Escolar Documentos
Profissional Documentos
Cultura Documentos
Manifestarile DZ Asupra Tractului Gastrointestinal
Enviado por
Georgiana DimulescuTítulo original
Direitos autorais
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Manifestarile DZ Asupra Tractului Gastrointestinal
Enviado por
Georgiana DimulescuDireitos autorais:
Review
G astrointestinal Manifestations of
Diabetes Mellitus
SHELDON TAUB, ANTHONY MARIANI, AND JAMIE S. BARKIN
This review briefly outlines the gastrointestinal manifestations of diabetes mellitus. Usually, gastro-
intestinal abnormalities are asymptomatic. Severe gastrointestinal dysfunction may be quite debilitating,
however. Gastrointestinal symptoms that are a consequence of diabetes may be confused with other
primary gastrointestinal disorders. We attempt to provide a clinical approach to differentiating the basis of
symptoms and outline the therapeutic plan that we generally employ. Much additional research is
necessary to clarify our understanding of the pathophysiology of the gastrointestinal complications of
diabetes and to develop improved therapeutic strategies, DIABETES CARE 2.- 437-447, SEPTEMBER-OCTOBER 1979.
D
iabetes mellitus is a protean disease that affects which along with hypoglycemia and hyperglycemia have been
every organ in the body. Since it is a common shown to have variable effects on the gut motility.3 These
disorder, affecting approximately 10 million effects are best demonstrated during diabetic ketoacidosis,
Americans,1 one would expect to frequently which is often accompanied by marked gastric distention
encounter complications as a result of diabetic gastro- secondary to acute gastric retention. A fourth mechanism
enteropathy.2"3 However, this is not true. It is noteworthy that may be responsible for the abnormalities of the GI tract
that despite extensive pathologic involvement of the gut, in diabetes is the altered hormone production of insulin and
there are frequently no clinical manifestations indicating glucagon. Elevated levels of these hormones usually result in a
diabetic involvement, with symptoms occurring in only a depression of GI motility and secretion. A fifth possible
small percentage of patients with gastrointestinal (GI) mechanism is the increased susceptibility of diabetic subjects
involvement. As a result of patients being asymptomatic for to secondary infection, as demonstrated by their higher
the most part, no definite data are available on the overall incidence of Candida esophagitis7 and ileocecal tuberculosis.3
frequency of GI manifestations in diabetes mellitus.
Five basic mechanisms have been proposed to account for
ESOPHAGUS
the functional and structural abnormalities associated with
the GI tract in diabetes mellitus.3 The first and most Of the GI manifestations associated with diabetes mellitus,
important is the visceral neuropathy that is responsible for those involving the esophagus are the most recently de-
motor weakness and hypotonia. Neuropathy may also affect scribed. Katz and Spiro, in their extensive review in 1966 of
secretory ability of such organs as the pancreas.3 Pathologic GI manifestations of diabetes,2 failed to even recognize the
abnormalities are noted in the sympathetic ganglion and effects that diabetes has on the esophagus. Mandelstam, in
visceral afferents, but there is a very poor correlation between 1967, was the first to adequately describe the relationship
structural changes and the functional aberrations.4'5 Second, between esophageal motility problems and diabetic neurop-
although diabetic microangiopathy might be responsible for athy.8 Since then, numerous studies have shown that
the disordered motor activity of the intestines, microscopic esophageal motor dysfunction is common in patients with
studies have failed to show microangiopathy in the mucosa associated diabetic neuropathy.9"11 Usually, diabetic periph-
of the GI tract.6 The third possible mechanism is the eral neuropathy precedes esophageal dysfunction.12 Cine
electrolyte imbalance that occasionally accompanies uncon- barium studies may reveal esophageal dilation, delayed
trolled diabetes, such as hypokalemia and hyperkalemia, esophageal emptying (especially when the subject is supine),
DIABETES CARE, VOL. 2 NO. 5, SEPTEMBER-OCTOBER 1979 437
GASTROINTESTINAL MANIFESTATIONS OF DIABETES MELLITUS/S. TAUB, A. MARIANI, AND J. S. BARKIN
and an increase in spontaneous contractions. Manometry formation.16 These findings are usually more prominent in
may indicate absence or reduction in number of primary the middle or lower third of the esophagus. Esophagoscopy
coordinated peristaltic waves that follow swallowing, as well often reveals a yellowish cheesy exudate, and a mucosa that
as the presence of tertiary contractions, which occur ranges from erythema with friability to frank ulceration.
randomly. Combinations of these manometric findings may Demonstration of yeast forms is often easier by smears of
also be seen. In the more severe lesions, the esophageal esophageal brushing than by biopsies.17 Cultures for Candida
motor abnormality may be identical with the defect found in may be positive. Serum agglutinin titers are usually of little
patients with diffuse esophageal spasm, showing both repeti- value, since many normal individuals harbor the organism
tive, nonperistaltic contractions during swallowing and without tissue invasion.
frequent spontaneous contractions. The differential diagnosis of monilial esophagitis in the
The esophageal musculature seems intact as demonstrated diabetic patient with odynophagia includes severe reflux
by the normal amplitude of esophageal contractions with a esophagitis and herpetic esophagitis. Treatment of monilial
decrease in esophageal contraction velocity. Sometimes the esophagitis generally consists of a trial with nystatin suspen-
lower esophageal sphincter is uninvolved, but usually a sion 250,000 U every 2 h in water for 1 wk. An alternative
reduced gastroesophageal sphincter pressure is noted by is nystatin suppositories that may be given orally every 4 h for
manometry.9 Manometric studies have been variously 1 wk. This regimen generally is effective in most cases.
described as showing a variety of different abnormalities, Patients who fail with this form of therapy may be treated
ranging from decreased frequency of peristalsis to increased systematically with flucytosine, low dose amphoterecin B,
repetitive contractions.9*13 or miconazole.
A recent study of esophageal motor function in a larger
group of unselected diabetic patients, without symptoms,
revealed that greater than half showed one or more of the STOMACH
signs of motility disorder, including absent peristaltic response
G
to swallowing and spontaneous and/or repetitive esophageal astric motor abnormalities occur in 20-30% of
contractions.14 In addition, there was a highly significant diabetic patients and, like esophageal motor
relationship between abnormal esophageal motility and the abnormalities, are often without clinical mani-
presence of peripheral neuropathy. Only 4 of 20 diabetic festations or symptomatology.3 On occasion,
patients without neuropathy had abnormal motility studies, delayed gastric emptying or gastric retention may cause vague
whereas 24 of 30 (80%) patients with neuropathy were abdominal discomfort, "heartburn," or protracted nausea,
found to have abnormal motility. Thus, if a diabetic patient vomiting, and halitosis. Anorexia and bloating are less
has peripheral neuropathy, there is an increased likelihood common, and weight loss is uncommon. Clinically, these
of an esophageal motor abnormality. manifestations are punctuated by symptom-free intervals.
Despite the fact that an autonomic abnormality in As with the esophagus, patients with gastric motility
esophageal motor function is common on esophageal problems usually have an associated peripheral neuropathy18
manometry in patients with diabetes mellitus, none of the or combined peripheral and autonomic neuropathy (orthostatic
patients in this study had symptoms of esophageal dysfunc- hypotension is the most common autonomic dysfunction).
tion.14 Symptoms, when present, usually are mild and Gastric neuropathy secondary to diabetes was first
consist of heartburn or rarely dysphagia. Many of these pa- recognized by Kassander in 1958, which he dubbed
tients also have a decrease of gastric acid secretion, which "gastroparesis diabeticorum" to describe the atony with
may account for their paucity of symptoms.14a Thus, a delayed gastric emptying seen in diabetic patients.19 The
diabetic patient with a complaint of dysphagia or heartburn basis of this delayed gastric emptying is probably vagal
should be thoroughly evaluated for other causes of dysphagia. neuropathy, since the vagi are normally involved in specific
Evaluation should include at least barium swallow with reflexes that regulate gastric motility and emptying. Support
cineradiography and possibly esophagoscopy. Motility studies for this hypothesis is that the normal stomach, because of its
also may be considered. Since treatment of these motor function as a reservoir, is able to accept increasing volumes
disorders is generally symptomatic, it is usually unnecessary. without increasing the intragastric pressure. This receptive
Monilial esophagitis usually only appears in patients who relaxation, mediated through the vagal nerves, is impaired
have an altered immune response, and thus may occur in after vagotomy as it is similarly impaired in diabetic patients.20
uncontrolled diabetes mellitus.15 The patient with monilial The motor activity of the stomach is also impaired, resulting
esophagitis often complains of dysphagia or odynophagia, in absence of the usual three contractions per minute, or
which may be so severe that he or she is unable to swallow. reduction of the peristaltic amplitude of those waves that are
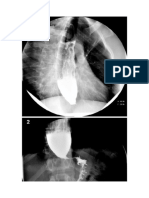
A barium swallow may show the findings of candidiasis, present.21 As a whole, irregularity and uncoordination
including a shaggy outline of the esophageal wall or frank characterize the motor activity of the stomach both after
ulcerations, intramucosal filling defects, and/or nodular vagotomy or as a consequence of diabetes. However,
438 DIABETES CARE, VOL. 2 NO. 5, SEPTEMBER-OCTOBER 1979
GASTROINTESTINAL MANIFESTATIONS OF DIABETES MELLITUS/S. TAUB, A. MARIANI, AND J. S. BARKIN
morphologic confirmation of a vagal lesion is lacking. The is guarded, as these patients usually have advanced complica-
histology of the gastric wall in patients with gastric retention tions of diabetes mellitus.
and diabetes mellitus reveals that both the plexus of Meissner Acute erosive gastritis, along with acute gastric dilation,
and that of Auerbach are normal. Other possible but unlikely may complicate diabetic ketoacidosis.3 Bleeding in this cir-
factors that may induce gastric atony are hyperglycemia cumstance is usually minor, and hardly ever due to duodenal
induced by a rapidly rising glucose level and an elevation of ulcer disease.3 The pathogenesis of this acute gastritis is
glucagon.21 uncertain, but several physiologic factors may play a role.
Gastric stasis results in the formation of coagulums of food, These include marked gastric retention with stasis, which may
which further coalesce and form gastric bezoars.22 Their stimulate an increased level of detergents in the:'gastric
symptoms are nausea, vomiting, and a sense of epigastric juices. This may facilitate back diffusion of acid and lead to
fullness. In addition, they can cause gastric outlet obstruc- the breakdown of the gastric mucosal barrier.3
tion. Thus, before making the "clinical" diagnosis of Until recently, the methods available for treating gastro-
gastroparesis diabeticorum, gastric bezoar function must be paresis diabeticorum have been less than gratifying. Strict
excluded. Fortunately, these bezoars usually respond to diabetic control is important. Cholinergic agents such as
mechanical or chemical dissolutions. bethanechol and the cholinesterase-inhibitor ambenonium
Gastric stasis may also result in bacterial overgrowth, as chloride have met with variable success, but are often
well as gastric candidiasis. Gastric candidiasis is usually associated with disturbing side effects.24 Operative^ drainage
localized, but there may be diffuse dissemination in 10%- of the atonic stomach, either with a gastroenteros^bmy or
15% of patients.23 The delayed emptying of the stomach may pyloroplasty, has occasionally been shown to be effective.26
result in the errative entry of food into the small intestine Predictably, the results are less than optimal, since it is a
to be absorbed. This erratic absorption may be responsible gastric motor abnormality and not a gastric outlet defect.
for frequent episodes of hypoglycemia and lability of Metoclopramide, a drug recently introduced into the United
diabetic control. States, but as yet not approved for use, stimulates contraction
Radiologic manifestations of diabetic gastric neuropathy of the smooth muscle of the GI tract. Metoclopramide is
have been well described.24'25 The most common radiologic thought to act by enhancing the local effect of acetylcholine
feature on upper GI series is the consistent and striking finding at postganglionic nerve endings.27 It has occasionally proved
of sluggish, ineffectual, and irregularly occurring gastric to be effective in diabetic gastroparesis. 28"29a
peristalsis.25 This may be accompanied by a significant Metoclopramide strikingly increases the rate of gastric
amount of solid gastric residue despite a 12-h fasting period emptying, as evidenced by (1) decreased gastric volumes,
prior to the x rays. This phenomenon is seen in up to 90% of (2) decreased recovery of ingested solid foods, and (3)
patients. Other features commonly noted on barium studies decreased half-time^'of gastric emptying as. shown by
include elongation of the stomach, giving it a "sausage- decreased recovery o^ fadiolabeled gastric markers.28 Unfor-
shaped" configuration, significant retention of ingested tunately, improvements with the drug have sometimes been
barium (defined as 50% or more of the ingested barium only temporary, and in the absence of controlled trials, the
present on the 30-min follow-up film), and duodenal bulb possibility of a "placebo effect" cannot be totally excluded.29
atony and dilation without evidence of organic obstruction. A decrease in gastric secretion has been described in
Several entities may radiographically simulate diabetic diabetes mellitus and may explain the reported decreased
gastric neuropathy,25 and include the following. (1) Auto- frequency of duodenal ulcer disease in diabetic patients.30'31
vagotomy that is due to tumor invasion of the vagus However, diabetic patients more commonly have complica-
nerve, originating from a pulmonary, esophageal, or gastric tions of duodenal ulcer disease.7 Basal and augmented acid
carcinoma. (2) Surgical vagotomy may temporarily simulate secretion are lower than normal in diabetic patients, and in
diabetic gastroparesis. However, the intramural nervous one study, approximately 17% of diabetic patients secreted
system of the stomach gradually takes over and furnishes no acid after histamine stimulation.31 This may be explained
enough parasympathetic activity to make peristalsis effective by the fact that gastric mucosa in diabetic individuals
again. (3) Drug-induced motility disturbance may occur. The becomes atrophic both at an earlier age and more frequently
drugs most commonly incriminated are tranquilizers (especially than in nondiabetic subjects.7 As many as 85% of diabetic
diazepam), anticholinergics, ganglion-blocking agents, and patients may have partial or complete atrophy of the gastric
tricyclic antidepressants. These are probably dose-related mucosa, as confirmed by biopsy.18 The gastritis that is found
effects, but occasionally an idiosyncratic reaction may be does not appear to be related to the severity or duration of
responsible. The effects are reversible upon discontinuation of the diabetes per se, but rather is age-related. The older the
the responsible agent, and therefore a detailed drug history is patient, the greater the frequency of either superficial or
imperative before the diagnosis of diabetic gastroparesis atrophic gastritis.18
is made. A recent study evaluated gastric function in diabetic
The prognosis of patients with gastroparesis diabeticorum patients with symptoms of gastroparesis diabeticorum (i.e.,
DIABETES CARE, VOL. 2 NO. 5, SEPTEMBER-OCTOBER 1979 439
GASTROINTESTINAL MANIFESTATIONS OF DIABETES MELLITUS/S. TAUB, A. MARIANI, ANDJ. S. BARKIN
nausea and recurrent vomiting) in comparison with long- involvement of the small bowel is often symptomatic,
standing but asymptomatic diabetic patients and nondiabetic resulting in diarrhea and/or steatorrhea.
controls.318 Both groups of diabetic patients had reduced acid The incidence of diarrhea as a complication of diabetes
secretory responses to sham feeding, consistent with vagal mellitus ranges from 0.001% to as high as 10%.35 Approxi-
neuropathy. On the other hand, acid secretory responses to mately half of those patients presenting with diarrhea have
homogenized food infused directly into the stomach were documented steatorrhea.35 Thus, patients with diabetic
normal, despite an enhanced serum gastrin response in the enteropathy may present with diarrhea alone or in combina-
diabetic patients. tion with steatorrhea.
Antibodies to various gastric components are more Diarrhea and/or steatorrhea in the diabetic patient may be
frequently found in diabetic than in nondiabetic individuals.32 caused by (1) diabetic diarrhea, the pathogenesis of which is
Up to 25% of diabetic patients may have circulating unclear; (2) bacterial overgrowth of jejunal segments, which
parietal cell antibodies compared with only about 8% of is associated with a slow transit time resulting in a "blind
controls. Parietal cell antibodies are especially prevalent in loop" syndrome; (3) chronic pancreatitis with exocrine
young female insulin-dependent diabetic patients.32 Intrinsic- insufficiency, an uncommon cause of diarrhea and steatorrhea
factor antibodies are also found more commonly in diabetes, in diabetes; and/or (4) superimposed adult celiac disease.3
occurring in up to 8% of those in whom it is searched for. Diabetic diarrhea is defined as the presence of chronic
Both intrinsic-factor and parietal cell antibodies are more diarrhea without steatorrhea.36 The diarrhea is usually brown,
frequent in insulin-dependent diabetic patients,3 especially in watery, voluminous, and may be associated with tenesmus.37
women with complications. In addition, latent pernicious It may occur during the day or at night, and when it occurs at
anemia appears to be more common in middle-aged and night it often presents with incontinence. Usually the
elderly diabetic patients, especially females, and affects up to affected patient is a middle-aged male who has long-standing
4%-5% of insulin-dependent diabetic patients in this group.3 diabetes, often poorly controlled on insulin. The patient may
It may be justifiable to do periodic Schilling tests in middle- be of normal weight or occasionally obese and often has
aged insulin-dependent diabetic patients to ascertain evidence of peripheral neuropathy, especially associated
potential need for parenteral vitamin B12. impotence. The diarrhea is typically episodic, with bouts
Two other entities are unique to the diabetic patient. The lasting days to weeks. These episodes are often followed by an
first is diabetic radiculopathy or diabetic plexus neuropathy,33 interval of weeks to months of either normal bowel habits or
resulting in abdominal pain secondary to diabetic neuropathy occasionally even constipation. Interestingly, as time passes
involving the thoracic nerve root area. This entity has been the diarrhea tends to become less severe.
incriminated as the cause of chronic abdominal pain The pathogenesis of diabetic diarrhea is still not totally
stimulating an abdominal malignancy and occasionally resolved. It appears that diabetic autonomic neuropathy plays
resulting in unneeded exploratory laparotomy. These patients a fundamental, although poorly understood, role.36 The only
may have anorexia and weight loss, and are often extensively significant autonomic pathologic finding, however, is a non-
evaluated in pursuit of a malignancy, especially pancreatic specific dendritic swelling in the sympathetic prevertebral
carcinoma. Patients usually have insulin-dependent diabetes and paravertebral ganglia, which is often present in diabetes
and often have diffuse peripheral neuropathies. Frequently, whether there is diarrhea or not.38 The ganglion cells of the
the pain gradually subsides on its own over 6-20 mo. In the plexuses of Auerbach and Meissner are normal. The intestinal
interim, treatment is symptomatic. The diagnosis may be mucosa in this group is usually normal, despite significant
made by electromyographic examination. steatorrhea.3 Although pancreatic exocrine insufficiency has
The second entity is postprandial facial sweating, which is a been proposed as a cause of diabetic diarrhea, most patients
manifestation of diabetic autonomic neuropathy.34 The have normal exocrine pancreatic function as determined by
patient describes drenching facial sweats occurring a few the secretion tests.4 Serum carotene levels may be normal
seconds after starting to chew food. The stimulus to this or elevated.3
gustatory sweating is the chewing of tasty food and this In approaching the differential diagnosis of diarrhea in a
symptom appears to be inhibited effectively by anti- diabetic patient, differentiation should be made between
cholinergic agents. diarrhea and steatorrhea complicating diabetes. This is best
accomplished by measuring 72-h fecal fat while the patient
consumes a diet with a minimum of 75-100 g fat daily.
SMALL INTESTINE
Radiologic features of diabetic diarrhea relate to associated
autonomic neuropathy and include delayed gastric emptying
L
ike the esophagus and stomach, the small bowel (vide supra), variation in luminal caliber with dilated loops of
may be extensively involved in diabetes mellitus. small bowel, segmentation, and mucosal swelling with
While the disorders involving the esophagus and coarsening of mucosal folds. There is considerable variability
and stomach may often go unnoticed, the diabetic in intestinal transit time, varying from shortening to con-
440 DIABETES CARE, VOL. 2 NO. 5, SEPTEMBER-OCTOBER 1979
GASTROINTESTINAL MANIFESTATIONS OF DIABETES MELLITUS/S. TAUB, A. MARIAN!, AND J. S. BARKIN
siderable prolongation.39 The history of small bowel biopsy long-chain fatty acids, which may also incite a secretory type
is usually normal, although occasionally a mild lymphocytic of diarrhea, similar to that seen after castor oil.43 The
infiltrate is present in the lamina propria.40 diagnosis of bacterial overgowth syndrome is usually made
Therapeutic approaches in patients with diabetic diarrhea clinically with gastric or small bowel cultures. It is possible
are somewhat limited, but include (1) strict control of the to determine the magnitude of bile acid deconjugation
diabetes with carefully regulated insulin dosages; (2) possibly induced by intestinal bacterial overgrowth by means of the
14
restriction of iced beverages; (3) use of psyllium hydrophilic C-glycocholate ( 14 C-GCA). test.43'4311 The test involves
mucilloid (Metamucil) powder in conjunction with di- demonstration of increased breath 14CO2 in patients with
phenoxylate hydrochloride (Lomotil) or codeine sulfate, a bacterial overgrowth, with the response normalized after
regime that has met with variable success. antibiotic therapy.43a An empiric trial of antibiotics may be
Steatorrhea may complicate diabetic enteropathy, and is warranted in the diabetic patient with diarrhea. A clinical
present in as many as 75% of diabetic patients with diarrhea. response reaffirms the diagnosis. Therapy should be with a
Significant steatorrhea should be evaluated as to its cause. As broad-spectrum antibiotic such as tetrqpycline or ampicillin.
noted, a 72-h fecal fat collection is used to quantitate the The dosage and duration of therapy should be individualized
steatorrhea. Conventional evaluation for etiology includes if the initial response is favorable. We have found that
upper GI series and small bowel barium studies, malabsorption ampicillin 250 mg four times daily for 1 wk of each month is
studies (i.e., D-xylose, folate levels, Schilling test), and small usually sufficient to control the diarrhea secondary to a blind
bowel biopsy. In most respects, the patient with diabetic loop syndrome.
steatorrhea is indistinguishable from the diabetic patient with A third mechanism of steatorrhea in diabetic patients is
diarrhea alone, except for the fecal fat content of the stool.4 celiac disease. Although the association of diabetes mellitus
As mentioned, earlier, patients with diabetic diarrhea and and celiac disease has been widely reported for many years,
steatorrhea usually have normal jejunal biopsies, the differ- the number.of patients in whom the diagnosis of celiac
ence between patients with and without steatorrhea usually disease has been fully confirmed is small.44 It is entirely
being one of degree.35 The mechanism responsible for possible that diabetes mellitus and adult celiac disease do not
steatorrhea in diabetes is usually unexplained, although the occur anymore frequently than they would by chance alone.44
following causes may be seen in patients with diabetes: (1) However, in children there does appear to be a more frequent
pancreatic exocrine insufficiency, (2) bacterial overgrowth association between these two disease entities than would
secondary to motility disturbance, or (3) concomitant adult occur by chance alone, although even these conclusions are
celiac disease.41 based on very small numbers.44 If there is a common linkage
Abnormalities of pancreatic exocrine function may occur or bridge between diabetes mellitus and celiac disease, it
in 20%-70% of diabetic patients, including those with appears to be connected in some way with histocompatability
juvenile-onset diabetes mellitus.42 The abnormalities which (HLA) antigens. This could imply some type of common
may be found after secretin stimulation (secretin test) are a genetic predisposition to both diabetes and celiac disease. A
low pancreatic volume and/or low exocrine secretion in significantly higher incidence of HLA B8 antigens has been
duodenal aspirates.7 Gross pancreatic exocrine insufficiency demonstrated in patients with celiac disease (80%) com-
is a rare sequel of the diabetic state, and thus pancreatic pared with controls (29.5%).45>56 Likewise, HLA B8 antigen
enzyme replacement usually does not ameliorate the occurs more commonly in patients with juvenile-onset
steatorrhea even when abnormalities are detected in the diabetes mellitus than in controls.47
secretin test. Diabetic diarrhea has many similarities to celiac disease: In
A small percentage of diabetic patients demonstrate both, the diarrhea may be intermittent, preceded by
bacterial overgrowth in the proximal small intestine. This is borborygmi, or associated with steatorrhea.. In addition, the
usually seen in those patients who have slow transit time. diarrhea may be most troublesome at night and associated
The bacterial overgrowth may lead to a type of "blind loop" with incontinence.44
syndrome, with resultant diarrhea and possible steatorrhea.43 Diabetic diarrhea is more common in men and occurs
Abnormal bacterial cultures include increased concentra- mostly in patients in whom diabetes was diagnosed many
tions of Escherichia coli, enterococci, aerobacter, and years previously. These patients are usually on insulin and
staphylococci, which may be found in the small bowel in often their diabetes is rather poorly controlled. Peripheral
concentrations of lOVrnl. A few patients, especially those neuropathy is found in most cases and autonomic neuropathy
with gastroparesis diabeticorum, demonstrate an overgrowth is often demonstrable. The patient is often able to maintain
of bacteria in the stomach.35 However, abnormal bacterial a remarkably good nutritional state despite frequent bowel
concentrations are found in less than 20% of patients in movements, and features of malabsorption are not usually
whom such clinical suspicion precipitates investigation. apparent. Also, in diabetic diarrhea, a tendency toward
Bacterial overgrowth leads to defective micellar formation hypoglycemia during episodes of diarrhea is not a feature.
and resultant fat malabsorption and formation of hydroxy In contrast, celiac disease occurs more commonly in
DIABETES CARE, VOL. 2 NO. 5, SEPTEMBER-OCTOBER 1979 441
GASTROINTESTINAL MANIFESTATIONS OF DIABETES MELLITUS/S. TAUB, A. MARIANI, AND J. S. BARKIN
women and has its onset before or coincident with the onset The presence of a fatty liver in juvenile diabetes usually
of diabetes. These patients often have features of malabsorp- implies metabolic decompensation and is frequently asso-
tion such as bone pain and peripheral edema. If neuropathy ciated with an enlarged liver that is tender to palpation and
is present in a diabetic patient with celiac disease, it is most may be painful. These symptoms improve following better
likely to be a manifestation of the diabetes.44 Likewise, the diabetic control.45 In maturity-onset diabetes, there is no
absence of a neuropathy in a diabetic patient with diarrhea correlation between either duration of diabetes or degree of
should arouse suspicion that the diarrhea is not of diabetic diabetic control and amount of fat in the liver. Fatty liver in
origin. An important clue that diarrhea in a diabetic patient this group is usually asymptomatic and rarely is associated with
may be due to celiac disease is a history of repeated episodes elevated bilirubin or transaminase activity. Obesity and
of hypoglycemia, particularly when the diarrhea is trouble- maturity-onset diabetes are closely linked in the causation of
some.44 Adherence to a strict, gluten-free diet in patients fatty liver.50 Loss of weight in these patients is usually followed
with celiac disease often results in a decrease of these hypo- by improvement in the fatty liver, as well as improved con-
glycemic episodes, even though insulin requirements are trol of the diabetes. Tolbutamide alone has not been shown to
often increased. In general, it is easier to control the diabetes reduce the steatosis of diabetes.49 The histologic picture of
following therapy of the associated celiac disease.35 Labora- fatty liver in maturity-onset diabetes consists of large fat
tory evaluation consistent with malabsorption such as a low globules having the composition of adipose tissue fat. Electron
carotene, albumin, and/or a low serum folate, or an elevated microscopy shows abnormal mitochondria and displacement
alkaline phosphatase may increase the suspicion of the of organelles by the fat globules.51
presence of celiac disease. The small bowel biopsy will Glycogen content of the liver. The liver of diabetic patients
enable one to diagnose celiac disease.44 Typical jejunal biopsy is rich in glycogen, although there is no correlation between
features of celiac disease are varying degrees of villous the hepatic glycogen content and the fasting blood glucose,
atrophy, cellular infiltrates in the lamina propria, and hyper- type of diabetes, degree of ketosis, or fat content of hepato-
plasia of the cells in the basal portions of the crypts of cytes.49 Hepatomegaly due to glycogen deposition may be
Lieberkuhn. A favorable response to a gluten-free diet, and seen in brittle diabetes, especially in children. At this same
occasionally the addition of steroids, is reasonably specific time a tendency toward hypoglycemia may develop. These
for the diagnosis of sprue.35 Likewise, if the small bowel features appear to be the result of chronic use of excess
biopsy is normal or shows a mild lymphocytic infiltrate in the insulin.49
lamina propria, celiac disease is unlikely, and one is probably Glycagon is found in the nuclei of hepatocytes in 60%-
dealing with diabetic diarrhea. 75% of diabetic patients. It is present in 30% of patients
without fatty liver and is found with greater frequency in
patients with increased amounts of cytoplasmic fat. In pa-
LARGE INTESTINE
tients with massive fatty liver, glycogenated nuclei are
invariably present.52 Glycogenated nuclei also may occur in
C
onstipation can be seen in as many as 20% of
diabetic patients who have neuropathy.3 Whether some normal subjects as well as in Wilson's disease, tuber-
this is increased above the normal population is culosis, cirrhosis, and hepatitis.49'53
uncertain, since both entities have a relatively Cirrhosis. It is felt that the fatty liver of diabetes does not
frequent occurrence. Occasionally, a massive amount of fecal progress to cirrhosis, although isolated case reports have
material may be found in the huge, atonic and dilated colon. appeared.54 On the other hand, as many as 80% of patients
This may simulate intestinal obstruction or fecal impaction with established cirrhosis develop carbohydrate intolerance,8
with the impaired mass evacuation of contents into the rectal including overt diabetes with fasting hyperglycemia and
ampulla.3 Occasionally, stercoral ulcerations have been glucosuria in 12%-32%. 55 It would appear that there is a
reported in diabetes mellitus in association with colonic correlation between severity of liver disease and degree of
distension and subsequent mucosal erosion.48 impairment of glucose tolerance. In fact, lowered serum
Like the other intestinal motor abnormalities, large bowel albumin is correlated with abnormal glucose tolerance. In
motility problems are usually seen in patients with associated addition, there is a greater frequency of glucose intolerance
peripheral neuropathies.35 Treatment is basically sympto- in patients with portal-systemic shunting, whether the shunt-
matic, with administration of laxatives and/or stool softeners. ing be surgically induced or spontaneous.56
The hyperglycemia of hepatic cirrhosis is associated with
hyperinsulinemia and insulin resistance. The cause of
LIVER
impaired glucose tolerance in cirrhosis is unclear, although
Fatty liver. The incidence of fatty liver in diabetes mellitus hyperglucagonemia appears to play an important role in its
ranges from 21% to 78%. 49 Whereas biopsy-proved fatty liver development. Recent studies demonstrated increased levels
is present in up to 44% of diabetic patients over 60 yr of age, of glucagon in cirrhosis, particularly in association with
it is found in only 4.5% of patients with juvenile diabetes. portal-systemic shunting.57'58 The hepatocellular damage
442 DIABETES CARE, VOL. 2 NO. 5, SEPTEMBER-OCTOBER 1979
GASTROINTESTINAL MANIFESTATIONS OF DIABETES MELLITUS/S. TAUB, A. MARIANI, AND J. S. BARKIN
resulting from cirrhosis may impair the ability of the liver to BILIARY TRACT
assimilate large glucose loads, thus leading to postprandial
T
hyperglycemia, hyperinsulinemia, and insulin resistance.59 here appears to be an increased incidence of
Other factors that have been implicated in the glucose cholelithiasis in patients with diabetes mellitus.72
intolerance of cirrhosis include hypokalemia,60 chronic Lieber73 found gallstones in 11.6 of 29,779
pancreatitis,61 and hemosiderosis.62 Idiopathic hemochroma- autopsies; however, in a selected group of
tosis affects the pancreas and the liver. Thus, it is not sur- diabetic subjects, the frequency was approximately 30%.
prising that glucose intolerance or overt diabetes is observed Warren74 and Robertson75 also reported a similar association
in up to 80% of these patients.63 Typical diabetic complica- between cholelithiasis and diabetes. Other authors, however,
tions such as retinopathy, nephropathy, and peripheral have been unable to demonstrate this increase.76'77 Studies in
neuropathy may occur in diabetes secondary to hemochroma- Pima Indians have been contradictory, although it is note-
tosis, but are not common in cirrhotic patients with worthy that this population has perhaps the highest incidence
diabetes.64 The diabetes in patients with idiopathic hemo- in the world of both diabetes and gallstones.78 More studies
chromatosis is not solely due to pancreatic islet cell damage are needed to clarify whether there is an increased incidence
but may be contributed to by cirrhosis and genetic influences.65 of cholelithiasis in diabetic patients, but the association
appears to be more than fortuitous.
Other states. There is an increased frequency of hepatitis
Recently, Ponz de Leon et al.79 reported that bile from
in diabetic patients, which may be due to decreased resistance
patients with maturity-onset diabetes was supersaturated with
to infection, but is more likely due to repeated hospital
cholesterol, and the absolute values for biliary bile acid con-
exposure. Madalinski et al. reported a 6.5% frequency of
centrations were significantly reduced. Both trends would
hepatitis B antigen.66
favor cholesterol precipitation and stone formation. The
Glucose tolerance tests are often impaired during the acute
above changes were not apparent in juvenile-onset diabetes.
phase of viral hepatitis. One hundred cases of clinical diabetes
The size of the bile acid pool and the proportion of the
have been reported to occur during viral hepatitis, excluding
individual biliary bile acids were normal in both types of
corticosteroid treatment. The pathogenesis of this glucose
diabetes.
intolerance remains unclear. However, most authors
postulate the existence of a viral endocrine pancreatitis or an The gallbladder of diabetic patients tends to be larger in
insulin-resistant state related either to hepatic cell insuf- size than that of nondiabetic individuals.80 Radiologic
ficiency or an increase in free fatty acid concentration.67 studies have demonstrated that the diabetic gallbladder con-
Several oral hypoglycemic agents may cause hepato- tracts less vigorously after a fatty meal than does a normal
toxicity. The incidence of chlorpropamide-induced cholestatic gallbladder. These findings are not in themselves indications
hepatitis is 0.5%, although transient but significant eleva- for cholecystectomy.
tions of the serum alkaline phosphatase level have been Acute cholecystitis. Acute cholecystitis is particularly lethal
observed in as many as 25% of patients under treatment with in patients with diabetes mellitus in whom a high incidence
this drug.68 Tolbutamide is less likely to cause hepatic of suppurative complications accounts for a mortality of about
dysfunction. Patients who recover from chlorpropamide 20%. Thus, diabetic patients with acute cholecystitis should
hepatitis generally do not relapse when given tolbutamide. undergo cholecystectomy early in the course of the latter
Foster et al.69 reported the occurrence of diabetes mellitus disease. It has been postulated that arterial ischemia of the
in association with hepatic cell adenomas in four family gallbladder wall may be an etiologic factor in the acute
members. They postulated that a defect in carbohydrate cholecystitis of diabetic patients.81 Some authors also suggest
metabolism may play a role in the development of hepatic elective cholecystectomy in diabetic patients with asympto-
cell adenomas. matic cholelithiasis in order to minimize potentially life-
Diabetic ketoacidosis is believed to have been the etiologic threatening complications.82
factor in the hepatic infarction suffered by a young man with Emphysematous cholecystitis is a form of acute cholecystitis
brittle diabetes mellitus.70 Plasma norepinephrine and that derives its name from the occurrence of gas within the
epinephrine levels are usually elevated in patients with gallbladder lumen. Twenty percent of such patients also suffer
diabetic ketoacidosis. Ng et al. suggested that this from diabetes mellitus. The gas-forming organisms that have
catecholamine elevation may have caused vasoconstriction been implicated in the pathogenesis of emphysematous
sufficient to produce hepatic ischemia and infarction in their cholecystitis include Clostridium welchii, which has been
patient.70 cultured most often, as well as anaerobic streptococci,
Holt et al.71 found that 5 of 14 patients with solitary Escherichia coli, Staphylococcus aureus, Pseudomonas, and
liver abscesses had diabetes, whereas none of 22 patients with Kkbsiella.83 Clinically, these patients exhibit a more toxic
multiple liver abscesses had diabetes. Biliary tract infection course; their pain may be more severe than expected in
was also present in the 5 diabetic patients with liver uncomplicated acute cholecystitis. The majority of patients
abscesses. with emphysematous cholecystitis are men in their sixth or
DIABETES CARE, VOL. 2 NO. 5, SEPTEMBER-OCTOBER 1979 443
GASTROINTESTINAL MANIFESTATIONS OF DIABETES MELLITUS/S. TAUB, A. MARIANI, AND J. S. BARKIN
seventh decade of life. The initial management of these When insulin is used in these patients, caution should be
patients consists of high doses of antibiotics (e.g., ampicillin taken to avoid hypoglycemia. Bank et al. suggest the use of a
12 g daily) and general supportive measures to stabilize the glucose tolerance test and possibly insulin studies 3 mo after
patient. This is followed early by laparotomy and chol- an attack of acute pancreatitis to detect any deterioration
ecystectomy. In those cases where the patient remains in the endocrine function of the pancreas.92
unstable despite supportive therapy, cholecystostomy for The use of phenformin therapy in the management of
drainage may be preferable to cholecystectomy.82 maturity-onset diabetes has been associated with acute
pancreatitis.93 Discontinuation of phenformin will avoid
PANCREAS progression of this drug-induced pancreatitis.
A
bnormalities of pancreatic exocrine secretion are Chronic pancreatitis. There is no evidence of an increased
seen in 20%-70% of diabetic patients.84 These incidence of chronic pancreatitis in patients with genetic
abnormalities, usually not severe, are clinically diabetes mellitus. However, idiopathic diabetes mellitus and
inapparent and may be due to vagal neuropathy, chronic pancreatitis are frequently seen in association with
deficient stimulating action of insulin, or glucagon many disease states, e.g., cystic fibrosis and idiopathic
inhibition.85 hemochromatosis.
The diabetic patient is at increased risk for acute pan- The diabetes secondary to chronic pancreatitis represents
creatitis86 and pancreatic carcinoma. On the other hand, 0.3% of all cases of diabetes. In patients with chronic
acute pancreatitis, chronic pancreatitis, and carcinoma of the pancreatitis followed from 1 to 15 yr who had no detectable
pancreas may themselves cause or exacerbate glucose pancreatic calcifications, Bank et al. noted overt diabetes in
intolerance and diabetes. 30% and an abnormal glucose tolerance in another 20%.92
Autopsy studies on patients over 20 yr of age have shown When pancreatic calcifications were present radiologically,
that pancreatitis occurs twice as frequently in diabetic as in overt diabetes was found in 70%, and a further 20% had an
nondiabetic patients, and the damage appears to result from abnormal glucose tolerance. The frequency of glucose
vascular lesions of the diabetic pancreas.86'87 There also intolerance in patients with chronic pancreatitis progressively
appears to be an increased incidence of acute pancreatitis in increases with duration of the disease.94
patients with juvenile-onset diabetes mellitus.88 Patients with Asymptomatic diabetes is most likely to occur in alcohol-
acute pancreatitis complicating diabetic ketoacidosis have a induced pancreatitis, alcohol-induced calcific pancreatitis,
high risk of mortality because of the frequently associated and in nonalcoholic tropical pancreatitis. This last entity is
shock and precipitous hypoglycemia.85 seen especially in Uganda, India, and Indonesia and appears
Acute pancreatitis may induce glucose intolerance. to result from nutritional deficiency.95'96 The occurrence of
Johansen and Ornsholt89 reported an 18% incidence of mild diabetes is lower in idiopathic chronic pancreatitis and
diabetes among 22 patients studied 7-48 mo after an attack of pancreatitis due to various other causes, e.g., biliary calculi
acute pancreatitis. Those patients who developed diabetes or trauma. Alcohol is the etiologic factor in 90% of patients
mellitus were less than 35 yr old, did not require insulin with chronic calcific pancreatitis in most Western countries.
therapy, and had abnormally low and delayed insulin re- The typical patient with pancreatic diabetes gives a history of
sponses after oral glucose intake. Knight and co-workers90 alcohol overindulgence for 5-15 yr, followed by recurrent
reported high serum glucagon levels during the 24 h mild abdominal pain, then by the appearance of diabetes
immediately following an attack of acute pancreatitis, with 1-20 yr later. Steatorrhea occurs 10-25 yr after onset of the
such values falling rapidly thereafter. Hyperglycemia has been disease.92
found in 10%-79% of patients experiencing an episode of Patients with pancreatic diabetes are unusually sensitive
acute pancreatitis. Berkowitz and Glassman suggested the to insulin and therefore may be more susceptible to
use of the intravenous tolbutamide test to aid in the earlier hypoglycemia.
diagnosis of acute pancreatitis when faced with difficult acute Linde et al. noted hypoglycemic episodes in 14 of 18
abdominal presentation.91 insulin-treated patients with chronic pancreatitis and in 3
Rarely, acute hemorrhagic pancreatitis may result in patients in whom severe hypoglycemia was believed to be
diabetes severe enough to cause ketoacidosis and even the cause of their death.97 The hypoglycemia may be due to
isolated cases of diabetic coma (both the ketotic and non- the impaired glucagon and growth hormone response to
ketotic hyperosmolar variety).92 Apart from these cases of insulin-induced hypoglycemia, which is commonly seen in
diabetic coma, there is no correlation between the severity of patients with pancreatic diabetes. The fasting serum insulin
hyperglycemia or the diabetic state and the mortality from levels of patients with chronic calcific pancreatitis are
acute pancreatitis. Thus, the carbohydrate intolerance of approximately half that of normal. The mean peak increment
acute pancreatitis usually is asymptomatic, temporary, and in serum insulin level after oral glucose loading was only
may subside rapidly. Insulin treatment is considered only in 20-25% of normal in patients with pancreatic diabetes. The
the presence of symptoms of diabetes or if ketosis develops. insulin response was also delayed.62 Because of the low
444 DIABETES CARE, VOL. 2 NO. 5, SEPTEMBER-OCTOBER 1979
GASTROINTESTINAL MANIFESTATIONS OF DIABETES MELLITUS/S. TAUB, A. MARIANI, AND J. S. BARKIN
incidence of diabetic vasculopathy and the tendency toward high circulating VIP level. U p to two-thirds of these islet cell
hypoglycemia, most authors advocate erring on the side of tumors are malignant.
undertreatment of pancreatic diabetes.97'98 Higher caloric
intake may be required because of the frequent finding of From the University of Miami School of Medicine, Division of
Gastroenterology, Miami, Florida.
weight loss in these patients. This weight loss may be Address reprint requests to Jamie S. Barkin, M.D., University
secondary to malabsorption from the associated pancreatic of Miami School of Medicine (D-49), P.O. Box 016960, Division
exocrine insufficiency. Bank et al. suggest a trial of oral of Gastroenterology, Miami, Florida 33101.
hypoglycemic agents before insulin therapy is instituted.
Glucagon may help in managing patients with pancreatic REFERENCES
diabetes complicated by drug-induced hypoglycemia.97 1
Crofford, O., et al.: Report of the National Commission on
Carcinoma of the pancreas. The frequency of carcinoma of Diabetes. Washington, D. C , DHEW Publication (NIH) 76-1018,
the pancreas in patients with diabetes mellitus appears to be 1976.
about twice that in the general population.97'98 The increased 2
Katz, L. A., and Spiro, H. M.: Gastrointestinal manifestations
frequency appears to be less marked in men, although this of diabetes. N. Engl. J. Med. 275: 1350-64, 1966.
3
probably reflects earlier death of men from other causes. It is Goyal, R. K., and Spiro, H. M.: Gastrointestinal manifestations
unknown why there is an increased frequency of carcinoma of of diabetes mellitus. Med. Clin. N. Am. 55: 1031-44, 1971.
3a
the pancreas in diabetes. It has been suggested that there are Scarpello, J. H. B., and Sladen, G. E.: Progress report:
functional links between the endocrine and exocrine pancreas diabetes and the gut. Gut 19: 1153-62, 1978.
4
Whalen, G. E., Soergel, K. H., and Geenen, J. E.: Diabetic
and that these may explain the above association. An
diarrhea. A clinical and pathological study. Gastroenterology
alternative proposal is that animal insulin or an impurity 56: 1021-32, 1969.
thereof may be carcinogenic to the pancreas.99'100 5
Hensley, G. T., and Soergel, K. H.: Neuropathologic findings
Diabetes was present before the onset of symptoms of in diabetic diarrhea. Arch. Pathol. 85: 587-97, 1968.
6
pancreatic carcinoma in 4.3% of patients with pancreatic Bojsen-Muller, F., Gronback, P., and Rostgaard, J.: Light
cancer, and diabetes was detected after the onset of symptoms microscopic study of gastrointestinal and skin capillaries in diabetes
in a further 15.3%.101>102 Eighty percent of patients with mellitus. Diabetes 12: 429-32, 1963.
7
pancreatic cancer have their diabetes discovered within a year Sleisenger, M. H., and Fordtran, J. S.: Gastrointestinal
of the time that the cancer becomes manifest, suggesting Disease: Pathophysiology, Diagnosis, Management. Philadelphia,
that the presence of the malignancy causes the diabetes in W. B. Saunders, 1978.
8
Mandelstam, P., and Lieber, A.: Esophageal dysfunction in
many of these patients.102 Therefore, when diabetes develops
diabetic neuropathy-gastroenteropathy, clinical and roentgeno-
in a middle-aged patient without a family history of the logical manifestations. JAMA 201: 88-92, 1967.
disease and with unexplained systemic symptoms, pancreatic 9
Mandelstam, P., Siegel, C. I., Lieber, A., and Siegel, M.: The
carcinoma should be sought. swallowing disorder in patients with diabetic neuropathy-gastro-
Glucagonoma. The glucagonoma syndrome results from enteropathy. Gastroenterology 56: 1-12, 1969.
10
functioning A-cell tumors of the pancreas. Hypergluca- Vela, A. R., and Balart, L.: Esophageal motor manifestations
gonemia is associated with an insulin level that is normal or in diabetes mellitus. Am. J. Surg. 118: 21-29, 1970.
11
markedly reduced depending on how much of the pancreatic Stewart, I. M., Hoaking, D. J., Preston, B. J., and Atkinson,
tissue is replaced by tumor. Associated carbohydrate in- M.: Oesophageal motor changes in diabetes mellitus. Thorax 31:
tolerance may range from mild to severe. Relative insulin- 278-83, 1976.
12
Hollis, J. B., Braddon, R. L., and Castell, D. O.: Esophageal
resistant diabetes may be present.49 Half of the patients with motor function in diabetes mellitus and its relation to peripheral
these tumors have a skin disease referred to as erythema neuropathy. Gastroenterology 66: 713, 1974 (abstract).
necrolytica migrans. Oral crusting and painful glossitis may 13
Horgan, J. H., and Doyle, J. S.: A comparative study of
also be present. esophageal motility in diabetics with neuropathy. Chest 60:
Verner-Morrison syndrome.103 The clinical features of this 170-74, 1971.
14
syndrome include profuse choleralike diarrhea (usually tea- Hollis, J. B., Castell, D. O., and Braddon, R. L.: Esophageal
colored), hypokalemia, basal achlorhydria in more than 50% function in diabetes mellitus and its relation to peripheral
of patients, diabetes, hypercalcemia in 25%, and cutaneous neuropathy. Gastroenterology 73: 1098-1102, 1977.
14a
flushing episodes. These patients also have an increased Hoskin, D. J., Bennett, T., and Hampton, J. R., et al.:
incidence of gallstones and large atonic gallbladders. This Diabetic autonomic neuropathy. Diabetes 27: 1043-54, 1978.
15
Eras, P., Goldstein, M. J., and Sherlock, P.: Candida infection
syndrome is believed to result from the presence of a non-beta of the gastrointestinal tract. Medicine 51: 367-79, 1972.
islet cell tumor of the pancreas secreting vasoactive intestinal 16
Lewicki, A. M., and Moore, J. P.: Esophageal moniliasis.
peptide (VIP). Animal experiments have demonstrated that Am. J. Roentgenol. Radium Ther. Nucl. Med. 125: 218-25, 1975.
the biologic actions of VIP fit closely the described features of 17
Kodsi, B. E., Wickremesinghe, P. C., Kozinn, P. J., Iswara, K.,
these patients.104 The hyperglycemic state appears to result and Goldberg, P. K.: Candida esophagitis. Gastroenterology 71:
from an increased hepatic glucose output, which is due to a 715-19, 1976.
DIABETES CARE, VOL. 2 NO. 5, SEPTEMBER-OCTOBER 1979 445
GASTROINTESTINAL MANIFESTATIONS OF DIABETES MELLITUS/S. TAUB, A. MARIANI, AND J. S. BARKIN
18 39
Glouberman, S.: Diabetes mellitus a n d t h e gastrointestinal Marshak, R. H . , and Linder, A . E.: Radiology of t h e small
tract. Part 1. Esophagus and stomach. Ariz. Med. 34(2): 1 0 1 - 0 2 , intestine. Philadelphia, W . B. Saunders, 1970.
1977. 40
Mehrotra, M . P . , Patney, N . L , W a h a l , P. K., et al.: Small
19
Kassander, P . : Asymptomatic gastric retention in diabetes intestinal functions in diabetes mellitus. Indian J. M e d . Sci. 30:
(gastroparesis diabeticorum). A n n . Intern. Med. 48: 7 9 7 - 8 1 2 , 45-50, 1976.
1958. 41
Wruble, L. D., and Kaiser, M. H.: Diabetic steatorrhea: a
20
Liavag, I., and Tonjum, S.: Gastric retention in diabetes distinct clinical entity. Am. J. Med. 37: 118-29, 1964.
42
mellitus. Acta Chir. Scand. 137: 593-99, 1971. Blumenthal, H . T . , Probsein, J. G . , a n d Berns, A . W . : Inter-
21
Aylett, P.: Gastric emptying and change in the blood sugar relationships of diabetes mellitus a n d pancreatitis. A r c h . Surg.
level as affected by glucagon and insulin. Clin. Sci. 22: 171-78, 87: 844-50, 1963.
43
1962. Goldstein, F., Wins, C. W., and Kowlessar, O. D.: Diabetic
22
Brady, P. G., and Richardson, K.: Gastric bezoar formation diarrhea and steatorrhea, microbiologic and clinical observations.
secondary to gastroparesis diabeticorum. Arch. Intern. Med. 137: Ann. Intern. Med. 72: 215-18, 1970.
43a
1729, 1977. Scarpello, J. H. B., Hague, R. V., Cullen, D. R., andSladen,
23
Piken, E., Dwyer, R., and Zablen, M. A.: Gastric candidiasis. G. E.: The 14C-glycocholate test in diabetic diarrhoea. Br. Med. J.
JAMA 240: 2181-82, 1978. 2: 673-75, 1976.
24 44
Zitomer, B. R., G r a m m , H . F . , a n d Koxak, G . P . : Gastric Walsh, C. H., Cooper, B. T., Wright, A. D., et al.: Diabetes
neuropathy in diabetes mellitus, clinical a n d radiologic observa- mellitus and coeliac disease: a clinical study. Q. J. Med., New
tions. Metabolism J 7: 1 9 9 - 2 1 1 , 1968. Series XLVU: 89-100, 1978.
25 45
Gramm, H . P . , Reuter, K., and Castello, P . : T h e radiologic Falchuk, Z. M . , Bogentine, G . N . , a n d Strober, W . :
manifestations of diabetic gastric neuropathy a n d its differential Predominance of histocompatibility antigen H L - A 8 in patients with
diagnosis. Gastroenterol. Radiol. 3: 1 5 1 - 5 5 , 1978. gluten-sensitive enteropathy. J. Clin. Invest. 5 / : 1 6 0 2 - 0 5 , 1972.
26 46
Roon, A . J., and Mason, G. R.: Surgical management of Stokes, P. L , Asquith, P., Holmes, G. K. T . , et al.: Histo-
gastroparesis diabeticorum. Calif. Med. 116: 5 8 - 6 1 , 1972. compatibility antigens associated with adult celiac disease. Lancet
27
Campbell, I. W . , Heading, R. C , Tothill, P. et al.: Gastric 2: 162-64, 1972.
47
emptying in diabetic autonomic neuropathy. G u t 18: 4 6 2 - 6 7 , 1977. Cudworth, A . G . , and Woodrow, J. C . : H L - A system a n d
28
Longstreth, G . F., Malagelada, J. R., and Kelly, K. A . : diabetes mellitus. Diabetes 24: 2 4 5 - 3 4 9 , 1975.
48
Metoclopramide stimulation of gastric motility and emptying in P a l e y , R. G . , Mitchell, W . , a n d Watkinson, G . : Terminal
diabetic gastroparesis (letter). A n n . Intern. Med. 86(2): 1 9 5 - 9 6 , colonic dilatation following intractable diarrhea in diabetics.
1977. Gastroenterology 41: 4 0 1 - 0 7 , 1961.
29 49
Brownlee, M . , and Kroopf, S. S.: Metoclopramide for Creufzfeldt, W . , Frerichs, H . , and Sickinger, K.: Liver diseases
gastroparesis diabeticorum. N . Engl. J. Med. 291: 1 2 5 7 - 5 8 , 1974. and diabetes mellitus. In Progress a n d Liver Disease, Vol. 3 . Popper,
29a
Braterman, D . , and Bogoch, A . : Metoclopramide for H., and Schaffner, F., Eds. New York, Grune & Stratton, 1970,
gastroparesis diabeticorum. Diabetes Care 1: 3 5 6 - 5 9 , 1978. pp. 371-407.
30 50
Dotevall, G . : Incidence of peptic ulcer disease in diabetes Petersen, P.: Fatty liver in patients with moderate alcohol
mellitus. Acta Med. Scand. J64: 4 6 3 - 7 7 , 1959. consumption, diabetes mellitus and overweight. Scand. J.
31
Dotevall, G . : Gastric secretion of acid in diabetes mellitus Gastroenterol. 12: 781-84, 1977.
51
during basal conditions and after maximum histamine stimulation. Petersen, P.: Abnormal mitochondria in hepatocytes in human
Acta Med. Scand. 170: 59-69, 1961. fatty liver. Acta Pathol. Microbiol. Scand. Sect. A 85: 413-20,
3Ia
Feldman, M., Corbett, D. B., Ramsey, E. J., Walsh, J. H., 1977.
52
and Richardson, C. G.: Abnormal gastric function in longstanding, Glouberman, S.: Diabetes mellitus a n d t h e gastrointestinal
insulin-dependent diabetic patients. Gastroenterology 77: 12-17, tract. Part III. Pancreas and liver. Ariz. Med. 31 (4): 2 6 3 - 6 4 , 1977.
53
1979. Scheuer, P. J.: T h e liver in systemic disease, pregnancy, and
32
Irvine, W . J., Clarke, B. F . , Scarth, L , C u l l e n , D . R . , a n d organ transplantation. In Liver Biopsy Interpretation, Vol. 14.
Duncan, L. J. P.: Thyroid a n d gastric autoimmunity in patients Scheuer, P. J., Ed. Baltimore, Williams & Wilkins, 1973, p . 128.
54
with diabetes mellitus. Lancet 2: 1 6 3 - 6 8 , 1970. Leevy, C . M.: Fatty liver: A study of 270 patients with biopsy
33
Longstreth, G . F., and Newcomer, A . D . : Abdominal pain proven fatty liver and a review of the literature. Medicine 41: 249,
caused by diabetic radiculopathy. A n n . Intern. Med. 86: 1 6 6 - 6 8 , 1962.
1977. 55
Conn, H. O . , Shreiker, W., and Elkington, S. G.: Association
34
Watkins, T . S.: Facial sweating after food: a n e w sign of of impaired glucose tolerance with portal-systemic shunting in
diabetic neuropathy. Br. Med. J. I: 5 8 3 - 8 7 , 1973. Laennec's cirrhosis. Am. J. Dig. Dis. 16: 2 2 7 - 3 9 , 1971.
35 56
Glouberman, S.: Diabetes mellitus a n d t h e gastrointestinal Blei, A. T . , Atterburg, C. E., Ringler, D., and Conn, H. O.:
tract. Part II. Small and large intestine and gallbladder. Ariz. Med. Diabetes and portocaval anastomosis: a prospective, controlled
34(3): 1 7 4 - 7 5 , 1977. investigation. Gastroenterology 75(5): 956, 1978.
36 57
Forgacs, S., Halmos, T . , and Salamon, F.: Diabetic diarrhea. Marco, J., Diego, J., and Villanueva, M. L.: Elevated plasma
Isr. J. Med. Sci. 8: 7 8 4 - 8 6 , 1972. glucagon levels in cirrhosis of the liver. N . Engl. J. Med. 289:
37
McNally, E. F., Reinhard, A . E., and Schwartz, P. E.: Small 1107-11, 1973.
58
bowel motility in diabetics. A m . J. Dig. Dis. 14: 1 6 3 - 6 9 , 1969. Sherwin, R., Joshi, P., Hendler, R., Felig, P., and Conn,
38
Malins, S. M., and Mayne, N . : Diabetic diarrhea: a study of 13 H. O . : Hyperglucagonemia in Laennec's cirrhosis. The role of portal
patients with jejunal biopsy. Diabetes 18: 8 5 8 - 6 6 , 1969. systemic shunting. N . Engl. J. Med. 290: 2 3 9 - 4 2 , 1974.
446 DIABETES CARE, VOL. 2 NO. 5, SEPTEMBER-OCTOBER 1979
GASTROINTESTINAL MANIFESTATIONS OF DIABETES MELLITUS/S. TAUB, A. MAR1ANI, ANDJ. S. BARK1N
59 81
Felig, P., and Sherwin, R.: Carbohydrate homeostasis, liver Schein, C. J.: Acute cholecystitis in the diabetic. Am. J.
and diabetes. In Progress in Liver Disease, Vol. 5. Popper, H., and Gastroenterol. 51: 511, 1969.
82
Schaffner, F., Eds. Grune &. Stratton, 1976, pp. 149-71. W a y , L. W . , a n d Sleisenger, M . H . : A c u t e cholecystitis. In
00 G a s t r o i n t e s t i n a l Disease, Pathophysiology, Diagnosis, M a n a g e -
Podolsky, S., Zimmerman, H. J., Burrows, B. A., Cardavelli,
J. A., and Patlairna, C. G.: Potassium depletion in hepatic cirrhosis: m e n t . Sleisenger, M . H . , a n d Fordtran, J. S . , Eds. Philadelphia.
a reversible cause of impaired growth hormone and insulin W. B. Saunders, 1978, pp. 1302-13.
83
response to stimulation. N. Engl. J. Med. 288: 644-48, 1973. Bonnabeau, R. C , Tenekjian, V., and Djadalizadeh, M.:
61
Peters, N., Dick, A. P., Hales, C. N., Orrell, D. H., and Emphysematous cholecystitis: report of a case. Am. Surg. 42:
Sarner, M.: Exocrine and endocrine pancreatic function in 352-54, 1976.
84
diabetes mellitus and chronic pancreatitis. Gut 7: 277-81, 1966. Chey, W. Y., Shay, H., and Shuman, C. R.: External pancreatic
02
Conn, H. O.: Porta-caval anastomosis and hepatic hemosiderin secretion in diabetes mellitus. Ann. Intern. Med. 59: 812-21, 1963.
85
deposition: a prospective controlled investigation. Gastroenterology Goyal, R. K., and Spiro, H. M.: Gastrointestinal manifesta-
62: 61-72, 1972. tions of diabetes mellitus. Med. Clin. N. Am. 55: 1031-44, 1971.
6:1 86
Grace, N. D., and Powell, L. W.: Iron storage disorder of the Blumenthal, H. T., Probstein, J. G., and Berns, A. W.: Inter-
liver. Gastroenterology 64:1257-83, 1974. relationship of diabetes mellitus and pancreatitis. Arch. Surg. 87:
64
Stocks, A. E., and Powell, L. W.: Carbohydrate intolerance 844-50, 1963.
87
in idiopathic hemochromatosis and cirrhosis of the liver. Q. J. Med. Goodhead, B.: Vascular factors in the pathogenesis of acute
42: 733-49, 1973. hemorrhagic pancreatitis. Ann. R. Coll. Surg. (Eng.) 45: 80-97,
65
Walsh, C. H., Malins, J. M., and Bloom, S. R.: Diabetes 1969.
88
mellitus in idiopathic hemochromatosis. Br. Med. J. 2: 1267-68, Malone, J. I.: Juvenile diabetes and acute pancreatitis. J.
1978. Pediatr. 85: 825-27, 1974.
66 89
M a d a l i n s k i , K., Brzosko, W . J., Malczewski, B . , et al.: J o h a n s e n , K., a n d O r n s h o l t , J.: Frequency of diabetes after
Au/HAA in sera of diabetic patients. Lancet J: 701-02, 1971. acute pancreatitis. Metabolism 21 (4): 291-96, 1972.
67 90
C h u p i n , M . , Charbonnel, B . , Le Bodic, W . , et al.: Glucose Knight, M . : Glucagon in t h e pathogenesis of acute pancreatitis.
tolerance in viral hepatitis: a study of twenty patients during t h e Br. J. Surg. 59: 9 1 2 , 1972.
91
acute phase a n d after recovery. Diabetes 27(6): 6 6 1 - 6 9 , 1978. Berkowitz, D . , and Glassman, S.: T h e intravenous tolbutamide
68
Klatskin, G . : Toxic a n d drug-induced hepatitis, 4 t h ed. In test as a diagnostic aid in acute pancreatitis. A m . J. Med. Sci. 246:
Diseases of t h e Liver, Vol. 20. Schiff, L. J. B . , Ed. N e w York, 439-42, 1963.
92
Lippincott, 1975, pp. 671-73. Bank, S., Marks, I. N., and Vinik, A. I.: Clinical and
69
Foster, J. H . , D o n o h u e , T . A . , a n d B e r m a n , M . M . : Familial hormonal aspects of pancreatic diabetes. Am. J. Gastroenterol.
liver-cell a d e n o m a s a n d diabetes mellitus. N . Engl. J. M e d . 2 9 9 ( 5 ) : 64(1): 13-22, 1975.
93
239-41, 1978. Graebner, G. M., Marmor, B. M., Hendel, R. C , and Gregg,
70
N g , R . C . K . , S i g m u n d , C . J . , a n d Lagos, C . M . : H e p a t i c R. O.: Pancreatitis and severe metabolic abnormalities due to
infarction and diabetic ketoacidosis. Gastroenterology 73: 804-07, phenformin therapy. Arch. Surg. Ill: 1014-16, 1976.
94
1977. G a m b i l l , E. E.: Pancreatitis. S t . Louis, C . V . Mosby, 1 9 7 3 ,
71
Holt, J. M., and Spry, C. J. F.: Solitary pyogenic liver p. 126.
95
abscess in patients with diabetes mellitus. Lancet 2: 198-200, 1966. Geevarghese, P. J.: Pancreatic Diabetes. Bombay, India,
72
Wheeler, H. O.: Pathogenesis of gallstones. In Gastrointestinal Popular Prakashan, 1968.
96
Disease, Pathophysiology, Diagnosis, Management. Sleisenger, M c M i l l a n , D . E . , a n d G e e v a r g h e s e , P . J.: D i e t a r y c y a n i d e a n d
M. H., Ed. Philadelphia, W. B. Saunders, 1978, pp. 1284-93. tropical malnutrition diabetes. Diabetes Care 2: 202-08, 1979.
73 97
Lieber, M. M.: The incidence of gallstones and their correla- Linde, J., Nilsson, L. H . , a n d Berany, F. R.: Diabetes a n d
tions with other diseases. Ann. Surg. J35: 394-405, 1952. hypoglycemia in chronic pancreatitis. Scand. J. Gastroenterol.
74
Warren, S.: The pathology of diabetes mellitus, 2nd ed. Phila- 12(3): 369-73, 1977.
98
delphia, Lea & Febiger, 1938, p. 106. Bank, S . , Marks, I. N . , a n d Barbezat, G . O . : T r e a t m e n t of
75
Robertson, H. E.: The preponderance of gallstones in women. acute and chronic pancreatitis. Drugs 13: 373-81, 1977.
99
Int. Abstr. Surg. 80: 1-23, 1945. Morgan, R. C. H., and Wormsley, K. G.: Cancer of the
76
Feldman, M., and Feldman, M., Jr.: The incidence of pancreas. Gut 18: 580-96, 1977.
100
cholelithiasis, cholesterolosis, and liver disease in diabetes mellitus: Kessler, I. I.: C a n c e r mortality a m o n g diabetics. J. N a t l .
an autopsy study. Diabetes 3: 305-07, 1954. Cancer Inst. 44: 673-86, 1970.
77 101
Zahor, Z . , Sternby, N . H . , Kagon, A . , U e n u e r a , K., V a n a c e k , Green, R. C , Baggenstoss, A. H., and Sprague, R. G.:
R., a n d V i c h e r t , A . M . : Frequency of cholelithiasis in Prague a n d Diabetes mellitus in association with primary carcinoma of the
Malmoe: an autopsy study. Scand. J. Gastroenterol. 9: 3-7, 1974. pancreas. Diabetes 7: 308-11, 1958.
78 102
Heaton, K. W.: Clinic in gastroenterology, disease of the K a r m o d y , A . J., a n d Kyle, J.: T h e association b e t w e e n
biliary tract. Philadelphia, W. B. Saunders, 1972, p. 251. carcinoma of the pancreas and diabetes mellitus. Br. J. Surg. 56:
79
Ponz de L e o n , M . , Ferenderes, R . , a n d Carulli, N . : Bile lipid 362-64, 1969.
103
c o m p o s i t i o n a n d bile acid pool size in diabetes. A m . J. Dig. Dis. Verner, J. V., and Morrison, A. B.: Endocrine pancreatic
23(8): 710-16, 1978. islet disease with diarrhea. Arch. Intern. Med. 133: 492-500, 1974.
80 104
Gitelson, S., Oppenheim, D., and Schwartz, A.: Size of the M o d l i n , I. M . , Bloom, S. R . , a n d M i t c h e l l , S. J.: Experi-
gallbladder in patients with diabetes mellitus. Diabetes 18: 493-98, mental evidence for vasoactive intestinal peptide as the cause of the
1969. watery diarrhea syndrome. Gastroenterology 75 (6): 1051-54, 1978.
DIABETES CARE, VOL. 2 NO. 5, SEPTEMBER-OCTOBER 1979 447
Você também pode gostar
- Gastroparesis: Pathophysiology, Clinical Presentation, Diagnosis and TreatmentNo EverandGastroparesis: Pathophysiology, Clinical Presentation, Diagnosis and TreatmentRichard MccallumAinda não há avaliações
- Gastrointestinal Health: The Self-Help Nutritional Program That Can Change the Lives of 80 Million AmericansNo EverandGastrointestinal Health: The Self-Help Nutritional Program That Can Change the Lives of 80 Million AmericansAinda não há avaliações
- Gastropathy DMDocumento7 páginasGastropathy DMIlham Suryo Wibowo AntonoAinda não há avaliações
- Diabetic Gastropathy: BG, BK T, Uc KDocumento7 páginasDiabetic Gastropathy: BG, BK T, Uc KdenishAinda não há avaliações
- Small Intestinal Manifestations Diabetes Mellitus: ProfessorDocumento5 páginasSmall Intestinal Manifestations Diabetes Mellitus: ProfessorkgsamAinda não há avaliações
- Case ReportDocumento6 páginasCase ReportMae GandaAinda não há avaliações
- Gastrointestinal Fabry DiseaseDocumento14 páginasGastrointestinal Fabry DiseasedanradulescuAinda não há avaliações
- Gastrointestinal Disorders in DiabetesDocumento20 páginasGastrointestinal Disorders in Diabetesdoctor327810Ainda não há avaliações
- 7-Diabetes MelitusDocumento8 páginas7-Diabetes MelitusFatimatus Zahroh IchsanAinda não há avaliações
- CTC Gerd FinalDocumento36 páginasCTC Gerd FinaljaipreyraAinda não há avaliações
- A Practical Approach To Gastrointestinal Complications of DiabetesDocumento8 páginasA Practical Approach To Gastrointestinal Complications of DiabetesNovita ApramadhaAinda não há avaliações
- Vitamin B12 Malabsorption Syndrome Diarrhea Celiac Disease Iga Screening Test IgaDocumento18 páginasVitamin B12 Malabsorption Syndrome Diarrhea Celiac Disease Iga Screening Test IgaPerplexed CeleryAinda não há avaliações
- Gato Poul Ou 2012Documento7 páginasGato Poul Ou 2012Anonymous BPSZDTAinda não há avaliações
- 01Documento12 páginas01whackjack_696977Ainda não há avaliações
- Diabetes Mellitus and The StomachDocumento14 páginasDiabetes Mellitus and The StomachyuriAinda não há avaliações
- Chapter 13. Heartburn and DyspepsiaDocumento22 páginasChapter 13. Heartburn and DyspepsiaMonica CiorneiAinda não há avaliações
- 1 Problemas de Motilidad GIDocumento13 páginas1 Problemas de Motilidad GIFlor De LotoAinda não há avaliações
- Gastroparesis: Etiology, Clinical Manifestations, and DiagnosisDocumento30 páginasGastroparesis: Etiology, Clinical Manifestations, and DiagnosissebastianAinda não há avaliações
- Nejm 2015 Functional DyspepsiaDocumento12 páginasNejm 2015 Functional DyspepsiarisewfAinda não há avaliações
- Gastroesophageal Reflux Disease PharmacotherapyDocumento22 páginasGastroesophageal Reflux Disease PharmacotherapyrinirhynAinda não há avaliações
- NAFLD HarrisonDocumento2 páginasNAFLD HarrisonJesly CharliesAinda não há avaliações
- Background: Afferent Loop Syndrome AnemiaDocumento7 páginasBackground: Afferent Loop Syndrome AnemiaClara PangaribuanAinda não há avaliações
- DispepsiaDocumento11 páginasDispepsiaPendikAinda não há avaliações
- Therapy Insight: Gastrointestinal Complications of Diabetes-Pathophysiology and ManagementDocumento10 páginasTherapy Insight: Gastrointestinal Complications of Diabetes-Pathophysiology and ManagementNovita ApramadhaAinda não há avaliações
- Dumping Syndrome: Nutrition Issues in Gastroenterology, Series #35Documento8 páginasDumping Syndrome: Nutrition Issues in Gastroenterology, Series #35ismuAinda não há avaliações
- Pancreatitis: Pancreatitis Is Inflammation of The Pancreas. It Occurs When Pancreatic Enzymes (Especially Trypsin)Documento6 páginasPancreatitis: Pancreatitis Is Inflammation of The Pancreas. It Occurs When Pancreatic Enzymes (Especially Trypsin)tianallyAinda não há avaliações
- DyspepDocumento2 páginasDyspepGeorge WinchesterAinda não há avaliações
- Dumping Syndrome - Background, Pathophysiology, EtiologyDocumento6 páginasDumping Syndrome - Background, Pathophysiology, EtiologyW CnAinda não há avaliações
- GerdDocumento10 páginasGerdMulia NtiAinda não há avaliações
- Pancreatic Diabetes Mellitus: Frequency and DiagnosisDocumento10 páginasPancreatic Diabetes Mellitus: Frequency and DiagnosisRutDanAinda não há avaliações
- Functional Dyspepsia: Review ArticleDocumento11 páginasFunctional Dyspepsia: Review Articlejenny puentesAinda não há avaliações
- Gastroparesia PDFDocumento19 páginasGastroparesia PDFCristian SanchezAinda não há avaliações
- Motility Disorders of The GITDocumento57 páginasMotility Disorders of The GITMahmoud AjinehAinda não há avaliações
- Functional Dyspepsia: Recent Advances in Pathophysiology: Update ArticleDocumento5 páginasFunctional Dyspepsia: Recent Advances in Pathophysiology: Update ArticleDen BollongAinda não há avaliações
- GI - Pathophysiology, Evaluation, and Treatment of BloatingDocumento11 páginasGI - Pathophysiology, Evaluation, and Treatment of BloatingTriLightAinda não há avaliações
- Pseudo ObstructionDocumento13 páginasPseudo ObstructionAnonymous ZUaUz1wwAinda não há avaliações
- Gastroesophageal Reflux Disease Control of Symptoms, Prevention of ComplicationsDocumento9 páginasGastroesophageal Reflux Disease Control of Symptoms, Prevention of ComplicationsTiurma SibaraniAinda não há avaliações
- Protein Losing EnteropathyDocumento9 páginasProtein Losing EnteropathyrajibAinda não há avaliações
- Ulkus PeptikDocumento26 páginasUlkus PeptikKang MunirAinda não há avaliações
- Acalasia PDFDocumento19 páginasAcalasia PDFsolidchessAinda não há avaliações
- Assessment of Metabolic and GastrointestinalDocumento3 páginasAssessment of Metabolic and GastrointestinalClark SavageAinda não há avaliações
- Constipation in The Elderly - American Family PhysicianDocumento9 páginasConstipation in The Elderly - American Family PhysicianMudassar SattarAinda não há avaliações
- 4 U1.0 B978 1 4160 6189 2..00012 3..DOCPDFDocumento9 páginas4 U1.0 B978 1 4160 6189 2..00012 3..DOCPDFJuan HernandezAinda não há avaliações
- Urinary TractDocumento3 páginasUrinary Tractsurfer_Ainda não há avaliações
- Pancreatic Diabetes Mellitus: Frequency and DiagnosisDocumento10 páginasPancreatic Diabetes Mellitus: Frequency and Diagnosisbalthier37Ainda não há avaliações
- Sindrome de DumpingDocumento8 páginasSindrome de DumpingJose Lopez FuentesAinda não há avaliações
- Irritable Bowel Syndrome and Other Functional Gastrointestinal DisordersDocumento3 páginasIrritable Bowel Syndrome and Other Functional Gastrointestinal DisordersdavpierAinda não há avaliações
- Review: Epidemiology, Mechanisms, and Management of Diabetic GastroparesisDocumento8 páginasReview: Epidemiology, Mechanisms, and Management of Diabetic GastroparesisIrma KurniawatiAinda não há avaliações
- Comprehensive Resume On Hepatitis ADocumento9 páginasComprehensive Resume On Hepatitis AGeoffrey MasyhurAinda não há avaliações
- Gastric Outlet Obstruction Due To Peptic Ulcer DiseaseDocumento4 páginasGastric Outlet Obstruction Due To Peptic Ulcer DiseaseTeguh RahAinda não há avaliações
- Abdominal Bloating PDFDocumento4 páginasAbdominal Bloating PDFYusuf Budi HermawanAinda não há avaliações
- Laporan Jaga DispepsiaDocumento4 páginasLaporan Jaga DispepsiaTeguh YukiAinda não há avaliações
- OesophagusDocumento15 páginasOesophagusIBRAHEM JUMAHAinda não há avaliações
- Achalasia CardiaDocumento3 páginasAchalasia CardiaAnonymous KaJImISFAinda não há avaliações
- Case 5 - Pancreatitis-1Documento4 páginasCase 5 - Pancreatitis-1ngAinda não há avaliações
- Small Bowel Obstruction - Clinical Diagnosis and TreatmentDocumento11 páginasSmall Bowel Obstruction - Clinical Diagnosis and TreatmentVigariooAinda não há avaliações
- GerdDocumento8 páginasGerdLoms KotopAinda não há avaliações
- Peptic Ulcer DiseaseDocumento3 páginasPeptic Ulcer DiseaseABEL KETEMAAinda não há avaliações
- Achalasia: Common Features of An Uncommon Disease: Gerd in The ST Century, Series #Documento7 páginasAchalasia: Common Features of An Uncommon Disease: Gerd in The ST Century, Series #Andreea ElenaAinda não há avaliações
- Esophageal Disease in Scleroderma: A T: C RDocumento7 páginasEsophageal Disease in Scleroderma: A T: C REva OretlaAinda não há avaliações
- Normal GFR in ChildDocumento8 páginasNormal GFR in ChildbobbypambudimdAinda não há avaliações
- Phil Airlines v. NLRCDocumento1 páginaPhil Airlines v. NLRCAlec VenturaAinda não há avaliações
- Health EducationDocumento8 páginasHealth EducationJamie Rose FontanillaAinda não há avaliações
- CCL-81 Product Sheet - VeroDocumento5 páginasCCL-81 Product Sheet - VeroKrishnan KrishnanAinda não há avaliações
- Effectiveness of Maitland vs. Mulligan Mobilization Techniques in (Ingles)Documento4 páginasEffectiveness of Maitland vs. Mulligan Mobilization Techniques in (Ingles)mauricio castroAinda não há avaliações
- DSM 5Documento33 páginasDSM 5Grace Marie100% (2)
- Eular References For Website 2015Documento240 páginasEular References For Website 2015Antonio BernalAinda não há avaliações
- Trasturel 440mg Anti CancerDocumento3 páginasTrasturel 440mg Anti CancerRadhika ChandranAinda não há avaliações
- Coca Cola Fairlife CaseDocumento4 páginasCoca Cola Fairlife Caseapi-315994561Ainda não há avaliações
- Relationship Between Interpupilary Distance and Vertical Dimensions of OcclusionDocumento6 páginasRelationship Between Interpupilary Distance and Vertical Dimensions of OcclusionIJAR JOURNALAinda não há avaliações
- Periodontal AbscessDocumento27 páginasPeriodontal AbscessAhmed Tawfig GamalAinda não há avaliações
- Investigatory Project On Malaria: Name: M.Bhavya Class: XI C' Year: 2018 - 2019Documento18 páginasInvestigatory Project On Malaria: Name: M.Bhavya Class: XI C' Year: 2018 - 2019Muramsetty Bhavya0% (1)
- Starlight ProgramsDocumento12 páginasStarlight Programsllacara@nncogannett.comAinda não há avaliações
- NPD Phase 1Documento2 páginasNPD Phase 1Abdullah ZahidAinda não há avaliações
- Wiley Veterinary GynaecologyDocumento2 páginasWiley Veterinary Gynaecologysanath0% (1)
- 2022 India Accessibility Standards and Guidelines For Civil Aviation - EnglishDocumento110 páginas2022 India Accessibility Standards and Guidelines For Civil Aviation - EnglishDisability Rights AllianceAinda não há avaliações
- Disaster Readiness Risk Reduction: Quarter 2-Module 13: DRR-related Laws and PoliciesDocumento16 páginasDisaster Readiness Risk Reduction: Quarter 2-Module 13: DRR-related Laws and PoliciesUel Cabz LaquihonAinda não há avaliações
- 23 Medicinal Plants The Native Americans Used On A Daily Basis - MSYCDocumento15 páginas23 Medicinal Plants The Native Americans Used On A Daily Basis - MSYCLeandro RodriguesAinda não há avaliações
- Dapus FixDocumento2 páginasDapus FixIkrima MuhdarmuhallyAinda não há avaliações
- Stacey Waters CVDocumento5 páginasStacey Waters CVapi-479651018Ainda não há avaliações
- 2014-1 Mag LoresDocumento52 páginas2014-1 Mag LoresLi SacAinda não há avaliações
- MSDS 15w-40Documento7 páginasMSDS 15w-40Farhat AzharAinda não há avaliações
- KLP 5 - Nutrition Intake and MedicationDocumento4 páginasKLP 5 - Nutrition Intake and MedicationdzakyAinda não há avaliações
- Nursing Care Plan: Phinma University of PangasinanDocumento1 páginaNursing Care Plan: Phinma University of PangasinanShaira De La CruzAinda não há avaliações
- Public Opinion On Idea of Digitalising Rural CommunityDocumento11 páginasPublic Opinion On Idea of Digitalising Rural CommunityINSTITUTE OF LEGAL EDUCATIONAinda não há avaliações
- Case Group 1Documento10 páginasCase Group 1JASMEEN RAVALAinda não há avaliações
- CLRDP 2017-2022 Midterm Update - FInalDraftDocumento328 páginasCLRDP 2017-2022 Midterm Update - FInalDraftemeyarayieAinda não há avaliações
- A Smart Gym Framework: Theoretical ApproachDocumento6 páginasA Smart Gym Framework: Theoretical ApproachciccioAinda não há avaliações
- Be Yourself: Questions & Answers For Gay, Lesbian, Bisexual & Transgender YouthDocumento15 páginasBe Yourself: Questions & Answers For Gay, Lesbian, Bisexual & Transgender YouthJosé Leote PaixãoAinda não há avaliações
- Epiglottitis: Assessment Nursing Diagnosis Scientific Explanatio N Planning Nursing Intervention Rationale EvaluationDocumento3 páginasEpiglottitis: Assessment Nursing Diagnosis Scientific Explanatio N Planning Nursing Intervention Rationale EvaluationfifiAinda não há avaliações