Escolar Documentos
Profissional Documentos
Cultura Documentos
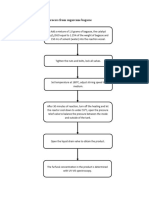
Method 1: (Method I Am Using Now, But Not Sure It Is Correct or Not, If Wrong I Can Redo The Experiment)
Enviado por
Yu HuiTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Method 1: (Method I Am Using Now, But Not Sure It Is Correct or Not, If Wrong I Can Redo The Experiment)
Enviado por
Yu HuiDireitos autorais:
Formatos disponíveis
Method 1: (method I am using now, but not sure it is correct or not,if wrong I can redo the
experiment)
Preparation of 30wt% phosphoric acid
Density of 85% H3PO4=1.689g/ml
Density of 30% H3PO4=1.181g/ml
Molarity of 85% H3PO4:
85𝑔 1.689𝑔 1000𝑚𝑙 𝑚𝑜𝑙
M= × × × 98𝑔 = 14.6494𝑀
100𝑔 𝑚𝑙 1𝐿
Molarity of 30% H3PO4
30𝑔 1.181𝑔 1000𝑚𝑙 𝑚𝑜𝑙
M= × × × 98𝑔 = 3.6153𝑀
100𝑔 𝑚𝑙 1𝐿
M1V1=M2V2
14.6494V1=3.6153(200)
V1=49ml of phosphoric acid is needed
Volume of water added = 151ml
𝑚𝑙
However, 50g of 30wt% H3PO4 = 42ml , 50𝑔 × 1.181𝑔 = 42𝑚𝑙
it is not enough to impregnate the biomass. SO I add 500ml of water.
I use 50g of 30wt% H3PO4, and dilute with 500ml water , the H3PO4 is further
diluted.
Method 2: Fix the volume of solution
𝑚𝑙
Since 50g of 85wt% is equal 30ml, 50𝑔 × 1.689𝑔 = 30𝑚𝑙
In order to make sure the biomass can be fully covered, I use 500ml of solution.
M1V1=M2V2
14.6294(30ml)= M2 (500ml)
V2 = 0.88 M
I use 50g of 85wt% H3PO4 and make up to 500ml solution
30ml of H3PO4 is added into 460ml of water to make up 500ml solution
For each experiment, I will use 0.88M H3PO4.
Method 3: Fix the molarity of H3PO4 (1M)
𝑚𝑙
Since 50g of 85wt% is equal 30ml, 50𝑔 × 1.689𝑔 = 30𝑚𝑙
M1V1=M2V2
14.6294(30ml)= 1 V2
V2 = 438.88 ml (volume of solution)
Volume of water added = 438.88 – 30 = 408.88 ml
I use 50g of 85wt% H3PO4, and dilute it to 1M for each set of experiment.
Você também pode gostar
- Exp 6Documento5 páginasExp 6Andrea CastroAinda não há avaliações
- Metode Analisis TitrimetriDocumento28 páginasMetode Analisis TitrimetriDeki TamonobAinda não há avaliações
- CABARLE, Elisha Mae - GenChemII (Act 3)Documento5 páginasCABARLE, Elisha Mae - GenChemII (Act 3)Elisha Mae CabarleAinda não há avaliações
- Presentation Expressing Solution PDFDocumento30 páginasPresentation Expressing Solution PDFNSTI WELEAREGAYAinda não há avaliações
- Exp 9 Kut101Documento17 páginasExp 9 Kut101hannan sharizalAinda não há avaliações
- Lec 5Documento35 páginasLec 5ibrahimomer922Ainda não há avaliações
- Manual Biochemistry-10-2017Documento65 páginasManual Biochemistry-10-2017Dental LecturesMMQAinda não há avaliações
- Percent Composition Chemical Formulas Lesson 4Documento16 páginasPercent Composition Chemical Formulas Lesson 4Mary Aurielle Barroga Nalus100% (1)
- Empirical Molecular FormulasDocumento51 páginasEmpirical Molecular FormulasArah Mae BonillaAinda não há avaliações
- Microsoft PowerPoint - Drug CalculationDocumento29 páginasMicrosoft PowerPoint - Drug CalculationShawn Hill100% (1)
- Calibration CurveDocumento3 páginasCalibration CurveJaldeep JasoliyaAinda não há avaliações
- SolutionskianDocumento4 páginasSolutionskianKiana RodriguezAinda não há avaliações
- Solution:: Combination ProductsDocumento3 páginasSolution:: Combination ProductsAnel Viridiana Alfonso BocarandoAinda não há avaliações
- Stoichiometry and SolutionsDocumento17 páginasStoichiometry and SolutionsyoonglespianoAinda não há avaliações
- ACFrOgDrPz9FkAZt1-Q ASQh5VuH69nlQsZ0GewzhdqS7HEsHUOSfrFDTblaWuNMxJ Z O1v RlvnBJYgK9MTPS kln8mxnEDs1sxKm1s9fB-7N9r7i39N8tW1QetLVbWg1Baz4f4DNbVLHPX2Lf-1 PDFDocumento23 páginasACFrOgDrPz9FkAZt1-Q ASQh5VuH69nlQsZ0GewzhdqS7HEsHUOSfrFDTblaWuNMxJ Z O1v RlvnBJYgK9MTPS kln8mxnEDs1sxKm1s9fB-7N9r7i39N8tW1QetLVbWg1Baz4f4DNbVLHPX2Lf-1 PDFNissah MhaeAinda não há avaliações
- Chapter 1 HW Sheet Answer KeyDocumento23 páginasChapter 1 HW Sheet Answer Keyjaivalpatel4226Ainda não há avaliações
- Interactive Examples Chapter 4Documento52 páginasInteractive Examples Chapter 4Mohammad Y Abu AyyashAinda não há avaliações
- Concentrations of SolutionsDocumento5 páginasConcentrations of SolutionsLaiba KhalidAinda não há avaliações
- SPT 1201 Tut 04Documento6 páginasSPT 1201 Tut 04tafadzwachikuni21septemberAinda não há avaliações
- Answers - Exercises Part 2Documento3 páginasAnswers - Exercises Part 2haqqiAinda não há avaliações
- Solvent: Meoh Instructions:: Sample PreparationDocumento10 páginasSolvent: Meoh Instructions:: Sample PreparationJosé CâmaraAinda não há avaliações
- Unit 2 Solutions UST TemplateDocumento15 páginasUnit 2 Solutions UST TemplateCess MontemayorAinda não há avaliações
- Empirical & Molecular FormulasDocumento28 páginasEmpirical & Molecular FormulasMenaga A/P Ilangkovan100% (2)
- Empirical & Molecular FormulasDocumento28 páginasEmpirical & Molecular FormulasGlenn ClementeAinda não há avaliações
- Concentration of SolutionDocumento13 páginasConcentration of SolutionRuth Francesca Tiamzon100% (1)
- Analytical ChemistryDocumento7 páginasAnalytical ChemistryLouisa WongAinda não há avaliações
- Protein EstimationDocumento4 páginasProtein Estimationmdanasd123Ainda não há avaliações
- Applications-Application - C.2.1. Ammonium in Solid Samples - EnglishDocumento3 páginasApplications-Application - C.2.1. Ammonium in Solid Samples - EnglishGabriela Alejandra Benito MoralesAinda não há avaliações
- Lec 4Documento14 páginasLec 4أمير حامدAinda não há avaliações
- 4CHM211Documento10 páginas4CHM211sukude303Ainda não há avaliações
- Experiment #2 Limiting ReactantDocumento26 páginasExperiment #2 Limiting Reactanthassan salehAinda não há avaliações
- Bab 2, KONSENTRASI LARUTAN Newest1Documento35 páginasBab 2, KONSENTRASI LARUTAN Newest1yohanesAinda não há avaliações
- Lab 6 - QUANTIFICATION OF TOTAL LIPID CONTENT - Vũ Trọng Thức PDFDocumento17 páginasLab 6 - QUANTIFICATION OF TOTAL LIPID CONTENT - Vũ Trọng Thức PDFTu HaAinda não há avaliações
- ApendixDocumento74 páginasApendixAnuradhaAinda não há avaliações
- mcb3020l Final ReviewDocumento32 páginasmcb3020l Final Reviewapi-413521508Ainda não há avaliações
- Calculations PharmaDocumento24 páginasCalculations PharmaAhmed YTAinda não há avaliações
- Stoichiometric CalculationDocumento8 páginasStoichiometric CalculationSobana KanthiAinda não há avaliações
- T NG H P FurfuralDocumento5 páginasT NG H P FurfuralTHỦYAinda não há avaliações
- BocalanBC BSP3-6 LabaratoryActivity2Documento4 páginasBocalanBC BSP3-6 LabaratoryActivity2ANA MARIA BETHINNA BOCALANAinda não há avaliações
- Stoichiometric CalculationsDocumento33 páginasStoichiometric CalculationsHazrati Ummi100% (1)
- March 07 2024 - Expressing Concentration of Solution QuantitativelyDocumento10 páginasMarch 07 2024 - Expressing Concentration of Solution QuantitativelyDavid BucoyAinda não há avaliações
- CarrieDocumento1 páginaCarrieCarrie RandleAinda não há avaliações
- Experiment 3 Lab ReportDocumento10 páginasExperiment 3 Lab ReportLilo KuleAinda não há avaliações
- 3 Solution ConcentrationsDocumento30 páginas3 Solution ConcentrationsB R YAinda não há avaliações
- Analytical Method of Fertilizer Grade Potassium Dihydrogen PhosphateDocumento6 páginasAnalytical Method of Fertilizer Grade Potassium Dihydrogen PhosphateGenaro PalacioAinda não há avaliações
- Lecture2and3 150321032756 Conversion Gate01Documento50 páginasLecture2and3 150321032756 Conversion Gate01Raja VeluAinda não há avaliações
- Mass & Molar Mass IIDocumento32 páginasMass & Molar Mass IInosirat aladeAinda não há avaliações
- Practical 3: Analysis of Solubilised Protein-Biuret Reagent MethodDocumento8 páginasPractical 3: Analysis of Solubilised Protein-Biuret Reagent MethodainakmliaAinda não há avaliações
- Analytical Chemistry 1Documento10 páginasAnalytical Chemistry 1Lai BotenganAinda não há avaliações
- Module 4 - CONCENTRATION OF SOLUTIONSDocumento8 páginasModule 4 - CONCENTRATION OF SOLUTIONSGabo Alfonso100% (2)
- Lab Report of BinderDocumento10 páginasLab Report of BinderJamal Haider HasibAinda não há avaliações
- ATK - MateriDocumento16 páginasATK - MateriYanto Guru TikAinda não há avaliações
- RX Pricing To Rules On Calculating RX OrdersDocumento4 páginasRX Pricing To Rules On Calculating RX OrdersIan GabritoAinda não há avaliações
- Lesson 6:: Percentage CompositionDocumento22 páginasLesson 6:: Percentage CompositionNikko SebastianAinda não há avaliações
- 11.1 - Concentration TermsDocumento25 páginas11.1 - Concentration TermsGreeshma ColumbusAinda não há avaliações
- BIOCHEM ACTIVITY 2b-EditDocumento1 páginaBIOCHEM ACTIVITY 2b-EditSharleneCherryM.SuratzkieAinda não há avaliações
- Lab 2Documento4 páginasLab 2Serdar Abdulkerim GulliAinda não há avaliações
- Name: Choi Yu Hui ID: 1402693Documento1 páginaName: Choi Yu Hui ID: 1402693Yu HuiAinda não há avaliações
- Time TableDocumento3 páginasTime TableYu HuiAinda não há avaliações
- Effect of Pretreatment On Synthesis of Oil Palm Frond Derived Catalyst For Biodiesel ProductionDocumento1 páginaEffect of Pretreatment On Synthesis of Oil Palm Frond Derived Catalyst For Biodiesel ProductionYu HuiAinda não há avaliações
- Yu Hui PDDocumento21 páginasYu Hui PDYu HuiAinda não há avaliações
- A Review: Bioprocess of Microalgae Using Ionic Liquid-Based Aqueous Two-Phase SystemDocumento1 páginaA Review: Bioprocess of Microalgae Using Ionic Liquid-Based Aqueous Two-Phase SystemYu HuiAinda não há avaliações
- Receipt of Lab Report Submission (To Be Keep by Student)Documento2 páginasReceipt of Lab Report Submission (To Be Keep by Student)Yu HuiAinda não há avaliações
- Presentation Rev02Documento41 páginasPresentation Rev02Yu HuiAinda não há avaliações
- Beth and AdamDocumento4 páginasBeth and AdamYu HuiAinda não há avaliações
- Universiti Tunku Abdul Rahman: UEMK 3223 Particle TechnologyDocumento6 páginasUniversiti Tunku Abdul Rahman: UEMK 3223 Particle TechnologyYu HuiAinda não há avaliações
- Heat-Exchanger Bypass Control: William L. LuybenDocumento9 páginasHeat-Exchanger Bypass Control: William L. LuybenYu Hui100% (1)
- References: Journal of Modern Engineering Research (IJMER), p.2Documento1 páginaReferences: Journal of Modern Engineering Research (IJMER), p.2Yu HuiAinda não há avaliações
- Wide Range Simulation Study of Taylor Bubbles in Circular Milli and MicrochannelsDocumento19 páginasWide Range Simulation Study of Taylor Bubbles in Circular Milli and MicrochannelsYu HuiAinda não há avaliações
- Imperfection in SolidDocumento11 páginasImperfection in SolidYu HuiAinda não há avaliações
- CL List of Companies 2015 and 2016Documento12 páginasCL List of Companies 2015 and 2016Yu HuiAinda não há avaliações
- Lab PreparationDocumento2 páginasLab PreparationYu HuiAinda não há avaliações
- (A.) (B.) Figure X: Carbon Steel Microstructure Before AnnealingDocumento2 páginas(A.) (B.) Figure X: Carbon Steel Microstructure Before AnnealingYu HuiAinda não há avaliações
- A CFD Simulation Study On Pressure Drop and Velocity Across Single Flow Microchannel Heat SinkDocumento10 páginasA CFD Simulation Study On Pressure Drop and Velocity Across Single Flow Microchannel Heat SinkYu HuiAinda não há avaliações
- Study of Numerical Simulation Applying To The Design of An Orifice With High-Velocity WaterjetDocumento10 páginasStudy of Numerical Simulation Applying To The Design of An Orifice With High-Velocity WaterjetYu HuiAinda não há avaliações
- NoseDocumento125 páginasNoseYu HuiAinda não há avaliações