Escolar Documentos
Profissional Documentos
Cultura Documentos
Distillation Report
Enviado por
weidatanDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Distillation Report
Enviado por
weidatanDireitos autorais:
Formatos disponíveis
School of Life Sciences & Chemical Technology
Diploma in Chemical & Biomolecular Engineering
Distillation Column
CBE Laboratory Module 3.1
Group D Tan Weili Tan Wen Guang Benjamin Tia Esmeralda Daniel Yu Shenghe Joshua Zulkifli Ali Amdan 03/05/11
S10075769H S10076805D S10077174H S10079152B S10076705C
Summary
The aim of this experiment is to determine the pressure drop, the boil-up rate, the purity and to observe the different degrees of foaming at different three power levels; 0.4kW, 0.6kW, 0.8kW in a distillation column. Temperatures at T2, T4, T6, and T8 are also taken to show the temperature difference throughout the column for a mixture of 20 vol% pure methanol and 80 vol% water. Four relationships are also observed and explained. Generally, as the pressure drop gets bigger, the boil-up rate increases. The purity remained the same for the first two boilup rates, at 95%, but changed at the third boil-up rate, at 89%. At the first boil-up rate when pressure was 29cmH2O, the degree of forming was gentle and localised to one stage of the column. At the second boil-up rate when pressure drop was 40cmH2O, the degree of foaming was still gentle but was spread out throughout the column. At the final boil-up rate when pressure drop was 76cmH2O, the degree of foaming had changed to being violent, but back to localise to only a single stage in the column. One glaring error in this experiment is the first boilup rate reading. A reason for this error is that small amounts of liquid built-up were taken into account, resulting into a wrong reading. This error was rectified in subsequent readings.
Introduction
Distillation is defined as a process where a liquid or vapour mixture is separated into its components through the adding and removing of heat to achieve a desired purity. It is based on the differences in boiling points between the since the more volatile liquid will be boiled off first, then condensed to achieve a higher purity. Distillation columns are designed to ensure that the separation is done efficiently. However, this process requires tremendous amounts of energy on both heating and cooling, and can contribute more than 50% of the operating costs in a plant. In the chemical industry, distillation is a very common method of separation since it can separate large quantities of mixtures. There two types of distillation the industry uses; continuous distillation and batch distillation. Batch distillation is more commonly used when small quantities of liquids need to be separated, like in the experiment.
Approach
10 litres of a mixture of 20 vol% pure methanol and 80 vol% water was prepared. This is transferred into the reboiler. There is a heating element in the reboiler that was used to heat up the mixture at three different powers; 0.4kW, 0.6kW and 0.8kW. The reboiler was set to the highest power setting first to allow the system to get heated up quickly, and then reduced to 0.4kW. The system is then allowed to achieve steady-state before readings are taken. For each power setting, four sets of temperature readings, the degree of foaming, pressure drop, boil-up rate and purity was taken.
Results and Analysis
Power(kW)
Temperature (oC) T2 T4 T6 T8
0.4 0.6 0.8
66 72 79
68 75 82
73 81 86
79 84 87
Degree of Foaming (Gentle/Violent; Spread out/ Localised) Gentle; Localised Gentle; Spread out Violent; Localised
Pressure Drop (cmH2O) 29 40 76
Boil-Up Rate (ml/s) 0.627 0.138 0.225
Purity (%) 95 95 89
From the results obtained, it is clear that the power supplied has affected, directly or indirectly, every result. As power increases, the temperature readings throughout the column get higher since more hot vapour rise through the column. Power also has a direct effect on boil-up rate since the more power supplied, the faster the liquid will reach boiling temperature for the methanol and the faster methanol and water will be boiled off. There is a slight error in the first boil-up rate. 5.50ml of liquid is collected in 8.77s when power was at 0.4kW, in contrast with the other readings when the powers of 0.6kW and 0.8kW collected 2.75ml and 4.50ml of liquid in 20.0s respectively. A possible reason for this error is that a small amount of liquid built-up was collected as well as the sample, leading to the inaccuracy. Theoretically, the boil-up rate is proportional to the pressure drop. When the boil-up rate is low, the pressure drop is small and the same is applied when both parameters are big. This is because as more liquid is boiled off, more vapour is formed and travels up the column. However, due to trays preventing the vapour from moving up except via small holes in the trays, the pressure below increases greatly while the pressure in the upper part of the column stays the same or increases slightly. Foaming refers to when the liquid, usually reflux liquid, expands due to vapour or gas passing through it. It occurs when agitation is caused by the pressure difference in the column as fast, pressurised vapour is trying to get through the orifices. The bigger the pressure drop, the more violent the foaming. Also, whether foaming is localised or spread out depends on the density of the vapour.
Discussion
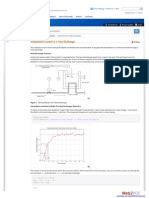
From the results, ignoring the error for the first value, the trend observed is that as the difference in pressure increases, the boil-up rate increases. Pressure can be defined as the amount of air molecules pushing down on the liquid and it suggests that the boil-up rate would slow down as the more energy is given to the mixture. When there is no liquid in the column, the trays of the column already provide intrinsic pressure and can be related to the equation:
hd=0.186Co2vlvhole2
The equation shows that the pressure difference depends on the differences in density between the vapour and liquid phases as well as velocity of vapour at the orifices.
From the graph, it can be seen that purity is affected by boil-up rate. Although purity is only affected by temperature, the graph shows differently. Methanol vaporizes at 650C and water at 1000C. Therefore, if the temperature of the distillation column is below the boiling point of water and above 650C, methanol will be able to vaporise and water will stay at liquid state, thus pure methanol will be obtained. However, from the graph, it is evident that purity is affected by boil-up rate. Some reasons for this difference in results may be: Water evaporates below boiling point therefore some water may have evaporated, affecting the concentration of methanol in top product receiver, thus explaining the differences in levels of purity. Some mixtures have boiling points that are really close, therefore increasing the difficulty for the mixture to me separated. Methanol and water have a difference of 350C in boiling points so as heat in the reboiler increases, so does the boil-up rate and this will definitely affect the purity of methanol in the top product receiver as some water might have vaporized and affected the purity. As the power supplied was increased to 0.8kW, it had been some time since the start of the experiment and therefore most the methanol in the reboiler might have been vaporized already causing the concentration to decrease. From the results, (ignoring the error of the first reading) as the boil up rate increases, the foaming will spread out and eventually become localize again. Foaming happens when there is expansion in the liquid which is due to the passage of the vapour. At 0.4 kW, the boil-up rate is assumed to be much lower than 0.627ml/s had gentle, localised foaming. At 0.6 kW, the boil-up was 0.138ml/s, and the foaming was still gentle but spread out. This is due to the decreasing density of the vapour that allows the vapour to flow faster hence forming a spread out formation. Eventually the density of the vapour will increase the vapour settles down and becomes denser which causes the foaming to be localised again. From the results, as pressure drop increases, the foaming gets more violent. Foaming occurs when there is agitation which is caused by the difference in pressure which also will increase the vapour velocity, forcing the vapour through the orifice trays. Therefore, the increment in the pressure drop foaming will become more violent. A variety of experimental errors occurred during this experiment. An inconsistent flow rate and error in determining when the system has reached steady-state as well as previous fluid built-up were the errors encountered. An inconsistent flow is troublesome in that it makes it harder to determine when steady-state has been reached. To solve this problem, adjust the valve controlling the tank or inlet pipe accordingly. Determining steady-state in this experiment is a problem because in is a batch process, and thus cannot perfectly be in steady state. To overcome this problem, when most parameters stabilise for about a minute, that is when readings should be taken. Previous fluid built-up from previous experiments as well as just before taking samples is a problem since it affects the purity of the sample taken. To overcome this problem, ensure that before running the experiment and before taking a sample, make sure the sample collection area is has been drained.
Conclusion
In conclusion, the higher the pressure drop, the more the boil-up rate as seen in the experiment. This is due to the differences in densities between the vapour and liquid phases and velocities of the of the vapour as it passes through the orifices of the trays. Although purity should only be affected by temperature, it is affected by boil-up rate. It could happen due to a variety of reasons, such as water evaporating and contaminating the sample. Boil-up rate affects foaming because of the passage of condensate liquid through vapour and their respective densities. A gentle and localised foaming suggests a low boil-up rate, while a violent, spread out foaming suggests a high boil-up rate.
References
http://lorien.ncl.ac.uk/ming/distil/distil0.htm http://en.wikipedia.org/wiki/Continuous_distillation http://ocw.korea.edu/ocw/college-of-engineering/process-control/lecture-notes-1/l7-dynrespii.pdf http://www.cheresources.com/invision/topic/3137-foaming-vs-pressure-drop/ http://www.cbu.edu/~rprice/lectures/distill7.html
Você também pode gostar
- High-Pressure Fluid Phase Equilibria: Phenomenology and ComputationNo EverandHigh-Pressure Fluid Phase Equilibria: Phenomenology and ComputationAinda não há avaliações
- 2423L3Documento8 páginas2423L3Ruben SyAinda não há avaliações
- Mechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesNo EverandMechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesAinda não há avaliações
- 900 1000 Sea Level Atmospheric Pressure Vapor Pressure, MM/HGDocumento5 páginas900 1000 Sea Level Atmospheric Pressure Vapor Pressure, MM/HGLexey Utlang100% (1)
- Distillation Column - Group5 - Eh2204aDocumento23 páginasDistillation Column - Group5 - Eh2204aatikah hasnorAinda não há avaliações
- Vapour Pressure LabDocumento3 páginasVapour Pressure Labapi-235688447Ainda não há avaliações
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsNo EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsNota: 5 de 5 estrelas5/5 (1)
- Experiment 1Documento13 páginasExperiment 1許書僑(乂傳說x飛龍乂)Ainda não há avaliações
- Engineering Bulletin No 1: Boiler and Furnace TestingNo EverandEngineering Bulletin No 1: Boiler and Furnace TestingNota: 4.5 de 5 estrelas4.5/5 (2)
- Punto de Ebullición - 1Documento8 páginasPunto de Ebullición - 1El Gil R GAinda não há avaliações
- Glass Transition and Phase Transitions in Food and Biological MaterialsNo EverandGlass Transition and Phase Transitions in Food and Biological MaterialsAinda não há avaliações
- Universiti Teknologi Mara Fakulti Kejuruteraan Kimia Chemical Engineering Laboratory Ii CHE523Documento14 páginasUniversiti Teknologi Mara Fakulti Kejuruteraan Kimia Chemical Engineering Laboratory Ii CHE523Heather Jarvis100% (2)
- Lab 2 - Distillation Column CompleteDocumento18 páginasLab 2 - Distillation Column CompleteHadiChan100% (1)
- Distillation Column Experiments: Pressure Drop & Composition AnalysisDocumento14 páginasDistillation Column Experiments: Pressure Drop & Composition AnalysisWahida Shukori67% (3)
- The Effects of Concentration of Glucose and Temperature On The Rate of OsmosisDocumento4 páginasThe Effects of Concentration of Glucose and Temperature On The Rate of OsmosisAisha Nicole Saito50% (2)
- Distillation Separates Liquid MixturesDocumento13 páginasDistillation Separates Liquid MixturesSanthosh KumarAinda não há avaliações
- Efficient Steam Accumulator Saves MoneyDocumento7 páginasEfficient Steam Accumulator Saves MoneyJaime ZeaAinda não há avaliações
- Determination of Boiling PointDocumento9 páginasDetermination of Boiling PointcrtgyhujikAinda não há avaliações
- ReportDocumento7 páginasReportShōyōHinataAinda não há avaliações
- Distillation Lab 9.10.2014Documento10 páginasDistillation Lab 9.10.2014Ahmed AliAinda não há avaliações
- Experiment 2: Fractional Distillation of A Mixture of Two UnknownsDocumento12 páginasExperiment 2: Fractional Distillation of A Mixture of Two UnknownsRahimi ShahimiAinda não há avaliações
- Batch Distillation ExperimentDocumento8 páginasBatch Distillation ExperimentJonelou CusipagAinda não há avaliações
- Theory: Factors Influencing Rate of EvaporationDocumento10 páginasTheory: Factors Influencing Rate of Evaporationviraj muzumdarAinda não há avaliações
- Lab+1 4309448 4309227Documento11 páginasLab+1 4309448 4309227Afwan IrfanAinda não há avaliações
- Experiment 5: Boiling Point and Melting Point DeterminationDocumento7 páginasExperiment 5: Boiling Point and Melting Point Determinationscsa31619Ainda não há avaliações
- Report Distillation ColumnDocumento20 páginasReport Distillation ColumnAzam Najmi33% (3)
- Distillation Separates Cyclohexane and Toluene MixtureDocumento8 páginasDistillation Separates Cyclohexane and Toluene MixturePeter Ickes100% (2)
- Distillation Column Lab ExperimentDocumento5 páginasDistillation Column Lab Experimentbigtommyk_0475% (4)
- Rate of Evaporation of LiquidsDocumento11 páginasRate of Evaporation of LiquidsNagraj leeAinda não há avaliações
- Fractional Distillation Lab ReportDocumento7 páginasFractional Distillation Lab ReportOmar AlasAinda não há avaliações
- EvaporationDocumento9 páginasEvaporationKim Tag-at YbañezAinda não há avaliações
- Pressure SaturationDocumento8 páginasPressure Saturationyumnaalhinai9Ainda não há avaliações
- Chem - 343 Lab 4 PDFDocumento10 páginasChem - 343 Lab 4 PDFAlyssa DewittAinda não há avaliações
- Modular Labaratory Program in Chemistry Separating Cyclohexane and Toluene by DistillationDocumento0 páginaModular Labaratory Program in Chemistry Separating Cyclohexane and Toluene by DistillationJoshua JohnsonAinda não há avaliações
- Distillation FractionalDocumento15 páginasDistillation FractionalLorena VivasAinda não há avaliações
- Objective of The Project: (3) Surface Area: Molecules That Escape The Surface of TheDocumento2 páginasObjective of The Project: (3) Surface Area: Molecules That Escape The Surface of ThePROKZZAinda não há avaliações
- Steam Distillation Vs Simple DistillationDocumento5 páginasSteam Distillation Vs Simple DistillationAde Hidayat ChaniagoAinda não há avaliações
- Petroleum Properties: Dr. Hanoon H. Mashkoor & Asst. Lect. Maryam J. JaafarDocumento35 páginasPetroleum Properties: Dr. Hanoon H. Mashkoor & Asst. Lect. Maryam J. JaafarMṜ ΛßßΛSAinda não há avaliações
- Experiment 6 - DistillationDocumento5 páginasExperiment 6 - DistillationRohit BiswasAinda não há avaliações
- V. Circulation SystemDocumento11 páginasV. Circulation SystemRaja RamAinda não há avaliações
- Distillation Column Full Report For CPE554Documento13 páginasDistillation Column Full Report For CPE554WanArifinAinda não há avaliações
- Chem 31.1 DistillationDocumento3 páginasChem 31.1 DistillationMonroe OrlinaAinda não há avaliações
- DistillationDocumento6 páginasDistillationpremise5274Ainda não há avaliações
- Study QuestionsDocumento10 páginasStudy QuestionsBithiah BidsAinda não há avaliações
- Bsed Sci Flores, Mark Brian Chapter2activityDocumento16 páginasBsed Sci Flores, Mark Brian Chapter2activityMark Brian FloresAinda não há avaliações
- Boiler experiment discussion on heating and cooling processesDocumento2 páginasBoiler experiment discussion on heating and cooling processesIfa Ismail100% (2)
- Vapor Pressure Lab: Effect of TemperatureDocumento7 páginasVapor Pressure Lab: Effect of TemperatureSuryansh KabraAinda não há avaliações
- Steam System of Power PlantDocumento34 páginasSteam System of Power PlantEjaz AhmedAinda não há avaliações
- Report On Fractional DistillationDocumento5 páginasReport On Fractional DistillationJackson YamangaAinda não há avaliações
- Experiment 2Documento11 páginasExperiment 2shathishAinda não há avaliações
- Circulation of BoilerDocumento11 páginasCirculation of BoilerJackSparrow86Ainda não há avaliações
- Simple Distillation of VodkaDocumento4 páginasSimple Distillation of VodkaKatrina TaracatacAinda não há avaliações
- Chemistry For PDFDocumento15 páginasChemistry For PDFvenka07Ainda não há avaliações
- Steam Accumulater 0909fultonwhitepaperDocumento10 páginasSteam Accumulater 0909fultonwhitepaperjmpbarrosAinda não há avaliações
- Measuring AssignmentDocumento3 páginasMeasuring AssignmentArnab BhattacharyaAinda não há avaliações
- CS5371 Theory of Computation: Lecture 1: Mathematics Review I (Basic Terminology)Documento23 páginasCS5371 Theory of Computation: Lecture 1: Mathematics Review I (Basic Terminology)Kamal WaliaAinda não há avaliações
- Solid angles in perspective: Ω, have a small but essential role in physics. For example, howDocumento8 páginasSolid angles in perspective: Ω, have a small but essential role in physics. For example, howashkarkabeer08Ainda não há avaliações
- Student's Error Analysis in Finishing Mathematic Word Problem Based Newman AnalysisDocumento11 páginasStudent's Error Analysis in Finishing Mathematic Word Problem Based Newman AnalysisguanyitorAinda não há avaliações
- Hysys For Aspen Plus Users PDFDocumento11 páginasHysys For Aspen Plus Users PDFKarim KholeifAinda não há avaliações
- CE-2101 Fluid Mechanics: Energy Consideration in Steady FlowDocumento53 páginasCE-2101 Fluid Mechanics: Energy Consideration in Steady FlowShaheer RizwanAinda não há avaliações
- Mid Drive Vs HubDocumento15 páginasMid Drive Vs HubRivan PamungkasAinda não há avaliações
- Unit Vi: Classification and PredictionDocumento29 páginasUnit Vi: Classification and PredictionpalaniappanAinda não há avaliações
- WWW Mathworks inDocumento7 páginasWWW Mathworks inRagini SharmaAinda não há avaliações
- Brushless DC Motor Control Using PLCDocumento6 páginasBrushless DC Motor Control Using PLCvuluyen6688Ainda não há avaliações
- Dynamic Programming Algorithm Explained in ECE 551 LectureDocumento11 páginasDynamic Programming Algorithm Explained in ECE 551 Lectureadambose1990Ainda não há avaliações
- Operation Manual Zoomlion QY40Documento133 páginasOperation Manual Zoomlion QY40Hải Tiến100% (1)
- Project PPTDocumento47 páginasProject PPTIshant KumawatAinda não há avaliações
- Graph Theory 6Documento30 páginasGraph Theory 6Zeeshan AhmedAinda não há avaliações
- Operating Instruction Precision Balance: Kern EwDocumento15 páginasOperating Instruction Precision Balance: Kern EwjohnAinda não há avaliações
- Understand The Standardization Protocol For Iot Understand The Concepts of Web of Things. Understand The Concepts of Cloud of Things With Understand The Basic Concepts of Aspect OrientedDocumento2 páginasUnderstand The Standardization Protocol For Iot Understand The Concepts of Web of Things. Understand The Concepts of Cloud of Things With Understand The Basic Concepts of Aspect OrientedShanthi GanesanAinda não há avaliações
- Add Nordic Semiconductor DFU To SDK Example: Bluetooth Low EnergyDocumento32 páginasAdd Nordic Semiconductor DFU To SDK Example: Bluetooth Low EnergyDaniel Ernesto EspitiaAinda não há avaliações
- Recurrent Neural Network-Based Robust NonsingularDocumento13 páginasRecurrent Neural Network-Based Robust NonsingularDong HoangAinda não há avaliações
- Teaching NLTK NorwegianDocumento68 páginasTeaching NLTK NorwegianRamesh AkulaAinda não há avaliações
- CSEC® Chemistry Past Papers January 2015Documento20 páginasCSEC® Chemistry Past Papers January 2015Jonathan RamsundarAinda não há avaliações
- Introduction to Nautilus 8 Mold Qualification and Design of Experiments SoftwareDocumento66 páginasIntroduction to Nautilus 8 Mold Qualification and Design of Experiments SoftwareJohn SuperdetalleAinda não há avaliações
- Size Reduction LectureDocumento28 páginasSize Reduction Lectureapsara karkiAinda não há avaliações
- Generator Protection Application GuideDocumento106 páginasGenerator Protection Application GuideJorge Alberto Chavarría Sacasa100% (1)
- Mechanical Technology Concepts: Applied ThermodynamicsDocumento45 páginasMechanical Technology Concepts: Applied ThermodynamicsLe0 GamingAinda não há avaliações
- Chapter 5 Refraction Through A Lens - Concise Physics Part II - Selina Solutions For Class 10 Physics ICSE - TopperLearningDocumento71 páginasChapter 5 Refraction Through A Lens - Concise Physics Part II - Selina Solutions For Class 10 Physics ICSE - TopperLearningHarsh AhirwarAinda não há avaliações
- TC 1800 QI 1 1 0 (User Manual)Documento25 páginasTC 1800 QI 1 1 0 (User Manual)Emman JimenezAinda não há avaliações
- TIMO Mock 2019 卷P3fDocumento9 páginasTIMO Mock 2019 卷P3fDo Yun100% (1)
- Eb 20 11Documento408 páginasEb 20 11henryAinda não há avaliações
- Bar BendingDocumento142 páginasBar Bendingjaffna100% (4)
- Area Under The CurveDocumento3 páginasArea Under The CurveReyland DumlaoAinda não há avaliações
- The Fabric of Civilization: How Textiles Made the WorldNo EverandThe Fabric of Civilization: How Textiles Made the WorldNota: 4.5 de 5 estrelas4.5/5 (57)
- Sully: The Untold Story Behind the Miracle on the HudsonNo EverandSully: The Untold Story Behind the Miracle on the HudsonNota: 4 de 5 estrelas4/5 (103)
- Dirt to Soil: One Family’s Journey into Regenerative AgricultureNo EverandDirt to Soil: One Family’s Journey into Regenerative AgricultureNota: 5 de 5 estrelas5/5 (124)
- Faster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestNo EverandFaster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestNota: 4 de 5 estrelas4/5 (28)
- Recording Unhinged: Creative and Unconventional Music Recording TechniquesNo EverandRecording Unhinged: Creative and Unconventional Music Recording TechniquesAinda não há avaliações
- Pale Blue Dot: A Vision of the Human Future in SpaceNo EverandPale Blue Dot: A Vision of the Human Future in SpaceNota: 4.5 de 5 estrelas4.5/5 (586)
- Highest Duty: My Search for What Really MattersNo EverandHighest Duty: My Search for What Really MattersAinda não há avaliações
- The Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaNo EverandThe Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaAinda não há avaliações
- The Weather Machine: A Journey Inside the ForecastNo EverandThe Weather Machine: A Journey Inside the ForecastNota: 3.5 de 5 estrelas3.5/5 (31)
- A Place of My Own: The Architecture of DaydreamsNo EverandA Place of My Own: The Architecture of DaydreamsNota: 4 de 5 estrelas4/5 (241)
- 35 Miles From Shore: The Ditching and Rescue of ALM Flight 980No Everand35 Miles From Shore: The Ditching and Rescue of ALM Flight 980Nota: 4 de 5 estrelas4/5 (21)
- Across the Airless Wilds: The Lunar Rover and the Triumph of the Final Moon LandingsNo EverandAcross the Airless Wilds: The Lunar Rover and the Triumph of the Final Moon LandingsAinda não há avaliações
- Packing for Mars: The Curious Science of Life in the VoidNo EverandPacking for Mars: The Curious Science of Life in the VoidNota: 4 de 5 estrelas4/5 (1395)
- The Future of Geography: How the Competition in Space Will Change Our WorldNo EverandThe Future of Geography: How the Competition in Space Will Change Our WorldNota: 4.5 de 5 estrelas4.5/5 (4)
- The Technology Trap: Capital, Labor, and Power in the Age of AutomationNo EverandThe Technology Trap: Capital, Labor, and Power in the Age of AutomationNota: 4.5 de 5 estrelas4.5/5 (46)
- Einstein's Fridge: How the Difference Between Hot and Cold Explains the UniverseNo EverandEinstein's Fridge: How the Difference Between Hot and Cold Explains the UniverseNota: 4.5 de 5 estrelas4.5/5 (50)
- Data-ism: The Revolution Transforming Decision Making, Consumer Behavior, and Almost Everything ElseNo EverandData-ism: The Revolution Transforming Decision Making, Consumer Behavior, and Almost Everything ElseNota: 3.5 de 5 estrelas3.5/5 (12)
- Broken Money: Why Our Financial System is Failing Us and How We Can Make it BetterNo EverandBroken Money: Why Our Financial System is Failing Us and How We Can Make it BetterNota: 5 de 5 estrelas5/5 (3)
- A Garden of Marvels: How We Discovered that Flowers Have Sex, Leaves Eat Air, and Other Secrets of PlantsNo EverandA Garden of Marvels: How We Discovered that Flowers Have Sex, Leaves Eat Air, and Other Secrets of PlantsAinda não há avaliações
- The End of Craving: Recovering the Lost Wisdom of Eating WellNo EverandThe End of Craving: Recovering the Lost Wisdom of Eating WellNota: 4.5 de 5 estrelas4.5/5 (80)
- ChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindNo EverandChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindAinda não há avaliações
- Artificial Intelligence: A Guide for Thinking HumansNo EverandArtificial Intelligence: A Guide for Thinking HumansNota: 4.5 de 5 estrelas4.5/5 (30)
- Reality+: Virtual Worlds and the Problems of PhilosophyNo EverandReality+: Virtual Worlds and the Problems of PhilosophyNota: 4 de 5 estrelas4/5 (24)