Escolar Documentos
Profissional Documentos
Cultura Documentos
The NORSE (New-Onset Refractory Status Epileptic Us) Syndrome
Enviado por
benghooi75Descrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
The NORSE (New-Onset Refractory Status Epileptic Us) Syndrome
Enviado por
benghooi75Direitos autorais:
Formatos disponíveis
The NORSE SyndromeEPV Wilder-Smith et al
417
Original Article
The NORSE (New-onset Refractory Status Epilepticus) Syndrome: Defining a Disease Entity
EPV Wilder-Smith,1MD, ECH Lim,1M Med (Int Med), HL Teoh,1MRCP, VK Sharma,1MRCP, JJH Tan,1MRCP, BPL Chan,1MRCP, BKC Ong,1FRCP
Abstract
Introduction: To characterise a homogeneous group of patients with new-onset refractory status epilepticus (NORSE syndrome). Materials and Methods: This is a descriptive, semiprospective review of all cases of NORSE syndrome seen between 2000 and 2004 at a tertiary care public hospital in Singapore. A review of the literature was performed to identify possible additional similar cases for comparison. Results: Seven patients with NORSE syndrome were identified. Characterising features were female gender, young age, previous good health, cerebrospinal fluid pleocytosis (in 4), antecedent febrile illness (in 5), extraordinarily prolonged status epilepticus (average 32 days), failure of extensive investigations to reveal an underlying cause, catastrophic outcome as well as temporal lobe and leptomeningeal abnormality on brain magnetic resonance imaging. A review of the literature identified 12 similar patients, comprising both adults and children. Conclusions: Based on our patients and those described in the literature, we characterise the NORSE syndrome. Increased recognition of this clinical entity is needed to help delineate the underlying aetiology of this unique severe illness. Ann Acad Med Singapore 2005;34:417-20 Key words: Epilepsy, Resistant, Status epilepticus, Treatment
Introduction Status epilepticus (SE) describes a clinical condition characterised by an epileptic seizure or a series of seizures that lasts for at least 30 minutes without consciousness being regained.1 Some authors have added a time line of 60 minutes. The incidence of generalised convulsive status epilepticus (GCSE) is between 40 and 80 per 100,000.2 The associated mortality rate has been estimated to be as high as 22%3 and may vary from 7.6% to 19% within the first 30 days.1,3,4 After 30 minutes to 60 minutes of continuous seizures, the physiologic compensatory mechanisms break down, resulting in an increased risk of neuronal damage as the seizures persist due to systemic and metabolic disturbances as well as a direct excitotoxic effect of neuronal discharges during the seizure.1 Refractory status epilepticus (RSE) is a life-threatening condition, which is characterised by the failure to respond to first- and second-line anticonvulsant therapy.5 Risk factors predisposing patients to RSE include delay in receiving treatment, infections of the central nervous system (CNS), metabolic encep1
halopathy and hypoxia.3,5 Most episodes of status are thought to develop without a prior history of epilepsy, and are almost always secondary to discernible underlying cerebral pathology.1 We describe a new clinical syndrome, consisting of cases with new-onset refractory status epilepticus (abbreviated to NORSE) seen in 7 patients over the last 3 years in our institution. The impressive clinical features of female gender, young age, previous good health, very long-lasting status epilepticus, extensive negative workup, including neuropathology in 2 patients and catastrophic outcome, caused us to review the literature in search of a possibly more widespread occurrence of this clinical syndrome. Materials and Methods After noticing 3 similar cases of young women with newonset RSE, with no discernible underlying cause and all of whom died within 3 months of presentation without abolition of seizures despite multiple medical interventions, we performed a retrospective review of the medical records of
Division of Neurology, Department of Medicine National University Hospital, Singapore Address for Reprints: A/Prof Einar P Wilder-Smith, Division of Neurology, Department of Medicine, National University Hospital, 5 Lower Kent Ridge Road, Singapore 119074. Email: mdcwse@nus.edu.sg
August 2005, Vol. 34 No. 7
418
The NORSE SyndromeEPV Wilder-Smith et al
all cases of SE seen between 2000 and 2002 at our tertiary care public hospital in Singapore, after which we prospectively assessed 4 more cases who presented within the next year. We defined SE as persistent clinical generalised epileptic activity lasting more than 30 minutes, without restoration of consciousness.1 RSE has widely varying definitions in the literature. We classified epilepsy as refractory when seizures did not respond or only partially responded to treatment with institution of 2 treatment regimens. All cases coded under the International Classification of Diseases (ICD) coding for SE (345.2, 345.3 and 345.7) were included for analysis. To identify similar cases of new-onset SE, the literature was reviewed using PubMed and the key index words of epilepsy, status epilepticus, seizures, continued seizures, refractory epilepsy and refractory status epilepticus. Results Patient Profiles Apart from the already identified 7 patients with NORSE syndrome, a retrospective case sheet analysis failed to pick up any additional cases. In total, we screened 355 patient files coded as SE. All of our patients were female, varying in age from 20 to 52 years, with a mean age of 33 years. Six patients were of Chinese ethnicity, and one was Malay. All save one (who had completed primary school) had completed secondary school (high school equivalent) education. None of the patients had previous seizures or a family history of epilepsy, nor a significant past medical or psychiatric history. Five out of 7 had fever in the week prior to the onset of seizures. All required ventilation during their prolonged admission. Except for extensor plantar responses, none of the patients had localising neurological signs. Table 1 is an overview list of the clinical characteristics. Six of the patients presented with a generalised tonic clonic seizure (GTCS), one with nonconvulsive SE manifesting as altered consciousness. Initial electroencephalogram (EEG) captured ictal discharges in all. In 3, initial EEG showed repeated ictal discharges originating from both fronto-temporal regions with no
Table 1. Clinical Characteristics of Patients with NORSE Patient 1 2 3 4 5 6 7 Preceding fever/illness Low-grade fever (1 week), transient diarrhoea Nil Fever (1 week), headache Fever (1 week), headache Nil Fever (3 days) Fever (3 days) Seizure type Multifocal/GTCS Multifocal/NCSE Multifocal/GTCS Multifocal/GTCS Multifocal/GTCS Multifocal/GTCS Multifocal/GTCS
clear side preference. In 1, there was continuous parasagital ictal discharge and 3 showed frontotemporal epileptiform waves originating from the right on 2 occasions and once from the left. In all patients, repeated EEGs showed the presence of multifocal SE. All patients were extensively investigated. The following tests were within normal limits in all patients: computed tomography (CT) scan of the brain (performed within 24 hours of admission), serum glucose, serum electrolytes including calcium, magnesium and phosphate, liver and thyroid function tests and collagen vascular workup (CRP, anti-dsDNA, anti-nuclear antibodies, Complement 3 & 4). Serum cryptococcal, herpes simplex virus 1 and 2 (HSV 1 and 2) and cytomegalovirus (CMV) IgG/M titres were within normal limits, as was venereal disease research laboratory (VDRL) testing. Cerebrospinal fluid (CSF) HSV 1 polymerase chain reaction, cryptococcal antigen testing and VDRL were within normal limits. CSF cultures were negative for mumps, measles, HSV 1 and 2, enteroviruses as for Mycobacterium tuberculosis, aerobic and anaerobic bacteria and fungi. CSF coagglutination was negative for Haemophilus influenza B, Streptococcus pneumoniae, Neisseria meningitides, Streptococcus group B and Escherischia coli K1 antigen. A toxicology screen was negative in all patients. In the initial phase of the EEG, 5 out of 7 patients had predominantly frontal ictal activity, which subsequently generalised. This later developed into multifocal ictal discharges. The significant positive investigations are listed in Table 2. Treatment and Outcome None of the initial presenting seizures were documented by medical staff, and firsthand witnesses to the initial presenting complaint described symptoms compatible with generalised tonic-clonic seizures. Subsequently, multifocal seizures were repeatedly seen. All our patients received intravenous benzodiazepines immediately (initial bolus of 5 mg to 10 mg followed by continuous intravenous delivery commencing at 3 mg to 5 mg midazolam/hour and titrating upwards), rapidly followed (within 10 to 30 minutes) by
Length of ICU stay (d) 92 11 24 15 7 11 65
Outcome Survived, vegetative, frequent seizures Survived, vegetative, frequent seizures Death Death Death Death Death
GTSE: generalised convulsive status epilepticus; ICU: intensive care unit; NCSE: non-convulsive status epilepticus; NORSE: new-onset refractory status epilepticus
Annals Academy of Medicine
The NORSE SyndromeEPV Wilder-Smith et al
419
Table 2. Abnormal Investigations in Patients with NORSE Patient WBC count x109/L 20 18 EEG pattern/Burst suppression achieved with ACD Multifocal seizures/ Achieved Multifocal seizures/ Achieved MRI brain (hours after admission) CSF studies (WBC in cells/uL)
1 2
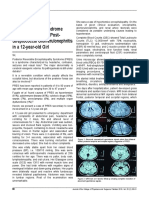
Biparietal and bitemporal FLAIR hyperintensities (no contrast) (7) Bilateral temporal, hippocampal, cerebellar penduncular FLAIR hyperintensities, leptomeningeal enhancement (24) Subarachnoid sulci FLAIR hyperintensities, leptomeningeal enhancement (72) Bilateral temporal and hippocampal FLAIR hyperintensities, leptomeningeal enhancement (24) Left temporal FLAIR and T2 hyperintensities (192) Bilateral temporal T2 and FLAIR hyperintensities (36) Bilateral corona radiata, centrum semiovale T2 and FLAIR hyperintensities, bilateral temporal FLAIR hyperintensities, leptomeningeal enhancement (48)
WBC 18 (polymorphs), normal glucose, protein 0.86 g/L WBC 63 (polymorphs), normal glucose, protein 0.25 g/L
3 4
16 9
Multifocal seizures/ Not achieved Multifocal seizures/ Achieved Multifocal seizures/ Not achieved Multifocal seizures/ Achieved Multifocal seizures/ Achieved
WBC 48 (lymphocytes), normal glucose, protein 0.27 g/L WBC 25 (lymphocytes), normal glucose, protein 1.77 g/L WBC 3 (lymphocytes), normal glucose, protein 0.16 g/L WBC 1 (lymphos), normal glucose, protein 0.51 g/L WBC 3 (polymorphs), normal glucose, protein 0.16 g/L
5 6 7
15 6 9
ACD: anticonvulsant drugs; CSF: cerebrospinal fluid; EEG: electroencephalogram; FLAIR: Fluid Attenuation Inversion Recovery Sequence; MRI: magnetic resonance imaging; WBC: white blood cell
maximal doses of intravenous phenytoin (slow bolus of 1g to 1.5 g) and valproate (800 mg 8 hourly), failing which they were treated with propafol. Four out of the 7 patients were given intravenous thiopentone when propafol failed to abort the seizures. Three received high-dose topiramate (1200 mg/day), and one of them (Patient 1) showed clinical improvement, without the complete abolition of seizures. In all patients, serum anticonvulsant levels of phenytoin and valproate were within the therapeutic range (>10 mg/L for phenytoin; >50 mg/L for valproate) during the first 2 days of treatment. If these values subsequently fell below these levels, extra dosing was given to achieve our laboratory therapeutic values. Two patients received maximal doses of levetiracetam (1500 mg bd), without obvious improvement. Three out of 7 patients received intravenous immunoglobulin therapy (IVIG) without effect (0.4 g/kg body weight per day for 3 days). Five of our 7 patients died while on intensive care. The cause of death was multi-organ failure. Two (Patients 1 and 2) survived in a vegetative state, with frequent daily seizures. We managed to obtain autopsy with neuropathology in 2 (Patients 5 and 6) of our patients. Brain histology was completely devoid of an inflammatory response, and apart from diffuse patchy neuronal cell loss, with reactive gliosis, no other abnormalities were detected. Autopsy excluded occult malignancy.
A literature review identified 2 groups of authors who recently described similar conditions in 6 adults (4 females and 2 males)6 and in a group of 6 children (4 males and 2 females) aged 5 months to 6 years.7 Discussion We describe a clinically distinct and homogeneous group of 7 previously healthy young female adults with generalised SE, refractory to aggressive antiepileptic treatment, with multifocal epileptiform discharges in the EEG and hyperintensities in the temporal lobe and leptomeninges on magnetic resonance imaging (MRI). Five out of 7 patients died despite intensive medical intervention and support, with the remaining 2 surviving with severe encephalopathy and recurrent seizures. The aetiology remains obscure, although we exhaustively searched for and failed to find any infectious, inflammatory, metabolic or toxic causes. The febrile start of the illness in 5 out of 7 of our patients could suggest a possible underlying infectious or inflammatory aetiology. However, the lack of an inflammatory response in the brains of 2 of the 7 cases, absent inflammatory serum and CSF markers as well as the lack of response to IVIG make this unlikely. In the face of negative markers of infection, lacking evidence of inflammation of the brain in 2 autopsies, the mild CSF pleocytosis is probably attributable to SE, as it can occur after SE of any type.8 Alternatively, CSF pleocytosis could
August 2005, Vol. 34 No. 7
420
The NORSE SyndromeEPV Wilder-Smith et al
be seen as a typical characteristic of this aetiologically enigmatous syndrome. The abnormalities seen on brain MRI are not sufficient to differentiate between an infectious, inflammatory or ictal encephalopathy. Two groups recently described similar patients in 6 adults (4 females and 2 males)6 and in a group of 6 children (4 males and 2 females) aged 5 months to 6 years.7 Extensive investigations, including brain neuropathology, were negative for infectious, inflammatory or structural brain abnormality. In these series, SE resistant to all types of antiepileptic treatment was documented with catastrophic outcome. Recent studies suggest that subtle immunological alterations in neurotransmitter function and altered drug clearance from the brain may play a hitherto underappreciated role in the aetiopathogenesis of RSE. Tanaka et al9 described associated intrathecal antibodies to inhibitory neurotransmitters such as glutamate in selected patients with RSE. Similarly, studies on animals and humans with refractory epilepsy have led to the discovery of a group of proteins that extrude drugs (in particular antiepileptics) from the CNS. These proteins have been shown to be overexpressed in the cerebral vascular and parenchymal tissue of both humans and rats.10,11 Recognition and further characterisation of this disease entity is crucial in gaining a better understanding of the reasons for its associated disastrous outcome and hopefully allowing for the development of better forms of treatment. Although it is a well-known fact that a large proportion of patients presenting with SE have no prior history of epilepsy, NORSE syndrome is distinct in that no identifiable underlying cause can be discerned. At present, only small numbers of patients with NORSE or a condition resembling it have been described, probably because it has yet to be recognised as a clinical entity. We speculate that anticonvulsants although reaching normal limits in the serum may not reach their brain target in sufficient concentrations, possibly because of a defect in the recently discovered group of efflux carrier proteins located at the blood-brain barrier. Apart from heightening awareness of this catastrophic condition, we suggest that future studies
should investigate whether antiepileptic drugs reach the CNS in sufficient concentrations in addition to whether the targets of antiepileptics, ion-channels and neurotransmitters show dysfunctional properties. Because of the early and aggressive treatment implicated both in our cases and those in the literature, it is unlikely that the timing and dosage of antiepileptics will play a major role in better managing this illness. Rather, a better understanding of the aetiopathology will result in the development of novel therapeutics useful for the treatment of RSE.
REFERENCES 1. Shorvon S. The management of status epilepticus. J Neurol Neurosurg Psychiatry 2001;70(Suppl):II22-7. 2. Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology 2003;61:1066-73. 3. Garzon E, Fernandes RM, Sakamoto AC. Analysis of clinical characteristics and risk factors for mortality in human status epilepticus. Seizure 2003;12:337-45. 4. Knake S, Rosenow F, Vescovi M, Oertel WH, Mueller HH, Wirbatz A, et al. Incidence of status epilepticus in adults in Germany: a prospective, population-based study. Epilepsia 2001;42:714-8. 5. Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol 2002;59:205-10. 6. Van Lierde I, Van Paesschen W, Dupont P, Maes A, Sciot R. De novo cryptogenic refractory multifocal febrile status epilepticus in the young adult: a review of six cases. Acta Neurol Belg 2003;103: 88-94. 7. Baxter P, Clarke A, Cross H, Harding B, Hicks E, Livingston J, et al. Idiopathic catastrophic epileptic encephalopathy presenting with acute onset intractable status. Seizure 2003;12:379-87. 8. Barry E, Hauser WA. Pleocytosis after status epilepticus. Arch Neurol 1994;51:190-3. 9. Tanaka Y, Nishida H, Yamada O, Takahashi Y, Moriwaki H. Intrathecal glutamate receptor antibodies in a patient with elderly-onset refractory epilepsy (Japanese). Rinsho Shinkeigaku 2003;43:345-9. 10. Marroni M, Marchi N, Cucullo L, Abbott NJ, Signorelli K, Janigro D. Vascular and parenchymal mechanisms in multiple drug resistance: a lesson from human epilepsy. Curr Drug Targets 2003;4:297-304. 11. Volk HA, Burkhardt K, Potschka H, Chen J, Becker A, Loscher W. Neuronal expression of the drug efflux transporter P-glycoprotein in the rat hippocampus after limbic seizures. Neuroscience 2004;123:751-9.
Annals Academy of Medicine
Você também pode gostar
- A "Malignant" Variant of Status EpilepticusDocumento8 páginasA "Malignant" Variant of Status Epilepticushijaugreen55Ainda não há avaliações
- Status Epilepticus in Adults: A Study From Nigeria: SciencedirectDocumento6 páginasStatus Epilepticus in Adults: A Study From Nigeria: SciencedirectLuther ThengAinda não há avaliações
- Posterior Reversible Encephalopathy Syndrome (PRES) : Electroencephalographic Findings and Seizure PatternsDocumento7 páginasPosterior Reversible Encephalopathy Syndrome (PRES) : Electroencephalographic Findings and Seizure PatternsJasper CubiasAinda não há avaliações
- The Importance of Genetic Testing in Adolescent-Onset Steroid-Resistant Nephrotic Syndrome - Case ReportDocumento9 páginasThe Importance of Genetic Testing in Adolescent-Onset Steroid-Resistant Nephrotic Syndrome - Case ReportAlrista MawarAinda não há avaliações
- Dysphagia in Lateral Medullary Infarction (Wallenberg's Syndrome)Documento8 páginasDysphagia in Lateral Medullary Infarction (Wallenberg's Syndrome)Dani PiñaAinda não há avaliações
- Acute Encephalitis Caused by Intrafamilial Transmission of Enterovirus 71 in AdultDocumento7 páginasAcute Encephalitis Caused by Intrafamilial Transmission of Enterovirus 71 in AdultAdhi SyukriAinda não há avaliações
- Huma Mir-1Documento51 páginasHuma Mir-1drw72409Ainda não há avaliações
- Evolution of Neurological, Neuropsychological and Sleep-Wake Disturbances After Paramedian Thalamic StrokeDocumento8 páginasEvolution of Neurological, Neuropsychological and Sleep-Wake Disturbances After Paramedian Thalamic Strokepokharelriwaj82Ainda não há avaliações
- The Neurology of Eclampsia: Some Observations: Views and ReviewsDocumento8 páginasThe Neurology of Eclampsia: Some Observations: Views and ReviewsFajar Rudy QimindraAinda não há avaliações
- Role of Lab Tests in First Febrile SeizuresDocumento4 páginasRole of Lab Tests in First Febrile SeizuresAlbertAinda não há avaliações
- Neuron-Specific Enolase As A Marker of The Severity and Outcome of Hypoxic Ischemic EncephalopathyDocumento5 páginasNeuron-Specific Enolase As A Marker of The Severity and Outcome of Hypoxic Ischemic EncephalopathyAgus WijataAinda não há avaliações
- Epilepticus: Morbidity and MortalityDocumento5 páginasEpilepticus: Morbidity and MortalityIndah Maharani NasutionAinda não há avaliações
- Posterior Reversible Encephalopathy Syndrome Secondary To Acute Post-Streptococcal Glomerulonephritis in A 12-Year-Old GirlDocumento2 páginasPosterior Reversible Encephalopathy Syndrome Secondary To Acute Post-Streptococcal Glomerulonephritis in A 12-Year-Old GirlwawaningAinda não há avaliações
- Guillain-Barré Syndrome in Children: Clinic, Laboratorial and Epidemiologic Study of 61 PatientsDocumento6 páginasGuillain-Barré Syndrome in Children: Clinic, Laboratorial and Epidemiologic Study of 61 PatientsFarah Basotjatjo KaharAinda não há avaliações
- A Case of Male Sle: An Unusual PresentationDocumento4 páginasA Case of Male Sle: An Unusual PresentationIJAR JOURNALAinda não há avaliações
- Sturge-Weber Syndrome. Study of 55 PatientsDocumento7 páginasSturge-Weber Syndrome. Study of 55 PatientsdzhzrnAinda não há avaliações
- Anti-N-methyl-D-aspartate Receptor (NMDAR) Encephalitis: A Series of Ten Cases From A University Hospital in MalaysiaDocumento6 páginasAnti-N-methyl-D-aspartate Receptor (NMDAR) Encephalitis: A Series of Ten Cases From A University Hospital in MalaysiaAbhishek KayalAinda não há avaliações
- Clinical Reasoning: A Young Man With Recurrent Paralysis, Revisable White Matter Lesions and Peripheral Neuropathy Word Count: 945Documento13 páginasClinical Reasoning: A Young Man With Recurrent Paralysis, Revisable White Matter Lesions and Peripheral Neuropathy Word Count: 945aileenzhongAinda não há avaliações
- 1 s2.0 S1875957210600198 MainDocumento9 páginas1 s2.0 S1875957210600198 MainTaddesse taddesse Taddesse taddesseAinda não há avaliações
- Prashant H 2006Documento6 páginasPrashant H 2006iradAinda não há avaliações
- Encephalitis and Aseptic Meningitis: Short-Term and Long-Term Outcome, Quality of Life and Neuropsychological FunctioningDocumento9 páginasEncephalitis and Aseptic Meningitis: Short-Term and Long-Term Outcome, Quality of Life and Neuropsychological FunctioningRoberto SoehartonoAinda não há avaliações
- Stroke JounalDocumento7 páginasStroke JounalTony HermawanAinda não há avaliações
- CCMDocumento28 páginasCCMSwati Pathak GiriAinda não há avaliações
- Hashimoto's Encephalopathy Case ReportsDocumento2 páginasHashimoto's Encephalopathy Case ReportsKantiNareshAinda não há avaliações
- Case ReportDocumento5 páginasCase ReportsyahputriAinda não há avaliações
- Pleds 2009 JamaDocumento7 páginasPleds 2009 JamaRosiane Da Silva FontanaAinda não há avaliações
- 1536 FullDocumento7 páginas1536 FullJosue LayedraAinda não há avaliações
- Status Epilepticus: Current Understanding: D P, M A M C, D GR M C, G, M PDocumento17 páginasStatus Epilepticus: Current Understanding: D P, M A M C, D GR M C, G, M PDMishraAinda não há avaliações
- Juvenile Lupus Erythematosus: Fourteen Years of Experience: Juvenil Lupus Eritematozus: On Dört Yıllık DeneyimDocumento8 páginasJuvenile Lupus Erythematosus: Fourteen Years of Experience: Juvenil Lupus Eritematozus: On Dört Yıllık DeneyimRahman SetiawanAinda não há avaliações
- A Cohort Study To Assess The New WHO Japanese Encephalitis Surveillance StandardsDocumento9 páginasA Cohort Study To Assess The New WHO Japanese Encephalitis Surveillance StandardsarmankoassracunAinda não há avaliações
- Misdiagnosis and Treatment Delays in Juvenile Myoclonic EpilepsyDocumento4 páginasMisdiagnosis and Treatment Delays in Juvenile Myoclonic EpilepsyPutu Filla JfAinda não há avaliações
- Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult PatientsDocumento8 páginasCauses and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult PatientschechenoveliaAinda não há avaliações
- Phi RythmDocumento6 páginasPhi RythmStanley IgweAinda não há avaliações
- Original Articles: Sydenham'S ChoreaDocumento4 páginasOriginal Articles: Sydenham'S ChoreaSabyasachi MukhopadhyayAinda não há avaliações
- Fneur 08 00507Documento8 páginasFneur 08 00507bozasnachoAinda não há avaliações
- Brit. J. vener. Dis. (1956), 32, 131. AbstractsDocumento5 páginasBrit. J. vener. Dis. (1956), 32, 131. AbstractsShaashi DamodaranAinda não há avaliações
- Journal of Medical Case ReportsDocumento4 páginasJournal of Medical Case ReportsSri Wahyuni YkAinda não há avaliações
- Case Report: A Case of Herpes Simplex Virus-1 Encephalitis From A Medicolegal Point of ViewDocumento4 páginasCase Report: A Case of Herpes Simplex Virus-1 Encephalitis From A Medicolegal Point of Viewzefri suhendarAinda não há avaliações
- Spectrum of Neurological Manifestations in Scorpion StingDocumento6 páginasSpectrum of Neurological Manifestations in Scorpion Stingiaset123Ainda não há avaliações
- Importance of Long-Term EEG in Seizure-Free PatienDocumento5 páginasImportance of Long-Term EEG in Seizure-Free PatienChu Thị NghiệpAinda não há avaliações
- Recurrent GBSDocumento14 páginasRecurrent GBSkashif mansoorAinda não há avaliações
- Neur CL in Pract 2013005694Documento10 páginasNeur CL in Pract 2013005694Haha RowlingAinda não há avaliações
- Neurosyphilis: The Reemergence of An Historical DiseaseDocumento2 páginasNeurosyphilis: The Reemergence of An Historical DiseaseDrashua AshuaAinda não há avaliações
- EncephalitisDocumento22 páginasEncephalitis2018 B01Seba AL-gunaidAinda não há avaliações
- Pathology I Review 10Documento30 páginasPathology I Review 10i_areinamoAinda não há avaliações
- Encefalitis NmdaDocumento9 páginasEncefalitis Nmdamariafernandasv98Ainda não há avaliações
- Rabin Stein 2010Documento6 páginasRabin Stein 2010luis fermin paredesAinda não há avaliações
- Treat SE with Benzos, AEDs, AnesthesiaDocumento15 páginasTreat SE with Benzos, AEDs, AnesthesiaKrisna DwiantamaAinda não há avaliações
- Basal Gangila CahngesDocumento12 páginasBasal Gangila CahngesJakkani RavikanthAinda não há avaliações
- Referat Status OkDocumento34 páginasReferat Status OkmarfirarizkiAinda não há avaliações
- Encefalitis Autoinmune 2011 PDFDocumento11 páginasEncefalitis Autoinmune 2011 PDFDiana Vanessa SuarezAinda não há avaliações
- Vol 54 No 2 Patel Selby YekkiralaDocumento5 páginasVol 54 No 2 Patel Selby YekkiralaDrashua AshuaAinda não há avaliações
- Journal Full Ayudita Silvia HasibuanDocumento29 páginasJournal Full Ayudita Silvia Hasibuanayudita silvia hasibuanAinda não há avaliações
- Jurnal 1 Ayudita Silvia HasibuanDocumento6 páginasJurnal 1 Ayudita Silvia HasibuanParutigapuluh NovemberAinda não há avaliações
- Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia From 2009-2013Documento14 páginasStevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia From 2009-2013LelyMariaApriliaAinda não há avaliações
- White Matter Disease: Neurology Rotation Lecture SeriesDocumento9 páginasWhite Matter Disease: Neurology Rotation Lecture SeriesSabrin BadarneAinda não há avaliações
- Status Epilepticus in Critically Ill PatientsDocumento11 páginasStatus Epilepticus in Critically Ill PatientsAkbal Nur KarimAinda não há avaliações
- Hypothermia For Neuroprotection in Convulsive Status EpilepticusDocumento11 páginasHypothermia For Neuroprotection in Convulsive Status EpilepticusismaangrianiAinda não há avaliações
- TB Meningitis Case Report: Isolated Oculomotor Nerve PalsyDocumento3 páginasTB Meningitis Case Report: Isolated Oculomotor Nerve PalsyRakasiwi GalihAinda não há avaliações
- Postural Tachycardia Syndrome: A Concise and Practical Guide to Management and Associated ConditionsNo EverandPostural Tachycardia Syndrome: A Concise and Practical Guide to Management and Associated ConditionsNicholas GallAinda não há avaliações
- A Predominantly Left Upper Limb Sensory ThyDocumento2 páginasA Predominantly Left Upper Limb Sensory Thybenghooi75Ainda não há avaliações
- CN-SFEMG For MGDocumento6 páginasCN-SFEMG For MGbenghooi75Ainda não há avaliações
- Uremic NeuropathyDocumento18 páginasUremic Neuropathybenghooi75Ainda não há avaliações
- Anticonvulsant Mechanisms of The Ketogenic DietDocumento16 páginasAnticonvulsant Mechanisms of The Ketogenic Dietbenghooi75Ainda não há avaliações
- Status Epilepticus in AdultsDocumento10 páginasStatus Epilepticus in AdultssiscaAinda não há avaliações
- Sedatives & Hypnotics - 17Documento76 páginasSedatives & Hypnotics - 17Raman KumarAinda não há avaliações
- Levetiracetam EpilepsiaDocumento6 páginasLevetiracetam EpilepsiaAndresPimentelAlvarezAinda não há avaliações
- Understanding Seizures and Epilepsy: Causes, Types, Diagnosis and TreatmentDocumento22 páginasUnderstanding Seizures and Epilepsy: Causes, Types, Diagnosis and TreatmentDr venkatesh jalluAinda não há avaliações
- Phenobarbital Sodium AHFS DIDocumento16 páginasPhenobarbital Sodium AHFS DIBrian HarrisAinda não há avaliações
- Jurnal Efficacy and Tolerability of Adjunctive Lacosamide in Pediatric Patients With Focal SeizuresDocumento15 páginasJurnal Efficacy and Tolerability of Adjunctive Lacosamide in Pediatric Patients With Focal SeizuresAnida HasnaAinda não há avaliações
- Clobazam For The Treatment ofDocumento3 páginasClobazam For The Treatment ofpronto4meAinda não há avaliações
- Formulary 2009Documento51 páginasFormulary 2009Wen ZhuAinda não há avaliações
- Vol 7 No. 3Documento99 páginasVol 7 No. 3G VenkateshAinda não há avaliações
- Med Chem IV Sem Pre RuhsDocumento1 páginaMed Chem IV Sem Pre Ruhsabhay sharmaAinda não há avaliações
- Top antiseptic, disinfectant and antibiotic brandsDocumento37 páginasTop antiseptic, disinfectant and antibiotic brandsgaurang mediaAinda não há avaliações
- Psychotropic DrugsDocumento60 páginasPsychotropic DrugsLaTasha Lindemann RNAinda não há avaliações
- Clinical and Hospital Pharmacy Questionnaire BLUE PACOPDocumento39 páginasClinical and Hospital Pharmacy Questionnaire BLUE PACOPSophia AndresAinda não há avaliações
- Central Nervous System MedicationsDocumento12 páginasCentral Nervous System MedicationsMARY JEANINA ALBAAinda não há avaliações
- Legal Summary-Chart English Final Tag508Documento2 páginasLegal Summary-Chart English Final Tag508Siska PindanAinda não há avaliações
- Adjuvant AnalgesicsDocumento44 páginasAdjuvant AnalgesicsZulfan EfendiAinda não há avaliações
- Erebral Palsy: An Information Guide For ParentsDocumento39 páginasErebral Palsy: An Information Guide For ParentsWilliam OttoAinda não há avaliações
- Anticonvulsant Activity of The Methanol Root Bark Extract of Ficus Sycomorus Linn. (Moraceae)Documento9 páginasAnticonvulsant Activity of The Methanol Root Bark Extract of Ficus Sycomorus Linn. (Moraceae)Journal of Pharmacy & Pharmacognosy ResearchAinda não há avaliações
- Clinical Aspects of Trigeminal Neuralgia: A Systematic ReviewDocumento5 páginasClinical Aspects of Trigeminal Neuralgia: A Systematic ReviewPratik ParikhAinda não há avaliações
- Refresher on epilepsy diagnosis and managementDocumento38 páginasRefresher on epilepsy diagnosis and managementB AuAinda não há avaliações
- JGPT - Development and Evaluation of Felbamate Loaded Solid Lipid NanoparticlesDocumento6 páginasJGPT - Development and Evaluation of Felbamate Loaded Solid Lipid NanoparticlesRamanuj SamalAinda não há avaliações
- Epilepsy: New Advances: Journal ReadingDocumento29 páginasEpilepsy: New Advances: Journal Readingastra yudhaTagamawanAinda não há avaliações
- Daftar Pustaka 1Documento4 páginasDaftar Pustaka 1dita pangestiAinda não há avaliações
- BCMJ 53 Vol6 Febrile SeizuresDocumento6 páginasBCMJ 53 Vol6 Febrile SeizuresdyyyaAinda não há avaliações
- A Case Report On Faropenem Induced Focal SeizuresDocumento3 páginasA Case Report On Faropenem Induced Focal SeizuresInternational Journal of Innovative Science and Research Technology100% (1)
- Drug Induced Optic NeuropathyDocumento23 páginasDrug Induced Optic NeuropathyFaizi JaniAinda não há avaliações
- DR Michael H Pfeiffer Website - Con-Drug-Scientist PDFDocumento5 páginasDR Michael H Pfeiffer Website - Con-Drug-Scientist PDFira roseAinda não há avaliações
- Kwan 2001Documento7 páginasKwan 2001Eliška VýborováAinda não há avaliações
- 11 Neurology PDFDocumento17 páginas11 Neurology PDFalialison7666100% (1)
- Evidence-Based Management Of: EpilepsyDocumento240 páginasEvidence-Based Management Of: Epilepsykhalid balshaAinda não há avaliações