Escolar Documentos
Profissional Documentos
Cultura Documentos
First Law tutorial problems
Enviado por
MiaHusnaTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
First Law tutorial problems
Enviado por
MiaHusnaDireitos autorais:
Formatos disponíveis
TUTORIAL 4
THE FIRST LAW
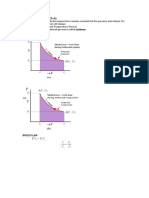
1. A sample of 4.50 g methane occupies 12.7 dm 3 at 310 K. (a) Calculate the work done when the gas
expands isothermally against a constant external pressure of 200 Torr until its volume has increased
by 3.3 dm 3. (b) Calculate the work that would be done if the same expansion occurred reversibly.
((a) w = – 87.99 J, (b) w = – 167.44 J)
2. A sample of argon of mass 6.56 g occupies 18.5 dm 3 at 305 K. (a) Calculate the work done when the
gas expands isothermally against a constant external pressure of 7.7 kPa until its volume has
increased by 2.5 dm 3. (b) Calculate the work that would be done if the same expansion occurred
reversibly. ((a) w = – 19.25 J, (b) w = – 52.71 J)
3. A sample of 1.00 mol H2O(g) is condensed isothermally and reversibly to liquid water at 100oC. The
standard enthalpy of vaporization of water at 100oC is 40.656 kJ mol–1. Find w, q, and for this
process. (w = 3.101 kJ, q = – 40.656 kJ, U = – 37.55 kJ, H = – 40.656 kJ)
4. A sample of 2.00 mol CH 3OH(g) is condensed isothermally and reversibly to liquid at 64oC. The
standard enthalpy of vaporization of methanol at 64oC is 35.3 kJ mol–1. Find w, q, and for this
process. (w = 5.603 kJ, q = – 70.6 kJ, U = – 64.996 kJ, H = – 70.6 kJ)
5. A strip of magnesium of mass 15.0 g is dropped into a beaker of dilute hydrochloric acid. Calculate
the work done by the system as a result of the reaction. The atmospheric pressure is 1.0 atm and the
temperature 25 C. (w = – 1548.48 J)
6. A piece of zinc of mass 5.0 g is dropped into a beaker of dilute hydrochloric acid. Calculate the work
done by the system as a result of the reaction. The atmospheric pressure is 1.1 atm and the
temperature 23 C. (w = – 188.26 J)
7. The constant-pressure heat capacity of a sample of a perfect gas was found to vary with temperature
according to the expression Cp/(J K–1) = 20.17 + 0.3665(T/K). Calculate q, w U H when the
temperature is raised from 25 C to 200 C (a) at constant pressure, (b) at constant volume
((a) q = 28254.76 J, w = – 1454.95 J, U = 26799.81 J, H = 28254.76 J
q = 26799.81 J, w = 0 J, U = 26799.81 J, H = 28254.76 J)
8. The constant-pressure heat capacity of a sample of a perfect gas was found to vary with temperature
according to the expression Cp/(J K–1) = 20.17 + 0.4001(T/K). Calculate q, w U H when the
temperature is raised from 0 C to 100 C (a) at constant pressure, (b) at constant volume.
((a) q = 14940 J, w = – 831.4 J, U = 14109 J, H = 14940 J
q = 14109 J, w = 0 J, U = 14109 J, H = 14940 J)
Você também pode gostar
- First Law tutorial problemsDocumento2 páginasFirst Law tutorial problemsFatin NurliyanaAinda não há avaliações
- Physical Chemistry II Thermodynamics Review ProblemsDocumento4 páginasPhysical Chemistry II Thermodynamics Review ProblemsMousa Floobert موسىAinda não há avaliações
- Taller Fisicoquimica TermoDocumento6 páginasTaller Fisicoquimica TermoWilo JaraAinda não há avaliações
- Thermodynamics Exercises: o A. e emDocumento2 páginasThermodynamics Exercises: o A. e emAnnisa Sylvia SimbolonAinda não há avaliações
- Thermodynam TasksDocumento1 páginaThermodynam TasksEhtıram SeyıdovAinda não há avaliações
- Asignment - Chapter 2Documento9 páginasAsignment - Chapter 2Nguyễn Đạt100% (1)
- 1999 HW 3 Key3 Physical ChemistryDocumento3 páginas1999 HW 3 Key3 Physical ChemistryJackHammerthornAinda não há avaliações
- THERMO AssignmentDocumento2 páginasTHERMO Assignmentaleena'Ainda não há avaliações
- Thermochemistry ProblemsDocumento2 páginasThermochemistry ProblemsAyush Chouhan100% (1)
- Tutorial 1 - Thermodynamics (2024)Documento4 páginasTutorial 1 - Thermodynamics (2024)kkhimatiAinda não há avaliações
- Tutorial 3 - First Law ProblemsDocumento1 páginaTutorial 3 - First Law ProblemsFatin NurliyanaAinda não há avaliações
- Tutorial Chapter 2Documento2 páginasTutorial Chapter 2Nur KamiliaAinda não há avaliações
- Tutorial 2 Spring 2018-19Documento3 páginasTutorial 2 Spring 2018-19ANMOLAinda não há avaliações
- 1Documento2 páginas1Bình TĩnhAinda não há avaliações
- BT Hoa Ly 1Documento22 páginasBT Hoa Ly 1Minh ThưAinda não há avaliações
- Chapter 03 The Second Law - HomeworkDocumento2 páginasChapter 03 The Second Law - HomeworkrottymarsellaAinda não há avaliações
- Homework 1Documento4 páginasHomework 1Itzel A. GuerreroAinda não há avaliações
- Problem set 1 covers energy, heat, gases, and thermodynamicsDocumento15 páginasProblem set 1 covers energy, heat, gases, and thermodynamicsFikret BazAinda não há avaliações
- PHYSICAL CHEMISTRY 1 Problem Set 2Documento1 páginaPHYSICAL CHEMISTRY 1 Problem Set 2Bryan bayAinda não há avaliações
- Chapter 5 - ThermochemistryDocumento54 páginasChapter 5 - ThermochemistryVarunesh MauthialaganAinda não há avaliações
- Pertemuan 7 ReviewDocumento45 páginasPertemuan 7 ReviewAna Sholikhatus Sa'diyah100% (1)
- Instructions: 1. Use Excel 2. Express Your Final Answer in 2 Decimal PlacesDocumento6 páginasInstructions: 1. Use Excel 2. Express Your Final Answer in 2 Decimal PlaceslukeAinda não há avaliações
- Tutorial 2-With AnswersDocumento11 páginasTutorial 2-With AnswersHayicAinda não há avaliações
- Tutorial Sheet On Thermodynamics 1Documento2 páginasTutorial Sheet On Thermodynamics 1Michelle MinduvalAinda não há avaliações
- Ideal Gas Equation and EntropyDocumento27 páginasIdeal Gas Equation and EntropyJude Roswel GenerilloAinda não há avaliações
- Fisicoquímica Problemario 1/3Documento1 páginaFisicoquímica Problemario 1/3Kari BustamanteAinda não há avaliações
- Homework 1 - Chem 113ADocumento1 páginaHomework 1 - Chem 113Azey2005Ainda não há avaliações
- CHEM 2820 Problem Set 1Documento3 páginasCHEM 2820 Problem Set 1Vicente JonathanAinda não há avaliações
- Tuttherm 2Documento6 páginasTuttherm 2Lin Xian XingAinda não há avaliações
- Soal Tugas Hukum I TermodinamikaDocumento2 páginasSoal Tugas Hukum I TermodinamikaRizqi SalsabilaAinda não há avaliações
- Hướng Dẫn Bài Tập Hoá Đại Cương 2Documento56 páginasHướng Dẫn Bài Tập Hoá Đại Cương 2Thái BảoAinda não há avaliações
- Processes of Fluids: Theories Values & DisciplineDocumento1 páginaProcesses of Fluids: Theories Values & DisciplineLester SamsonAinda não há avaliações
- Tuttherm2 PDFDocumento6 páginasTuttherm2 PDFPrabir BanerjeeAinda não há avaliações
- Second Law Thermodynamics Problems SolvedDocumento2 páginasSecond Law Thermodynamics Problems SolvedNikki ByrneAinda não há avaliações
- Day 1 CalculationsDocumento10 páginasDay 1 CalculationsAnonymous f4e1pzrwAinda não há avaliações
- Chem 73 PS2 2017 PDFDocumento2 páginasChem 73 PS2 2017 PDFImee Kassandra Estomo CachoAinda não há avaliações
- MIT ThermoDocumento6 páginasMIT ThermoTinray ReyesAinda não há avaliações
- B18pa1 NHN 08 PDFDocumento4 páginasB18pa1 NHN 08 PDFMohamed AbdullaAinda não há avaliações
- Thermodynamic 1Documento312 páginasThermodynamic 1Daniel Wang100% (5)
- Heat Capacity and Specific Heat Capacity ExplainedDocumento25 páginasHeat Capacity and Specific Heat Capacity ExplainedShiu Ping Wong100% (1)
- Thermochemistry (Solutions)Documento16 páginasThermochemistry (Solutions)MarikAinda não há avaliações
- Thermo 1 Isothermal ProcessDocumento3 páginasThermo 1 Isothermal ProcessMARIA RHODORA BITBITAinda não há avaliações
- Ass 6Documento2 páginasAss 6MayankAinda não há avaliações
- Chapter 1 and 2 AssignmentsDocumento24 páginasChapter 1 and 2 AssignmentsHà Thế VinhAinda não há avaliações
- Lista 1Documento2 páginasLista 1Daniel AlvesAinda não há avaliações
- Thermochemistry SolutionsDocumento8 páginasThermochemistry Solutionsnagendra_rdAinda não há avaliações
- Thermodynamics IntroductionDocumento13 páginasThermodynamics IntroductionGissela BTAinda não há avaliações
- HDC2 SolutionDocumento56 páginasHDC2 Solutionmanhhungntb1212Ainda não há avaliações
- Chapter 1-3Documento22 páginasChapter 1-3Aiman LatifAinda não há avaliações
- Thermodynamics Questions and AnswersDocumento5 páginasThermodynamics Questions and AnswersMD SHOEBUDDIN0% (1)
- Pchem ExamDocumento8 páginasPchem ExamDanielson CulanibanAinda não há avaliações
- Thermodynamics AssignmentDocumento1 páginaThermodynamics AssignmentSaransh KumarAinda não há avaliações
- UPCS Physics Problem SetDocumento13 páginasUPCS Physics Problem SetRicardo Jose BracamonteAinda não há avaliações
- Solutions Set 3 AtkinsDocumento16 páginasSolutions Set 3 AtkinsSakinah Himav RezeikaAinda não há avaliações
- Chem Principles 7e ISM Focus 04 Even FINALDocumento62 páginasChem Principles 7e ISM Focus 04 Even FINALSelma MeloAinda não há avaliações
- CalorimetryDocumento5 páginasCalorimetryJerich Ivan PaalisboAinda não há avaliações
- Problem Set 2Documento5 páginasProblem Set 2UnitedNationsAveAinda não há avaliações
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterNo EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterNota: 5 de 5 estrelas5/5 (1)
- Sharifah Athirah Sayed Alwi (S50691 - 5) 196Documento1 páginaSharifah Athirah Sayed Alwi (S50691 - 5) 196MiaHusnaAinda não há avaliações
- Pegukuhan - Introduction To Phase Diagram (S50362)Documento2 páginasPegukuhan - Introduction To Phase Diagram (S50362)MiaHusnaAinda não há avaliações
- Ways To Promote The Commercialization of The ProductDocumento2 páginasWays To Promote The Commercialization of The ProductMiaHusnaAinda não há avaliações
- Journal of Molecular StructureDocumento7 páginasJournal of Molecular StructureMiaHusnaAinda não há avaliações
- Tugasan 1Documento1 páginaTugasan 1MiaHusnaAinda não há avaliações
- Answer Test 2 KoloidDocumento3 páginasAnswer Test 2 KoloidMiaHusnaAinda não há avaliações
- Coronavirus ManusiaDocumento2 páginasCoronavirus ManusiaMiaHusnaAinda não há avaliações
- KIM3104Documento9 páginasKIM3104MiaHusnaAinda não há avaliações
- KIM3104Documento7 páginasKIM3104MiaHusnaAinda não há avaliações
- Example Kimia Fizik PDFDocumento32 páginasExample Kimia Fizik PDFMiaHusnaAinda não há avaliações
- Example Kimia Fizik PDFDocumento32 páginasExample Kimia Fizik PDFMiaHusnaAinda não há avaliações
- Laporan Amali 5 TranslatedDocumento10 páginasLaporan Amali 5 TranslatedMiaHusnaAinda não há avaliações
- Physic For Life Sciences - RPP DAU 34203Documento6 páginasPhysic For Life Sciences - RPP DAU 34203MiaHusnaAinda não há avaliações
- Enthalpy PDFDocumento119 páginasEnthalpy PDFEstuardo Javier Gan RodríguezAinda não há avaliações
- Thermostat: Data SheetDocumento10 páginasThermostat: Data SheetmabniAinda não há avaliações
- CBSE 8 Science CBSE - Combustion and Flame, Free Test Papers, Sample Questions, HOTS Questions and Notes, CBSE - Combustion and FlameDocumento9 páginasCBSE 8 Science CBSE - Combustion and Flame, Free Test Papers, Sample Questions, HOTS Questions and Notes, CBSE - Combustion and FlameR.Shruti 1040-12Ainda não há avaliações
- Abrasive Jet MachiningDocumento8 páginasAbrasive Jet MachiningRanjith KaruturiAinda não há avaliações
- Claudius Peters Pneumatic Conveying Brochure enDocumento16 páginasClaudius Peters Pneumatic Conveying Brochure enSen VanAinda não há avaliações
- Ifferent: How Things Are DifferentDocumento9 páginasIfferent: How Things Are DifferentnidyaAinda não há avaliações
- Compact pressure switches for gas and air monitoringDocumento6 páginasCompact pressure switches for gas and air monitoringCarlos BaezaAinda não há avaliações
- FSN Soot PDFDocumento17 páginasFSN Soot PDFShi LiangAinda não há avaliações
- Rolling Defects and Causes in Metal WorkingDocumento10 páginasRolling Defects and Causes in Metal WorkingHarish HAinda não há avaliações
- Chapter1 PDFDocumento64 páginasChapter1 PDFsgw67Ainda não há avaliações
- Amagat's Law: Ideal Gas MixtureDocumento2 páginasAmagat's Law: Ideal Gas MixtureAar AeyAinda não há avaliações
- Chemical World Lesson PlanDocumento9 páginasChemical World Lesson PlanellencolussoAinda não há avaliações
- Combined Gas Law Performance TaskDocumento10 páginasCombined Gas Law Performance TaskJoseph AlegadoAinda não há avaliações
- ThesisDocumento119 páginasThesisJoséAinda não há avaliações
- Model VOC Management Plan GuidanceDocumento31 páginasModel VOC Management Plan GuidanceCari RiveraAinda não há avaliações
- RK60000 Instruction ManualDocumento36 páginasRK60000 Instruction ManualsasahitoAinda não há avaliações
- DPP-2 - Is Matter Around Us Pure - 03-08-2021Documento1 páginaDPP-2 - Is Matter Around Us Pure - 03-08-2021Dheeraj SharmaAinda não há avaliações
- 10 Most Important Derivations With SolutionsDocumento18 páginas10 Most Important Derivations With SolutionsManish PattajoshiAinda não há avaliações
- Product Guide 2021 EN LRDocumento30 páginasProduct Guide 2021 EN LRCARLOS CHAVEZAinda não há avaliações
- Chemical Kinetics Rate Laws and Reaction MechanismsDocumento19 páginasChemical Kinetics Rate Laws and Reaction MechanismsJillian PeteAinda não há avaliações
- CO2 CorrosionDocumento18 páginasCO2 CorrosionShie OthongAinda não há avaliações
- EA Plasma, Laser, OxyfuelDocumento5 páginasEA Plasma, Laser, OxyfuelSaravanan VelayuthamAinda não há avaliações
- NCERTXtract ObjectiveChemistryforNEET PDFDocumento712 páginasNCERTXtract ObjectiveChemistryforNEET PDFMAPA art and crafts67% (3)
- CHAPTER 1: INTRODUCTION TO CHEMISTRY AND MEASUREMENTDocumento62 páginasCHAPTER 1: INTRODUCTION TO CHEMISTRY AND MEASUREMENTBobAinda não há avaliações
- ChemistryDocumento44 páginasChemistryRUPAMAinda não há avaliações
- Lesson 5.3 Ideal Gas LawDocumento6 páginasLesson 5.3 Ideal Gas LawAqshin BabaevAinda não há avaliações
- Best Practice Guide Impulse Lines For Differential-Pressure FlowmetersDocumento22 páginasBest Practice Guide Impulse Lines For Differential-Pressure FlowmetersamietkolteAinda não há avaliações
- User Guide BranzfireDocumento44 páginasUser Guide BranzfirePatricio Vladimir Valdes GacituaAinda não há avaliações
- 1Documento16 páginas1Neill TeodoroAinda não há avaliações
- Corrosion of Carbon Steels in Monoethylene Glycol (51300-00498-Sg)Documento13 páginasCorrosion of Carbon Steels in Monoethylene Glycol (51300-00498-Sg)Henny CasanovaAinda não há avaliações