Escolar Documentos
Profissional Documentos
Cultura Documentos
Lyofast MW 039 S
Enviado por
Nilo C Cervantes ChipaDescrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Lyofast MW 039 S
Enviado por
Nilo C Cervantes ChipaDireitos autorais:
Formatos disponíveis
Lyofast MW 039 S
Description Lyofast MW 039 S consists of undefined strains of Lactococcus lactis ssp. cremoris
and defined strains of Lactococcus lactis ssp. lactis biovar diacetylactis.
Lyofast MW 039 S ensures a uniform and controlled production of fresh cheese, and
soft cheese.
Application Sprinkle the culture powder directly into process milk under aseptic conditions ensuring
that the culture is well dispersed by gentle stirring. The following may be used as
inoculation guidelines:
Product UC/100 l Product UC/100 l
Fresh cheese 0.5-2.0 Soft cheese 0.7-2.0
Rotation The recommended rotation is available upon request.
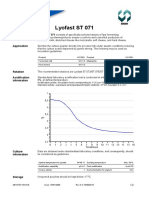
Acidification Standardised laboratory acidification test is conducted in milk powder, reconstituted at
information 9%, at defined temperature.
Acidification profile: inoculation level corresponding to 1 UC per 100 litres milk.
Standard activity: expressed as temperature/time/pH relations: 32°C/7.5 hours/pH 5.2 ± 0.1.
Culture Data are obtained under standardised laboratory conditions, and consequently, should
information be considered as guidelines.
Optimal temperature for growth 25-35 °C Scalding temperature Max. 39°C
Acidification capability pH 4.4 Gas production/citrate/urea +++
Storage Unopened pouches should be kept below -17°C.
Package data The freeze-dried culture is packed in waterproof and airproof aluminium pouches. The
packaging material is food grade. Lyofast MW 039 S is available in 10 and 50 UC.
M91MW039S/1/UK/0 Issue: 12/05/2011 Review: 1 of 17/04/2014 1/2
Lyofast MW 039 S
Shelf life 18 months when stored below -17°C.
Heavy metal Pb (lead) < 1 ppm
specification Hg (mercury) < 0.03 ppm
Cd (cadmium) < 0.1 ppm
* Analysed on regular basis.
Microbiological Bacillus cereus <100 CFU/g Method: Sacco M10 (1)
specification Coagulase positive staphylococci* <10 CFU/g Method: Sacco M11(2)
Enterobacteriaceae <10 CFU/g Method: Sacco M2 (3)
Escherichia coli <1 CFU/g Method: Sacco M27 (4)
Listeria monocytogenes* Not detected in 25 g Method: Sacco M13 (5)
Moulds & yeasts <10 CFU/g Method: Sacco M3 (6)
Salmonella spp* Not detected in 25 g Method: Sacco M12 (7)
* Analysed on regular basis. All analytical methods are available upon request.
(1)ISO 7932; (2)ISO 6888-1-2; (3)ISO 21528-1-2; (4)ISO11866-1-2/IDF 170-1-2; (5)ISO 11290-1-2; (6)ISO
6611/IDF 94; (7)ISO 6785/IDF 93.
GMO The microbial strains are not genetically modified (GMO) in accordance with the
European Directive 2001/18/EC. The strains are isolated from natural sources. The raw
materials used are also GMO free in accordance with Regulation (EC) No. 1829/2003
and Regulation (EC) No. 1830/2003. Statement available upon request.
Allergens The raw materials used are generally based on dairy ingredients. All materials are free
of the following components and their derivates: peanut, tree nut, sesame, egg, fish,
shellfish, mollusc, crustacean, sulphite, cereals containing gluten, celery, mustard, soy
and lupine. Statement available upon request.
Safety information Material Safety Data Sheet available on www.saccosrl.it
Certificate Lot certificate available upon request.
ISO Sacco S.r.l. is UNI EN ISO 9001:2008 certified since 1998. Sacco cultures are
Kosher approval generally Kosher approved except for surface ripening cultures.
Service Please contact your distributor for guidance and instructions for your choice of culture
and processing. Information about additional package sizes and sales units is also
available upon request.
Liability This information is based on our knowledge trustworthy and presented in good faith. No
guarantee against patent infringement is implied or inferred.
M91MW039S/1/UK/0 Issue: 12/05/2011 Review: 1 of 17/04/2014 2/2
Você também pode gostar
- Dokumen - Pub - Bobs Refunding Ebook v3 PDFDocumento65 páginasDokumen - Pub - Bobs Refunding Ebook v3 PDFJohn the First100% (3)
- Continental-4rev 0302 PDFDocumento24 páginasContinental-4rev 0302 PDFLuis Mejia AlbarracinAinda não há avaliações
- HACCP Plan For Plain-Flavored Ultra-High Temperature Processed SoymilkDocumento25 páginasHACCP Plan For Plain-Flavored Ultra-High Temperature Processed SoymilkXi YANGAinda não há avaliações
- A Simple, Fast and Green Titrimetric Method For The Determination of The Iodine Value of Vegetable Oils Without Wijs SolutionDocumento5 páginasA Simple, Fast and Green Titrimetric Method For The Determination of The Iodine Value of Vegetable Oils Without Wijs SolutionA Sierra100% (1)
- Yellow Cornstarch SpecDocumento1 páginaYellow Cornstarch SpecEdwin Biggie MwaleAinda não há avaliações
- Grindamyl A 14000Documento3 páginasGrindamyl A 14000Toni AcoskiAinda não há avaliações
- Mcdonald 2016Documento10 páginasMcdonald 2016Andrika SaputraAinda não há avaliações
- Mark Garside Resume May 2014Documento3 páginasMark Garside Resume May 2014api-199955558Ainda não há avaliações
- RTDM Admin Guide PDFDocumento498 páginasRTDM Admin Guide PDFtemp100% (2)
- TC Lyofast-Y 456-B EN 230616Documento2 páginasTC Lyofast-Y 456-B EN 230616Anel MamaniAinda não há avaliações
- Ficha Tecnica Sab 440ADocumento0 páginaFicha Tecnica Sab 440AAlexander GuzmanAinda não há avaliações
- Ficha Tecnica Cultivo para QuesoDocumento2 páginasFicha Tecnica Cultivo para QuesoLeyarope RoRoAinda não há avaliações
- Lyofast MTX 432 ENDocumento2 páginasLyofast MTX 432 ENLuisaGordonAinda não há avaliações
- Iso 6496 1999Documento9 páginasIso 6496 1999Andrea Del Pilar Grimaldo QuispeAinda não há avaliações
- ADocumento2 páginasAmaraki998Ainda não há avaliações
- The Effects of Oils and Frying Temperatures On The Texture and FatDocumento5 páginasThe Effects of Oils and Frying Temperatures On The Texture and FatAgus SupriatnaAinda não há avaliações
- 997.02 Yeast and Mold Count in FoodsDocumento2 páginas997.02 Yeast and Mold Count in FoodsJavier muñozAinda não há avaliações
- Promo Carfosel 950Documento3 páginasPromo Carfosel 950Srdjan JovanovicAinda não há avaliações
- Actividad Ureásica 22-90Documento2 páginasActividad Ureásica 22-90Sarah WellsAinda não há avaliações
- Biofine FDDocumento2 páginasBiofine FDMoisés LozanoAinda não há avaliações
- AOAC 960.39 Grasa en Carne CrudaDocumento1 páginaAOAC 960.39 Grasa en Carne CrudaCompras FisicoquimicoAinda não há avaliações
- Codex Blue Cheese New PDFDocumento5 páginasCodex Blue Cheese New PDFMohamad ArtaAinda não há avaliações
- Application Bulletin N°160 - CarraLact DPC LineDocumento4 páginasApplication Bulletin N°160 - CarraLact DPC LineYomo OuyaneAinda não há avaliações
- Thermophysical Properties of Orange JuiceDocumento14 páginasThermophysical Properties of Orange JuiceGladys González González100% (1)
- Citrato de SodioDocumento13 páginasCitrato de SodioIngridJohanaVergaraAinda não há avaliações
- AOC 92.159 Iodine Absorption NumberDocumento1 páginaAOC 92.159 Iodine Absorption NumberJuan Felipe Romero RojasAinda não há avaliações
- Quality Assurance of Selective Culture Media. Método Ecometrico Mossel Et Al 1983Documento15 páginasQuality Assurance of Selective Culture Media. Método Ecometrico Mossel Et Al 1983catabacteymicrobioloAinda não há avaliações
- D Study PlanDocumento4 páginasD Study Planhafeez soomroAinda não há avaliações
- 37.1.37 AOAC Official Method 942.15 Acidity (Titratable) of Fruit ProductsDocumento1 página37.1.37 AOAC Official Method 942.15 Acidity (Titratable) of Fruit ProductsJuan Alejandro Jiménez100% (1)
- Yo-Mix-883 Lyo 50 DcuDocumento2 páginasYo-Mix-883 Lyo 50 DcuEdwin Chila ChoqueAinda não há avaliações
- Fat Determination AoacDocumento2 páginasFat Determination AoacMervin RecolitoAinda não há avaliações
- NTP Iso 6658.Documento44 páginasNTP Iso 6658.Ebenezer Asiedu-AgyeiAinda não há avaliações
- Condensed Milk Production: Paul MatimbaDocumento32 páginasCondensed Milk Production: Paul Matimbamatthew matawoAinda não há avaliações
- Parctica Calificada 01Documento3 páginasParctica Calificada 01karla lozanoAinda não há avaliações
- AOAC 984.25. Moisture (Loss of Mass On Drying) in Frozen French-Fried PotatoesDocumento1 páginaAOAC 984.25. Moisture (Loss of Mass On Drying) in Frozen French-Fried PotatoesGerman AyalaAinda não há avaliações
- Elaboración de Hamburguesa A Partir De: Prochylodus Nigricans BOQUICHICODocumento9 páginasElaboración de Hamburguesa A Partir De: Prochylodus Nigricans BOQUICHICOESaesCruzAinda não há avaliações
- Especific HeatDocumento18 páginasEspecific HeatRafaela RibeiroAinda não há avaliações
- Verb To Be - ReadingDocumento1 páginaVerb To Be - ReadingManuelAinda não há avaliações
- BlanchingDocumento11 páginasBlanchingmse8710100% (1)
- Aoac 925.45Documento2 páginasAoac 925.45KiaraAinda não há avaliações
- 915.01 Cloruro en Plantas PDFDocumento1 página915.01 Cloruro en Plantas PDFlizeth rico quinteroAinda não há avaliações
- Aoac 962.09 Fibre Crude in Animal Feed ADocumento3 páginasAoac 962.09 Fibre Crude in Animal Feed Alaboratorium operasionalAinda não há avaliações
- Unit 11 - Session 1: Flashback FridayDocumento9 páginasUnit 11 - Session 1: Flashback FridayMaría Ruiz100% (1)
- CXS 282eDocumento4 páginasCXS 282eZac HernándezAinda não há avaliações
- Wyeast NutrientDocumento1 páginaWyeast NutrientJesso GeorgeAinda não há avaliações
- ISO 1735 Cheese Determination of Fat Content - Gravimetric MethodDocumento20 páginasISO 1735 Cheese Determination of Fat Content - Gravimetric MethodJocilene DantasAinda não há avaliações
- Cristalización de ChocolateDocumento12 páginasCristalización de ChocolateErika -Ainda não há avaliações
- Ramapet N1: Sales SpecificationsDocumento1 páginaRamapet N1: Sales SpecificationsJoshua DjohanAinda não há avaliações
- Proteolysis and Lipolysis of Goat Milk CheeseDocumento9 páginasProteolysis and Lipolysis of Goat Milk CheesePedro ParraAinda não há avaliações
- Unit 11 - Session 5: TV MemoriesDocumento9 páginasUnit 11 - Session 5: TV Memoriesruth eche100% (1)
- Production of Soft CheeseDocumento13 páginasProduction of Soft CheeseRose YacobAinda não há avaliações
- Colloidal Stability of BeerDocumento8 páginasColloidal Stability of BeerZach SmolskyAinda não há avaliações
- 33.2.27A AOAC of Fi Cial Method 2000.18 Fat Con Tent of Raw and Pas Teur Ized Whole MilkDocumento2 páginas33.2.27A AOAC of Fi Cial Method 2000.18 Fat Con Tent of Raw and Pas Teur Ized Whole MilkJavier muñoz100% (1)
- Characterization of PectinDocumento10 páginasCharacterization of PectinJames Aaron SantiagoAinda não há avaliações
- Recommended Methods of Analysis and Sampling: CODEX STAN 234-1999Documento48 páginasRecommended Methods of Analysis and Sampling: CODEX STAN 234-1999shraddhaJPAinda não há avaliações
- Aerated Food GelsDocumento12 páginasAerated Food GelsOana SilviaAinda não há avaliações
- Production of Traditional MozzarellaDocumento58 páginasProduction of Traditional MozzarellarajaytAinda não há avaliações
- Problema 10Documento1 páginaProblema 10Adahi0% (1)
- 1 Callejo2015 Bread - Selection, Training and Validation Process of A Sensory Panel For PDFDocumento8 páginas1 Callejo2015 Bread - Selection, Training and Validation Process of A Sensory Panel For PDFМария СтолповенкоAinda não há avaliações
- Preparación de La Muestra en Queso AOAC 955 - 30Documento1 páginaPreparación de La Muestra en Queso AOAC 955 - 30Yolby Milena Rodriguez ArizaAinda não há avaliações
- LyofastST071 TechDocumento2 páginasLyofastST071 Techchrist castroAinda não há avaliações
- Lyofast MO 242: DescriptionDocumento2 páginasLyofast MO 242: DescriptionMilena CastroAinda não há avaliações
- Lyofast ST 081Documento2 páginasLyofast ST 081jimeAinda não há avaliações
- Analysis of autochthonous lactic acid bacteria from mozzarella cheese productionNo EverandAnalysis of autochthonous lactic acid bacteria from mozzarella cheese productionAinda não há avaliações
- Arens - Auditing and Assurance Services 15e-2Documento17 páginasArens - Auditing and Assurance Services 15e-2Magdaline ChuaAinda não há avaliações
- Functions in C++Documento23 páginasFunctions in C++Abhishek ModiAinda não há avaliações
- AIA1800 Operator ManualDocumento184 páginasAIA1800 Operator ManualZain Sa'adehAinda não há avaliações
- Syllabus DresserDocumento2 páginasSyllabus DresserVikash Aggarwal50% (2)
- Emea 119948060Documento31 páginasEmea 119948060ASHUTOSH MISHRAAinda não há avaliações
- On Derridean Différance - UsiefDocumento16 páginasOn Derridean Différance - UsiefS JEROME 2070505Ainda não há avaliações
- What Is TranslationDocumento3 páginasWhat Is TranslationSanskriti MehtaAinda não há avaliações
- Tplink Eap110 Qig EngDocumento20 páginasTplink Eap110 Qig EngMaciejAinda não há avaliações
- .Urp 203 Note 2022 - 1642405559000Documento6 páginas.Urp 203 Note 2022 - 1642405559000Farouk SalehAinda não há avaliações
- 18 June 2020 12:03: New Section 1 Page 1Documento4 páginas18 June 2020 12:03: New Section 1 Page 1KarthikNayakaAinda não há avaliações
- Outdoor Air Pollution: Sources, Health Effects and SolutionsDocumento20 páginasOutdoor Air Pollution: Sources, Health Effects and SolutionsCamelia RadulescuAinda não há avaliações
- Sam Media Recruitment QuestionnaireDocumento17 páginasSam Media Recruitment Questionnairechek taiAinda não há avaliações
- Unit 16 - Monitoring, Review and Audit by Allan WatsonDocumento29 páginasUnit 16 - Monitoring, Review and Audit by Allan WatsonLuqman OsmanAinda não há avaliações
- Electro Fashion Sewable LED Kits WebDocumento10 páginasElectro Fashion Sewable LED Kits WebAndrei VasileAinda não há avaliações
- Principals' Leadership Styles and Student Academic Performance in Secondary Schools in Ekiti State, NigeriaDocumento12 páginasPrincipals' Leadership Styles and Student Academic Performance in Secondary Schools in Ekiti State, NigeriaiqraAinda não há avaliações
- PED003Documento1 páginaPED003ely mae dag-umanAinda não há avaliações
- CEE Annual Report 2018Documento100 páginasCEE Annual Report 2018BusinessTech100% (1)
- Lesson PlanDocumento2 páginasLesson Plannicole rigonAinda não há avaliações
- Iec TR 61010-3-020-1999Documento76 páginasIec TR 61010-3-020-1999Vasko MandilAinda não há avaliações
- Test ScienceDocumento2 páginasTest Sciencejam syAinda não há avaliações
- PhraseologyDocumento14 páginasPhraseologyiasminakhtar100% (1)
- Hdfs Default XML ParametersDocumento14 páginasHdfs Default XML ParametersVinod BihalAinda não há avaliações
- II 2022 06 Baena-Rojas CanoDocumento11 páginasII 2022 06 Baena-Rojas CanoSebastian GaonaAinda não há avaliações
- Snapdragon 435 Processor Product Brief PDFDocumento2 páginasSnapdragon 435 Processor Product Brief PDFrichardtao89Ainda não há avaliações
- NDY 9332v3Documento8 páginasNDY 9332v3sulphurdioxideAinda não há avaliações
- Activity # 1 (DRRR)Documento2 páginasActivity # 1 (DRRR)Juliana Xyrelle FutalanAinda não há avaliações