Escolar Documentos
Profissional Documentos
Cultura Documentos
Imaging Features of Sarcoidosis On MDCT, FDG PET, and PET/CT
Enviado por
ALE X RAYTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Imaging Features of Sarcoidosis On MDCT, FDG PET, and PET/CT
Enviado por
ALE X RAYDireitos autorais:
Formatos disponíveis
AJR Integrative Imaging
LIFELONG LEARNING
FOR RADIOLOGY
Imaging Features of Sarcoidosis on MDCT, FDG PET,
and PET/CT
Hima B. Prabhakar1, Chad B. Rabinowitz1, Fiona K. Gibbons2, Walter J. O’Donnell2, Jo-Anne O. Shepard3, and Suzanne L. Aquino3
Objective The imaging features of sarcoidosis are protean and can
The objectives of this article are to discuss the epidemiology be shown with a variety of imaging techniques. Diagnostic
and natural history of sarcoidosis; to review the classic imaging imaging can not only help suggest a diagnosis in asymptom-
features of sarcoidosis on radiography, CT, and 67Ga nuclear atic patients, but can also help in monitoring therapeutic
medicine scans; and to present clinical examples of sarcoidosis response in symptomatic patients. FDG uptake on PET in
as seen on PET and PET/CT in the chest, abdomen and pelvis, patients with sarcoidosis is nonspecific and can mimic that
and bones. in malignancies such as lymphoma and diffuse metastatic
disease [2].
Conclusion
The imaging features of sarcoidosis are diverse and can be Epidemiology
seen on a variety of imaging techniques. It is important for ra- Sarcoidosis has a worldwide distribution and typically af-
diologists and nuclear medicine physicians to recognize the com- fects young to middle-aged adults. The highest prevalence of
mon imaging features and patterns of sarcoidosis in order to the disease is found in African-Americans, Swedes, and Danes.
raise the possibility in the appropriate clinical setting. In the United States, the incidence rate of sarcoidosis is 35.5
cases per 100,000 in blacks and 10.9 cases per 100,000 in
Introduction whites. Additionally, the disease incidence is slightly higher in
Sarcoidosis is a multiorgan granulomatous disease with a women than in men [3].
wide variety of imaging features. Imaging abnormalities can
commonly be seen on chest radiography, MDCT, 67Ga scans, Clinical Presentation and Natural History
FDG PET, and PET/CT. FDG uptake from sarcoidosis is non- Because sarcoidosis affects multiple organ systems, presen-
specific and can mimic other disease processes, including lym- tation varies from nonspecific constitutional symptoms to
phoma and diffuse metastatic disease. When combined with those related to specific organ involvement. Symptoms related
imaging features on other techniques, such as MDCT, FDG to lung involvement (dyspnea and cough) can lead to chest
uptake can be useful in monitoring therapeutic response in radiographs that eventually yield the diagnosis of sarcoid.
patients with known sarcoidosis. Because imaging features of One third of patients have peripheral lymphadenopathy, most
sarcoidosis can overlap considerably with those of malignant commonly involving the cervical, axillary, and inguinal lymph
disorders, it is important for both radiologists and nuclear nodes. One quarter of patients show characteristic skin le-
medicine specialists to be aware of the many varied presenta- sions, including erythema nodosum and lupus pernio [3].
tions of sarcoidosis in order to suggest the diagnosis in the The natural history of sarcoidosis varies significantly
appropriate clinical setting. from patient to patient. The disease spontaneously remits in
Sarcoidosis is a systemic and chronic disease of unknown up to one third of patients, but is chronic and progressive in
cause [1]. The characteristic histologic lesion, a noncaseating up to 30%. There is a 1–5% fatality rate from the disease,
granuloma, has been described as affecting all organ systems, most commonly resulting from severe respiratory or cardiac
although they are most frequently seen affecting the lungs [2]. involvement [3].
Keywords: CT, FDG PET, MDCT, PET/CT, sarcoidosis
DOI:10.2214/AJR.07.7001
Received March 8, 2007; accepted after revision June 11, 2007.
1
Abdominal Imaging and Interventional Radiology, Department of Radiology, Massachusetts General Hospital, 55 Fruit St., FND 270, Boston, MA 02114. Address correspondence to
H. B. Prabhakar (himaprab@gmail.com).
Department of Pulmonary/Critical Care Medicine, Massachusetts General Hospital, Boston, MA.
2
Thoracic Radiology, Department of Radiology, Massachusetts General Hospital, Boston, MA.
3
AJR 2008;190:S1–S6 0361–803X/08/1903–S1 © American Roentgen Ray Society
AJR:190, March 2008 S1
Prabhakar et al.
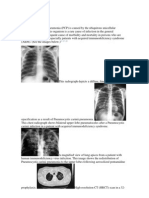
Fig. 1—Stage 1 sarcoidosis in 54-year-old man with biopsy-proven sarcoidosis. Fig. 2—Pulmonary nodules in peribronchovascular distribution in 44-year-old
Frontal chest radiograph shows right paratracheal and bilateral hilar lymphade- woman with sarcoidosis. High-resolution chest CT image shows multiple tiny pul-
nopathy (arrows) and clear lungs. monary nodules centered in peribronchovascular distribution (upper arrow). Small
pulmonary nodules can also be seen lining right major fissure (lower arrow).
Fig. 3—Abdominal lymphadenopathy in 38-year-old man with biopsy-proven Fig. 4—Lambda (λ) sign on 67Ga scan in 26-year-old man with biopsy-proven sar-
sarcoidosis. Contrast-enhanced axial CT image of upper abdomen shows mul- coidosis. Anterior image of chest shows increased tracer uptake in right paratra-
tiple periaortic lymph nodes (arrows). cheal and bilateral hilar lymph nodes, in configuration known as “lambda sign.”
Classic Imaging Features of Sarcoidosis MDCT
Radiography Lymphadenopathy and parenchymal involvement in the
Chest radiographic features include mediastinal and bi- neck and chest are more readily shown on MDCT. In the
lateral hilar lymphadenopathy, parenchymal opacities, and, neck, palpable cervical lymphadenopathy is identified in
in more advanced cases, parenchymal fibrosis. A clinical one third of patients, usually in the posterior triangle. In
staging system based on the chest radiograph has been de- the chest, paratracheal, mediastinal, and bilateral hilar
vised to monitor disease in patients with sarcoidosis as well lymphadenopathy are most commonly identified. Charac-
as to predict patient prognosis. The five-part staging sys- teristic parenchymal lesions include pulmonary nodules,
tem ranges from stage 0 (no radiographic abnormality) to typically in a peribronchovascular distribution or along fis-
stage 4 (pulmonary fibrosis), with varying degrees of sures [2] (Fig. 2). Less commonly, alveolar consolidation can
lymphadenopathy and pulmonary parenchymal abnormal- be seen with air bronchograms, cavitation, and fibrosis [4].
ities in between. Spontaneous remission is more commonly In the abdomen, lesions are less characteristic, mimick-
seen in patients with stage 1 disease (Fig. 1) than in patients ing systemic diseases such as lymphoma, diffuse metastatic
with more advanced stages [3]. disease, or granulomatous or mycobacterial infection. In
S2 AJR:190, March 2008
Imaging Features of Sarcoidosis
is nonspecific in both intensity and pattern, and is not gener-
ally useful in making an initial diagnosis. Additionally, marked
FDG uptake in lymph nodes and parenchymal organs can be
an important mimic of malignancy, specifically lymphoma
and diffuse metastatic disease. Despite this, FDG uptake can
decrease when sarcoidosis is treated, and PET can be useful in
monitoring the effectiveness of therapy [8, 9].
Although FDG uptake is nonspecific in sarcoidosis, com-
bining the imaging features of sarcoidosis on CT with up-
take on PET can make combined FDG PET/CT a useful
technique in monitoring disease progression or remission.
Additionally, if characteristic patterns of chest CT lesions
are identified (as described previously), along with typical
patterns of lymphadenopathy, the disease can be suggested
on the basis of FDG PET/CT findings. Histologic proof,
however, often is still required because of the importance of
excluding malignancies, particularly lymphoma [10].
Clinical Examples on FDG PET and PET/CT
Head and Neck
Head and neck involvement by sarcoidosis is usually iden-
tified as cervical lymphadenopathy, seen in approximately
one third of patients [2]. On FDG PET, increased uptake has
Fig. 5—Palpable submental lymph node with FDG uptake in 56-year-old woman been described in these lymph nodes (Fig. 5), as well as in the
with palpable submental lymph node. Axial fused contrast-enhanced PET/CT im- parotid glands, in a similar distribution to that seen with 67Ga
age shows enlarged left submental lymph node (arrow) with increased FDG up-
take. Lesion was biopsied and was consistent with sarcoidosis. scanning [7].
addition to diffuse lymphadenopathy (Fig. 3), nonspecific Chest
parenchymal lesions have been described, usually in the Although the radiographic and CT features of sarcoidosis
spleen and liver [4]. Diffuse hepatic involvement can prog- have been well described in the chest, few articles have spe-
ress in some cases to confluent hepatic fibrosis [2]. cifically addressed patterns of FDG uptake in the lungs. Me-
diastinal and hilar lymphadenopathy from sarcoidosis shows
Gallium-67 Scanning increased FDG uptake, as in other parts of the body (Fig. 6).
Gallium-67 imaging has been widely used in the diagnosis Lung parenchymal involvement and FDG uptake is less well
of sarcoidosis. Gallium-67 is taken up in lesions with in- described; however, it has been shown that FDG PET can
creased blood flow, typically in lesions having an inflamma- detect lung involvement by sarcoidosis in patients after trans-
tory or infectious cause. In sarcoidosis, a characteristic pat- plantation [9].
tern of uptake in the chest has been described as the “lambda
sign:” paratracheal and bilateral hilar uptake [5] (Fig. 4). An- Abdomen
other pattern of uptake is called the “panda sign,” caused by Again, as elsewhere in the body, abdominal lymph nodes
uptake in the lacrimal and parotid glands. Although this pat- secondary to sarcoidosis can show increased FDG activity
tern can be seen in other entities, such as lymphoma and [7]. Parenchymal lesions in the abdomen have also been de-
HIV, the bilateral symmetric involvement of the glands is scribed as showing increased FDG uptake. For example,
more typical of sarcoidosis [6]. Additionally, when the panda sarcoidosis is known to cause splenomegaly and low-density
sign is seen in conjunction with the lambda sign, it is highly focal lesions in the spleen that have been reported to have
specific for sarcoidosis [5]. increased FDG uptake on PET [11] (Fig. 7).
FDG PET and PET/CT Musculoskeletal
FDG PET is an important clinical tool in the evaluation of Bone involvement in sarcoidosis can be seen in up to one
known or suspected malignancy. Uptake of the tracer is non- third of patients, usually in the hands and feet. Less com-
specific, however, and is related to tissue metabolism. Thus, monly, axial skeletal involvement can be seen. In both cases,
the agent is also readily taken up in some infectious and in- lesions of sarcoid can be either osteolytic or osteosclerotic,
flammatory conditions. Prior studies show increased FDG and their nonspecific appearance can make diagnosis diffi-
uptake in active sarcoidosis [7, 8]. FDG uptake in sarcoidosis cult. Increased activity can be seen on bone scintigraphy.
AJR:190, March 2008 S3
Prabhakar et al.
A B
Fig. 6—Confluent parenchymal lung nodules and mediastinal and bilateral hilar
lymphadenopathy with increased FDG uptake in 56-year-old woman with biopsy-
proven sarcoidosis.
A–C, Axial CT image (A) shows confluent parenchymal lung nodules (yellow ar-
rows) and mediastinal and bilateral hilar lymphadenopathy (blue arrows). These
abnormalities show increased FDG uptake on fused PET/CT (B) and unfused
PET (C) images.
A B C
Fig. 7—Splenic lesions with uptake from sarcoidosis in 43-year-old woman with history of Hodgkin’s lymphoma.
A–C, Images from combined PET/CT show low-density lesions (arrows, A and C) in spleen on coronal CT image (A). Lesions show increased FDG uptake on fused PET/
CT (B) and unfused PET (C) images. Because of patient’s history of lymphoma, she underwent splenectomy to assess cause of lesion, and pathology revealed nonca-
seating granulomas consistent with sarcoidosis. Sarcoidosis is known to cause splenomegaly and low-density focal lesions in the spleen and has been reported to
have increased FDG uptake on PET scans [11].
S4 AJR:190, March 2008
Imaging Features of Sarcoidosis
A B
Fig. 8—Skeletal uptake in 56-year-old woman with known sarcoidosis in neck,
who presented with pelvic bone pain.
A–C, Images from combined PET/CT scan show multiple subtle sclerotic lesions
(arrows) in bilateral iliac bones on axial CT image (A). These lesions show in-
creased FDG uptake on fused PET/CT (B) and unfused PET (C) images. Biopsy of
left iliac bone lesion was consistent with sarcoidosis.
Fig. 9—Follicular lymphoma and asymptomatic pulmonary sarcoidosis in 44-year-old woman
with history of grade 3 follicular lymphoma that is now in remission. Patient underwent trans-
bronchial biopsy to evaluate small lymph nodes in chest, which revealed noncaseating granulo-
mas consistent with sarcoidosis. Whole-body PET image shows marked FDG uptake in bilateral
axillae and left paratracheal regions (upper arrows), as well as in abdomen (lower arrows). Distri-
bution of adenopathy is more consistent with lymphoma than with sarcoidosis, especially be-
cause of lack of significant hilar or mediastinal lymphadenopathy. Biopsy of left axillary lymph
node revealed follicular lymphoma.
AJR:190, March 2008 S5
Prabhakar et al.
Additionally, case reports have described increased FDG tures and patterns of sarcoidosis in order to raise the pos-
uptake in skeletal sarcoidosis (Fig. 8). In conjunction with sibility in the appropriate clinical setting.
the more characteristic findings of sarcoidosis, such as me-
diastinal lymphadenopathy, bone involvement from sarcoid References
can be suggested in patients with increased focal bone FDG 1. Cox CE, Davis-Allen A, Judson MA. Sarcoidosis. Med Clin North Am 2005;
89:817–828
uptake rather than diffuse metastatic disease [12, 13]. 2. Koyama T, Ueda H, Togashi K, Umeoka S, Kataoka M, Nagai S. Radiologic man-
ifestations of sarcoidosis in various organs. RadioGraphics 2004; 24:87–104
Sarcoidosis as a Mimic of Malignancy 3. Statement on sarcoidosis. Joint Statement of the American Thoracic Society
The most common radiologic finding in sarcoidosis is in- (ATS), the European Respiratory Society (ERS), and the World Association of
trathoracic lymphadenopathy, seen in up to 85% of patients Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the
ATS Board of Directors and by the ERS Executive Committee, February 1999.
[2]. Abdominal lymphadenopathy is seen 30% of cases, with Am J Respir Crit Care Med 1999; 160:736–755
massive lymphadenopathy (lymph nodes > 2 cm) seen in 10% 4. Warshauer DM, Lee JK. Imaging manifestations of abdominal sarcoidosis.
of patients [4]. Given the presence of lymphadenopathy in AJR 2004; 182:15–28
such a large percentage of patients with sarcoidosis, it is not 5. Sulavik SB, Spencer RP, Weed DA, Shapiro HR, Shiue ST, Castriotta RJ. Rec-
ognition of distinctive patterns of gallium-67 distribution in sarcoidosis. J Nucl
surprising that one of the more common differential consider-
Med 1990; 31:1909–1914
ations in these patients is lymphoma. Additionally, as de- 6. Kurdziel KA. The panda sign. Radiology 2000; 215:884–885
scribed previously, musculoskeletal involvement in sarcoidosis 7. Lewis PJ, Salama A. Uptake of fluorine-18-fluorodeoxyglucose in sarcoidosis.
can manifest as increased focal uptake throughout the skele- J Nucl Med 1994; 35:1647–1649
ton, which can mimic diffuse metastatic disease [12, 13]. 8. Nishiyama Y, Yamamoto Y, Fukunaga K, et al. Comparative evaluation of 18F–
FDG PET and 67Ga scintigraphy in patients with sarcoidosis. J Nucl Med 2006;
To further complicate matters, a known association exists
47:1571–1576
between sarcoidosis and lymphoma, described in 1986 by 9. Love C, Tomas MB, Tronco GG, Palestro CJ. FDG PET of infection and in-
Brinker and called “sarcoidosis–lymphoma syndrome” [14]. flammation. RadioGraphics 2005; 25:1357–1368
Several cases studies have been published describing the asso- 10. Hollister D Jr, Lee MS, Eisen RN, Fey C, Portlock CS. Variable problems in
ciation of chronic active sarcoidosis and systemic lymphoma, lymphomas: Case 2. Sarcoidosis mimicking progressive lymphoma. J Clin On-
col 2005; 23:8113–8116
both Hodgkin’s and non-Hodgkin’s lymphoma [15, 16] (Fig.
11. Vento JA, Arici M, Spencer RP, Sood R. F-18 FDG PET: mottled splenomega-
9). Using data from patients with respiratory sarcoidosis who ly with remission of symptoms after splenectomy in sarcoidosis. Clin Nucl
had registered with the Danish Institute of Clinical Epidemi- Med 2004; 29:103–104
ology, Brinker determined that patients with sarcoidosis are 12. Aberg C, Ponzo F, Raphael B, Amorosi E, Moran V, Kramer E. FDG positron
emission tomography of bone involvement in sarcoidosis. AJR 2004;
at 5.5 times increased risk of developing a lymphoprolifera-
182:975–977
tive disorder as other patients in the same age group [14]. 13. Ludwig V, Fordice S, Lamar R, Martin WH, Delbeke D. Unsuspected skeletal
sarcoidosis mimicking metastatic disease on FDG positron emission tomogra-
Conclusion phy and bone scintigraphy. Clin Nucl Med 2003; 28:176–179
The imaging features of sarcoidosis are diverse and can 14. Brinker H. The sarcoidosis-lymphoma syndrome. Br J Cancer 1986; 54:467–473
be shown on a variety of imaging techniques. FDG uptake 15. Schmuth M, Prior C, Illersperger B, Topar G, Fritsch P, Sepp N. Systemic sar-
on PET in patients with sarcoidosis is variable and can coidosis and cutaneous lymphoma: is the association fortuitous? Br J Dermatol
1999; 140:952–955
mimic malignancies such as lymphoma and diffuse meta- 16. Dunphy CH, Panella MJ, Grosso LE. Low-grade B-cell lymphoma and con-
static disease. It is important for radiologists and nuclear comitant extensive sarcoidlike granulomas: a case report and review of the lit-
medicine physicians to recognize the common imaging fea- erature. Arch Pathol Lab Med 2000; 124:152–156
S6 AJR:190, March 2008
Você também pode gostar
- Bookshelf NBK553857Documento12 páginasBookshelf NBK553857Sanda Škrinjarić-CincarAinda não há avaliações
- Pulmonary Sarcoidosis Presenting With Cannonball Pattern Mimicking Lung MetastasesDocumento5 páginasPulmonary Sarcoidosis Presenting With Cannonball Pattern Mimicking Lung MetastasesIJAR JOURNALAinda não há avaliações
- Tuberculosis: A Benign Impostor: Cher H. Tan Dimitrios P. Kontoyiannis Chitra Viswanathan Revathy B. IyerDocumento7 páginasTuberculosis: A Benign Impostor: Cher H. Tan Dimitrios P. Kontoyiannis Chitra Viswanathan Revathy B. IyerElza Astri SafitriAinda não há avaliações
- 1 s2.0 S2542454819300499 MainDocumento18 páginas1 s2.0 S2542454819300499 MainBhagya Narayan PanditAinda não há avaliações
- Detection and Monitoring of Interstitial Lung Disease in PatientsDocumento8 páginasDetection and Monitoring of Interstitial Lung Disease in PatientsdethachaniagoAinda não há avaliações
- Olfactory Neuroblastoma UP TO DATEDocumento22 páginasOlfactory Neuroblastoma UP TO DATEZuleibis Melean LopezAinda não há avaliações
- Transfer I RDocumento11 páginasTransfer I Rdaniester.bsrAinda não há avaliações
- Cardiac Sarcoidosis: Key Concepts in Pathogenesis, Disease Management, and Interesting CasesNo EverandCardiac Sarcoidosis: Key Concepts in Pathogenesis, Disease Management, and Interesting CasesAinda não há avaliações
- COVID 19PatientsAndTheRadiologDocumento7 páginasCOVID 19PatientsAndTheRadiologjturos2003Ainda não há avaliações
- Original Research PaperDocumento5 páginasOriginal Research PapersrijitAinda não há avaliações
- Areas Involved, Comprising 48% To 67% of LesionsDocumento5 páginasAreas Involved, Comprising 48% To 67% of LesionsFahmi Sulistyo HutomoAinda não há avaliações
- Extra Renal Rhabdoid Tumor in An Adult Presentin 2024 International JournalDocumento5 páginasExtra Renal Rhabdoid Tumor in An Adult Presentin 2024 International JournalRonald QuezadaAinda não há avaliações
- Acta 88 134Documento9 páginasActa 88 134Andre ErlisAinda não há avaliações
- Radiographic Signs of Pulmonar DiseaseDocumento10 páginasRadiographic Signs of Pulmonar DiseaseMarleeng CáceresAinda não há avaliações
- Astrocytoma 10.1007/s00401-015-1410-7Documento14 páginasAstrocytoma 10.1007/s00401-015-1410-7Rikizu HobbiesAinda não há avaliações
- Current Concepts in The Diagnosis and Staging of Lung CancerDocumento15 páginasCurrent Concepts in The Diagnosis and Staging of Lung CancerVictor ChiabaiAinda não há avaliações
- An Update On Polymyalgia RheumaticaDocumento16 páginasAn Update On Polymyalgia RheumaticaGian CarloAinda não há avaliações
- Bmhim 23 80 1 063-068Documento6 páginasBmhim 23 80 1 063-068Karina CamachoAinda não há avaliações
- International Journal of Infectious Diseases: Evangelia Skoura, Alimuddin Zumla, Jamshed BomanjiDocumento7 páginasInternational Journal of Infectious Diseases: Evangelia Skoura, Alimuddin Zumla, Jamshed BomanjiNita Julita CindayaAinda não há avaliações
- Journal of Internal Medicine - 2022 - Lundberg - An Update On Polymyalgia RheumaticaDocumento16 páginasJournal of Internal Medicine - 2022 - Lundberg - An Update On Polymyalgia RheumaticaDenisa Carmen ColiofAinda não há avaliações
- Rakovich Solitary Fibrous Tumour of The Pleura A Case Report 2010Documento2 páginasRakovich Solitary Fibrous Tumour of The Pleura A Case Report 2010JZAinda não há avaliações
- The Role of Imaging in Malignant Pleural Mesothelioma: An Update After The 2018 BTS GuidelinesDocumento10 páginasThe Role of Imaging in Malignant Pleural Mesothelioma: An Update After The 2018 BTS GuidelinesOncología CdsAinda não há avaliações
- Section II - Chest Radiology: Figure 1ADocumento35 páginasSection II - Chest Radiology: Figure 1AHaluk Alibazoglu100% (1)
- Covid 10Documento14 páginasCovid 10jenifer paathAinda não há avaliações
- Glimelius 2013Documento8 páginasGlimelius 2013Sofia SimpertigueAinda não há avaliações
- The Value of HRCT Chest in Diagnosis of COVID 19 and Its Correlation With RT-PCRDocumento5 páginasThe Value of HRCT Chest in Diagnosis of COVID 19 and Its Correlation With RT-PCREditor IJETBSAinda não há avaliações
- Articulo Tumor de Orbita1Documento16 páginasArticulo Tumor de Orbita1Dora Aranda VillalobosAinda não há avaliações
- Spinal Cord EnhancementDocumento3 páginasSpinal Cord EnhancementGabriel PinillaAinda não há avaliações
- Cancer de Pulmon Clinical KeyDocumento15 páginasCancer de Pulmon Clinical KeyMarcia MejíaAinda não há avaliações
- Article Imaging of Salivary Gland TumoursDocumento11 páginasArticle Imaging of Salivary Gland TumourskaryndpAinda não há avaliações
- 16 - Bab 2Documento3 páginas16 - Bab 2najibAinda não há avaliações
- Radiographics Sarcoidosis From Head To Toe 2018Documento21 páginasRadiographics Sarcoidosis From Head To Toe 2018wfranelicAinda não há avaliações
- Calcific Tendinitis of The Rotator Cuff State of The Art in Diagnosis PDFDocumento8 páginasCalcific Tendinitis of The Rotator Cuff State of The Art in Diagnosis PDFItai IzhakAinda não há avaliações
- Renal Cell Carcinoma in Young Patients: A Review of Recent LiteratureDocumento6 páginasRenal Cell Carcinoma in Young Patients: A Review of Recent LiteratureSAhand HamzaAinda não há avaliações
- 2015 PMR GuidelinesDocumento7 páginas2015 PMR GuidelinesAbdulAzizAinda não há avaliações
- OverviewDocumento2 páginasOverviewAlexa DivaAinda não há avaliações
- Ctoij MS Id 555925Documento8 páginasCtoij MS Id 555925lucasnegromonte0001Ainda não há avaliações
- Laryngeal Chondrosarcoma of The Thyroid Cartilage: Case ReportDocumento5 páginasLaryngeal Chondrosarcoma of The Thyroid Cartilage: Case ReportelvirAinda não há avaliações
- Glandula SalialDocumento45 páginasGlandula SalialJose Daniel Garcia AlatorreAinda não há avaliações
- Jurnal Lipoma Inggris 1Documento9 páginasJurnal Lipoma Inggris 1Yunawan Dwi PuspoAinda não há avaliações
- Reti No Blast OmaDocumento11 páginasReti No Blast OmaNiluh Ita PasyantiAinda não há avaliações
- Jurnal Patologi AnatomiDocumento5 páginasJurnal Patologi Anatomiafiqzakieilhami11Ainda não há avaliações
- Imaging of SpondylodiscitisDocumento17 páginasImaging of SpondylodiscitisRoxana StanciuAinda não há avaliações
- Fast Facts: Blastic Plasmacytoid Dendritic Cell Neoplasm: Shedding light on a rare diseaseNo EverandFast Facts: Blastic Plasmacytoid Dendritic Cell Neoplasm: Shedding light on a rare diseaseAinda não há avaliações
- Rosado-de-Christenson Localized Fibrous Tumors of The Pleura 2003Documento25 páginasRosado-de-Christenson Localized Fibrous Tumors of The Pleura 2003JZAinda não há avaliações
- PisahknDocumento13 páginasPisahknNurul Hazi putriAinda não há avaliações
- Radiologic Manifestations of Pulmonary Tuberculosis in Patients of Intensive Care UnitsDocumento6 páginasRadiologic Manifestations of Pulmonary Tuberculosis in Patients of Intensive Care UnitsAnonymous qwH3D0KrpAinda não há avaliações
- 10.1007@s00270 019 02259 WDocumento7 páginas10.1007@s00270 019 02259 WJuan Daniel Serrano GuerreroAinda não há avaliações
- Cancers 15 03981 v2Documento33 páginasCancers 15 03981 v2abhi16243Ainda não há avaliações
- Lymph Nodes Normal and MalignantDocumento12 páginasLymph Nodes Normal and MalignantMagzAinda não há avaliações
- Cópia de 1990, MorrisDocumento2 páginasCópia de 1990, MorrisAna Cecília RizzuttiAinda não há avaliações
- 風免journalDocumento45 páginas風免journalpeipeipoorAinda não há avaliações
- Pulmonary Pseudotumoral Tuberculosis in An Old Man: A Rare PresentationDocumento3 páginasPulmonary Pseudotumoral Tuberculosis in An Old Man: A Rare PresentationNurhasanahAinda não há avaliações
- Imaging-Guided Chest Biopsies: Techniques and Clinical ResultsDocumento10 páginasImaging-Guided Chest Biopsies: Techniques and Clinical Resultsweni kartika nugrohoAinda não há avaliações
- 264 2011 Article 1480Documento6 páginas264 2011 Article 1480Paul Mendez AguilarAinda não há avaliações
- Gupta Part 2Documento13 páginasGupta Part 2rrralexandrescuAinda não há avaliações
- Doble ArcoDocumento19 páginasDoble ArcoCorazon MabelAinda não há avaliações
- Pub1466 WebDocumento128 páginasPub1466 WebALE X RAY100% (1)
- Forensic Radiography Forensic Radiography Forensic Radiography Forensic Radiography Forensic Radiography Forensic RadiographyDocumento54 páginasForensic Radiography Forensic Radiography Forensic Radiography Forensic Radiography Forensic Radiography Forensic RadiographyALE X RAYAinda não há avaliações
- Herca Statement Body Scanners 101201 FinalDocumento2 páginasHerca Statement Body Scanners 101201 FinalALE X RAYAinda não há avaliações
- 09h10 DR D Bosch RM 11 Session 1 FridayDocumento7 páginas09h10 DR D Bosch RM 11 Session 1 FridayALE X RAYAinda não há avaliações
- DBT As1Documento2 páginasDBT As1ALE X RAYAinda não há avaliações
- LcserneticsDocumento20 páginasLcserneticsALE X RAYAinda não há avaliações
- WP-00007 Tomo 03-08Documento8 páginasWP-00007 Tomo 03-08ALE X RAYAinda não há avaliações
- Radiation BiologyDocumento166 páginasRadiation BiologyAnh Tran100% (4)
- Radiation BiologyDocumento166 páginasRadiation BiologyAnh Tran100% (4)
- Featured Case StudyDocumento2 páginasFeatured Case StudyALE X RAYAinda não há avaliações
- Procedure Guideline For FDG PET Brain ImagingDocumento12 páginasProcedure Guideline For FDG PET Brain ImagingALE X RAYAinda não há avaliações
- Guideline For Adult Gastric EmptyingDocumento5 páginasGuideline For Adult Gastric EmptyingALE X RAYAinda não há avaliações
- Society of Nuclear Medicine Procedure Guideline For Telenuclear MedicineDocumento4 páginasSociety of Nuclear Medicine Procedure Guideline For Telenuclear MedicineALE X RAYAinda não há avaliações
- Interventional Procedures - Avoiding Radiation InjuriesDocumento28 páginasInterventional Procedures - Avoiding Radiation InjuriesALE X RAYAinda não há avaliações
- Managing Patient Dose in Computed Tomography (CT)Documento28 páginasManaging Patient Dose in Computed Tomography (CT)ALE X RAY100% (1)
- IMCI Chart BookletDocumento66 páginasIMCI Chart Bookletnorwin_033875Ainda não há avaliações
- Bucks County Courier Times 01-03-2010Documento90 páginasBucks County Courier Times 01-03-2010ssinger04Ainda não há avaliações
- BDS 2nd Year DM NotesDocumento34 páginasBDS 2nd Year DM NotesPUBG VS FREE FIREAinda não há avaliações
- PLI Proposal FormDocumento8 páginasPLI Proposal FormPalakala NagarjunaAinda não há avaliações
- DolcetDocumento3 páginasDolcetConn_Casipe_8158100% (4)
- Business Proposal SampleDocumento3 páginasBusiness Proposal SampleIan TattaoAinda não há avaliações
- The Letter of Intent To Enter PracticeDocumento4 páginasThe Letter of Intent To Enter Practicekazniels100% (1)
- Chocolate and The Brain: Neurobiological Impact of Cocoa Flavanols On Cognition and BehaviorDocumento9 páginasChocolate and The Brain: Neurobiological Impact of Cocoa Flavanols On Cognition and BehaviorStefan AvramovskiAinda não há avaliações
- Orthofix Investor Presentation - January 2017Documento20 páginasOrthofix Investor Presentation - January 2017medtechyAinda não há avaliações
- Orientation To Blood Bank 2Documento24 páginasOrientation To Blood Bank 2Darshita SharmaAinda não há avaliações
- Mix It Up and SqueezeDocumento3 páginasMix It Up and Squeezeapi-233757247100% (1)
- Retinos PDFDocumento83 páginasRetinos PDFPaulo Gan100% (2)
- Jordan University of Science and TechnologyDocumento33 páginasJordan University of Science and TechnologyNourAldin AbuSalehAinda não há avaliações
- Herk Iames Pressley RidgeDocumento3 páginasHerk Iames Pressley Ridgeapi-350759206Ainda não há avaliações
- A Regenerative Interventional Approach To The Management of Degenerative Low Back PainDocumento16 páginasA Regenerative Interventional Approach To The Management of Degenerative Low Back PainAthenaeum Scientific PublishersAinda não há avaliações
- Times Leader 07-25-2011Documento27 páginasTimes Leader 07-25-2011The Times LeaderAinda não há avaliações
- OB Gyne HistoryDocumento2 páginasOB Gyne HistoryNeil Victor Ongco PajugotAinda não há avaliações
- Scientific Agenda 1st PAEC Virtual Breast Cancer Symposium 15-10-2022Documento4 páginasScientific Agenda 1st PAEC Virtual Breast Cancer Symposium 15-10-2022M NaveedAinda não há avaliações
- Hamstring Injuries in Football PDFDocumento276 páginasHamstring Injuries in Football PDFulfahrifAinda não há avaliações
- PhilHealth Circular No. 14 S. 2018 - CF4Documento3 páginasPhilHealth Circular No. 14 S. 2018 - CF4Toche Doce100% (1)
- Pemanfaatan Teknik Assisted Hatching Dalam Meningkatkan Implantasi EmbrioDocumento10 páginasPemanfaatan Teknik Assisted Hatching Dalam Meningkatkan Implantasi EmbrioMumutTeaAinda não há avaliações
- Classification of Drugs Are: Hepatoprotective Drugs E.g.: Silymarin Antibiotics E.G.Documento2 páginasClassification of Drugs Are: Hepatoprotective Drugs E.g.: Silymarin Antibiotics E.G.Navya Sara SanthoshAinda não há avaliações
- Lesson Plan For Anatomy and PhysiologyDocumento7 páginasLesson Plan For Anatomy and PhysiologyJamie Bagundol100% (1)
- Reliance Policy Extn Request FormDocumento1 páginaReliance Policy Extn Request Formanshu_204Ainda não há avaliações
- Tanggal Rujuk BalnamaDocumento7 páginasTanggal Rujuk BalnamapkmjemursariAinda não há avaliações
- NantesDiagnosticCriteria For Pudendal NeuralgiaDocumento5 páginasNantesDiagnosticCriteria For Pudendal NeuralgiaTeresa BeckAinda não há avaliações
- Drug InteractionsDocumento21 páginasDrug InteractionsPawan Deshmukh100% (1)
- Antidepression ReikiDocumento6 páginasAntidepression ReikiDoc Lyman88% (8)
- Reiki 1Documento19 páginasReiki 1api-246890707Ainda não há avaliações
- Burnout: From Popular Culture To Psychiatric Diagnosis in SwedenDocumento21 páginasBurnout: From Popular Culture To Psychiatric Diagnosis in SwedenRajan PandaAinda não há avaliações