há avaliações
- Cardiac Troponin and Defining Myocardial Infarction: Thomas E. Kaier, Bashir Alaour, and Michael MarberDocumento13 páginasCardiac Troponin and Defining Myocardial Infarction: Thomas E. Kaier, Bashir Alaour, and Michael MarberwiwiAinda não há avaliações
- Ajr 10 5540Documento9 páginasAjr 10 5540Pepe pepe pepeAinda não há avaliações
- BRONCHO ALVEOLAR CARCINOMA DIFFERENTIAL DIAGNOSISDocumento3 páginasBRONCHO ALVEOLAR CARCINOMA DIFFERENTIAL DIAGNOSISmarielleaudreeyAinda não há avaliações
- Traumatic Subarachnoid Hemorrhage Resulting From Posterior Communicating Artery RuptureDocumento5 páginasTraumatic Subarachnoid Hemorrhage Resulting From Posterior Communicating Artery RuptureIraAinda não há avaliações
- Tumores Cardiacos LerDocumento11 páginasTumores Cardiacos LerBeatriz Silva SilvaAinda não há avaliações
- Differentiation Between Calcification and Hemorrhage in Brain Tumors Using Susceptibility-Weighted Imaging: A Pilot StudyDocumento4 páginasDifferentiation Between Calcification and Hemorrhage in Brain Tumors Using Susceptibility-Weighted Imaging: A Pilot StudyDanaAmaranducaiAinda não há avaliações
- 5 Basic EchoDocumento64 páginas5 Basic Echoola adelAinda não há avaliações
- Nursing Care Plan For HELLP SyndromeDocumento17 páginasNursing Care Plan For HELLP SyndromeRosemarie Carpio75% (4)
- 1 Approachtoheadct 130418093230 Phpapp01Documento71 páginas1 Approachtoheadct 130418093230 Phpapp01Sikandar Shahzad YousafzaiAinda não há avaliações
- Sarcoidosis - A Diagnosis of Exclusion (Journal)Documento9 páginasSarcoidosis - A Diagnosis of Exclusion (Journal)fikryahAinda não há avaliações
- Spinal Hematoma EXPLAINEDDocumento20 páginasSpinal Hematoma EXPLAINEDcalustre2016Ainda não há avaliações
- Nasolacrimal Drainage ApparatusDocumento10 páginasNasolacrimal Drainage Apparatussem76Ainda não há avaliações
- Pictorial Essay: Cavernous Sinus SyndromeDocumento8 páginasPictorial Essay: Cavernous Sinus SyndromeDian DestriyanahAinda não há avaliações
- Barlinn, Vascular Imaging in Stroke Comparative AnalysisDocumento9 páginasBarlinn, Vascular Imaging in Stroke Comparative Analysislaila forestaAinda não há avaliações
- Small Vessel DiseaseDocumento21 páginasSmall Vessel Diseasepaola nabhanAinda não há avaliações
- Diagnostic Test FinalDocumento5 páginasDiagnostic Test FinalDivynne MadeloAinda não há avaliações
- CT Findings of Pulmonary NocardiosisDocumento7 páginasCT Findings of Pulmonary NocardiosisMANGAinda não há avaliações
- Role of CT and MRI in Stroke DiagnosisDocumento51 páginasRole of CT and MRI in Stroke DiagnosisBokuma KuciAinda não há avaliações
- Point of Care Echocardiography in The.5Documento10 páginasPoint of Care Echocardiography in The.5carlos GuzmánAinda não há avaliações
- Imaging of Intracranial Hemorrhage: Journal ReadingDocumento26 páginasImaging of Intracranial Hemorrhage: Journal ReadingMuhammad ZulfikarAinda não há avaliações
- Day 3-Neuroradology 4ADocumento5 páginasDay 3-Neuroradology 4AAla'a Emerald AguamAinda não há avaliações
- Survival After Surgical Excision OF Single Metastatic Brain TumorsDocumento6 páginasSurvival After Surgical Excision OF Single Metastatic Brain Tumorsfira rifaAinda não há avaliações
- Aneurysmal Subarachnoid HemorrhageDocumento4 páginasAneurysmal Subarachnoid Hemorrhagestudent_019Ainda não há avaliações
- Types of Cerebral Herniation and RXDocumento13 páginasTypes of Cerebral Herniation and RXJean Brando Torres GuerreroAinda não há avaliações
- Types of Cerebral Herniation and ImagingDocumento13 páginasTypes of Cerebral Herniation and ImagingPaulo LuizAinda não há avaliações
- Scientific WorldDocumento4 páginasScientific WorldNeet Aipg newstipsAinda não há avaliações
- Head CT Findings and InterpretationDocumento31 páginasHead CT Findings and InterpretationElisabeth F. Ojha100% (2)
- urv4.7Documento31 páginasurv4.7BALEWAinda não há avaliações
- Solid Renal Masses: What The Numbers Tell Us: Stella K. Kang William C. Huang Pari V. Pandharipande Hersh ChandaranaDocumento11 páginasSolid Renal Masses: What The Numbers Tell Us: Stella K. Kang William C. Huang Pari V. Pandharipande Hersh ChandaranaTạ Minh ZSAinda não há avaliações
- Adrenal Artery Embolization: Anatomy, Indications, and Technical ConsiderationsDocumento12 páginasAdrenal Artery Embolization: Anatomy, Indications, and Technical ConsiderationsChavdarAinda não há avaliações
- Imaging of Intracranial Hemorrhage: Jeremy J. Heit, Michael Iv, Max WintermarkDocumento21 páginasImaging of Intracranial Hemorrhage: Jeremy J. Heit, Michael Iv, Max WintermarkBrianAinda não há avaliações
- Ila Headache (1) 2Documento49 páginasIla Headache (1) 2Tengku Nur IffahAinda não há avaliações
- Cardiologi Imaging Part 1Documento8 páginasCardiologi Imaging Part 1Anonymous sTcHszTAinda não há avaliações
- Imaging ICHDocumento17 páginasImaging ICHCrhistian GarcíaAinda não há avaliações
- AJR Chest Radiography in The ICU Parte II 2012Documento10 páginasAJR Chest Radiography in The ICU Parte II 2012wfranelicAinda não há avaliações
- Ajr 137 2 245Documento6 páginasAjr 137 2 245Khalvia KhairinAinda não há avaliações
- White MatterDocumento7 páginasWhite MatterReny Wahyu SariAinda não há avaliações
- STR 0000000000000407Documento80 páginasSTR 0000000000000407husam husamAinda não há avaliações
- Cerebral Edema: ResidentsDocumento16 páginasCerebral Edema: ResidentswulanAinda não há avaliações
- Upper Extremity Venous Doppler Ultrasound PDFDocumento12 páginasUpper Extremity Venous Doppler Ultrasound PDFLayla Salomão0% (1)
- Neuro Module Part 3Documento31 páginasNeuro Module Part 3Gabriel GhiațăAinda não há avaliações
- Aneurisma IntracranealDocumento13 páginasAneurisma IntracranealEdgar CorbiéreAinda não há avaliações
- Lec 3 Raised Intracranial PressureDocumento9 páginasLec 3 Raised Intracranial PressureEmily MurrayAinda não há avaliações
- Winslow PathwayDocumento3 páginasWinslow PathwayMia MadalinaAinda não há avaliações
- NCPDocumento2 páginasNCPBella SalikAinda não há avaliações
- Case Apsc 2023 - FdaDocumento18 páginasCase Apsc 2023 - FdaFariz DwikyAinda não há avaliações
- Imaging in Subarachnoid Hemorrhage - Overview, Radiography, Computed TomographyDocumento13 páginasImaging in Subarachnoid Hemorrhage - Overview, Radiography, Computed TomographySaraAinda não há avaliações
- Hemorrhagic StrokeDocumento11 páginasHemorrhagic StrokeCassandra Mae PerochoAinda não há avaliações
- Journal Radiologi - SAH of Unknown CauseDocumento9 páginasJournal Radiologi - SAH of Unknown CauseObsgyn UKI 9 Aug - 14 Okt 21Ainda não há avaliações
- محاضرة النيوروDocumento37 páginasمحاضرة النيوروHala BahaaAinda não há avaliações
- Dimitri RenardDocumento11 páginasDimitri RenardRashellya RasyidaAinda não há avaliações
- Trove Research Carbon Credit Demand Supply and Prices 1 June 2021Documento51 páginasTrove Research Carbon Credit Demand Supply and Prices 1 June 2021Ceren ArkancanAinda não há avaliações
- Principles of Cost Accounting 1Documento6 páginasPrinciples of Cost Accounting 1Alimamy KamaraAinda não há avaliações
- Overview for Report Designers in 40 CharactersDocumento21 páginasOverview for Report Designers in 40 CharacterskashishAinda não há avaliações
- K Series Parts List - 091228Documento25 páginasK Series Parts List - 091228AstraluxAinda não há avaliações
- Seminar #22 Vocabury For Speaking PracticeDocumento7 páginasSeminar #22 Vocabury For Speaking PracticeOyun-erdene ErdenebilegAinda não há avaliações
- Donaldson 004117 PDFDocumento6 páginasDonaldson 004117 PDFNSAinda não há avaliações
- Electrophoresis and Fractionation of Wheat GlutenDocumento14 páginasElectrophoresis and Fractionation of Wheat GlutensecucaAinda não há avaliações
- Tutorial 3Documento2 páginasTutorial 3prasoon jhaAinda não há avaliações
- 3 Steel Grating Catalogue 2010 - SERIES 1 PDFDocumento6 páginas3 Steel Grating Catalogue 2010 - SERIES 1 PDFPablo MatrakaAinda não há avaliações
- Biomechanics of Advanced Tennis: January 2003Documento7 páginasBiomechanics of Advanced Tennis: January 2003Katrien BalAinda não há avaliações
- Brochure - Truemax Concrete Pump Truck Mounted TP25M4Documento16 páginasBrochure - Truemax Concrete Pump Truck Mounted TP25M4RizkiRamadhanAinda não há avaliações
- Write 10 Lines On My Favourite Subject EnglishDocumento1 páginaWrite 10 Lines On My Favourite Subject EnglishIrene ThebestAinda não há avaliações
- Raychem Price ListDocumento48 páginasRaychem Price ListramshivvermaAinda não há avaliações
- Principles of SamplingDocumento15 páginasPrinciples of SamplingziggerzagAinda não há avaliações
- Dr. Malik's Farms BrochureDocumento18 páginasDr. Malik's Farms BrochureNeil AgshikarAinda não há avaliações
- Legal Principles and The Limits of The Law Raz PDFDocumento33 páginasLegal Principles and The Limits of The Law Raz PDFlpakgpwj100% (2)
- Moderntheater 170210003221 PDFDocumento80 páginasModerntheater 170210003221 PDFDycan MikeAinda não há avaliações
- Analysis of VariancesDocumento40 páginasAnalysis of VariancesSameer MalhotraAinda não há avaliações
- Maximizing modular learning opportunities through innovation and collaborationDocumento2 páginasMaximizing modular learning opportunities through innovation and collaborationNIMFA SEPARAAinda não há avaliações
- Chapter 1 - IntroductionDocumento42 páginasChapter 1 - IntroductionShola ayipAinda não há avaliações
- SQL 1: Basic Statements: Yufei TaoDocumento24 páginasSQL 1: Basic Statements: Yufei TaoHui Ka HoAinda não há avaliações
- Critique On A Film Director's Approach To Managing CreativityDocumento2 páginasCritique On A Film Director's Approach To Managing CreativityDax GaffudAinda não há avaliações
- Keberhasilan Aklimatisasi Dan Pembesaran Bibit Kompot Anggrek Bulan (Phalaenopsis) Pada Beberapa Kombinasi Media TanamDocumento6 páginasKeberhasilan Aklimatisasi Dan Pembesaran Bibit Kompot Anggrek Bulan (Phalaenopsis) Pada Beberapa Kombinasi Media TanamSihonoAinda não há avaliações
- EG-45-105 Material Information Sheet (Textura) V2Documento4 páginasEG-45-105 Material Information Sheet (Textura) V2GPRAinda não há avaliações
- OLA CAB MARKET ANALYSIS AND TRENDSDocumento55 páginasOLA CAB MARKET ANALYSIS AND TRENDSnitin gadkariAinda não há avaliações
- Chapter 9-10 (PPE) Reinzo GallegoDocumento48 páginasChapter 9-10 (PPE) Reinzo GallegoReinzo GallegoAinda não há avaliações
- Wika Type 111.11Documento2 páginasWika Type 111.11warehouse cikalongAinda não há avaliações
- Levels of Attainment.Documento6 páginasLevels of Attainment.rajeshbarasaraAinda não há avaliações
- Lab ReportDocumento5 páginasLab ReportHugsAinda não há avaliações
- Chapter 08Documento18 páginasChapter 08soobraAinda não há avaliações