Escolar Documentos
Profissional Documentos
Cultura Documentos
Microbiology: August 24, 2011 Mary Ann C. Bunyi, MD
Enviado por
Lenard PlatonDescrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Microbiology: August 24, 2011 Mary Ann C. Bunyi, MD
Enviado por
Lenard PlatonDireitos autorais:
Formatos disponíveis
Microbiology
Mary Ann C. Bunyi, MD
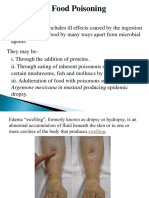
Genus Bacillus Large gram positive rods occurring in chains Most are saprophytic organisms (such as Bacillus subtilis and Bacillus cereus ) occurring in soil, water, air and on vegetation Bacillus anthracis is the principal pathogen of the genus and causes anthrax About 50 species Soil organisms Three (3) important Bacillus sp. o Bacillus subtilis o Bacillus cereus o Bacillus anthracis Two (2) species of major medical importance o Bacillus cereus o Bacillus anthracis
Lecture 14 (2-3): Bacillus August 24, 2011
EPIDEMIOLOGY Ubiquitous in the environment Found in many foods, especially rice, pulses (legumes), meat and vegetables Present in small numbers in raw, dried, and processed foods Spores are heat resistant therefore survive pasteurization, brief cooking or boiling Vegetative forms produce enterotoxins TRANSMISSION Ingestion of food/drinks contaminated with organism or toxin DISEASES Food poisoning from reheated cooked rice and meat/vegetables Gastroenteritis Two (2) clinical syndromes associated with Bacillus cereus food borne illness due to two (2) different toxins 1. Emetic syndrome o Acquired by eating food with preformed toxin most commonly fried rice or recooked rice o Occurs after a short incubation period (0.5 6 hours) o Nausea, vomiting, abdominal cramps o Diarrhea seen in one third of patients o Caused by heat-stable toxin 2. Diarrhea syndrome o Spore-associated disease caused by ingestion of contaminated meat or vegetables o Slightly longer incubation period (6 24 hours) o Predominantly characterized by moderate to severe abdominal cramps and watery diarrhea o Vomiting seen in one fourth of patients o Caused by heat-labile enterotoxin Both syndromes are mild, not associated with fever and abate within 24 hours Table 1. Summary of two (2) clinical syndromes associated with Bacillus cereus food borne illness
Emetic Syndrome Diarrhea Syndrome
** Bacillus subtilis was not discussed due to medical insignificance; however, it is included in this transcription for additional information. MORPHOLOGY Typical cells measure 1 x 3-4 m Have square ends Spores are in the center Aerobic, gram positive rods, occurring in chains See Figure 4, Color Plates, page 4 PHYSICAL PROPERTIES Saprophytic species use simple sources of nitrogen and carbon for energy and growth Spores are resistant to environmental changes such as dry heat and chemical disinfectants Animal products contaminated with anthrax spores can be sterilized only by autoclaving Bacillus subtilis Hay bacillus or grass bacillus Gram positive Catalase positive Model organism for laboratory studies Not considered a human pathogen; though it may contaminate food but rarely causes food poisoning Spores can survive the extreme heating that is often used to cook food, and it is responsible for causing ropiness a sticky, stringy consistency caused by bacterial production of long-chain polysaccharides
CHARACTERISTICS Large, gram-positive, spore-forming rod Motile Non-encapsulated Respires aerobically Bacillus cereus CHARACTERISTICS Large, gram-positive, spore-forming rod Motile Non-encapsulated Respires aerobically Self-limited LABORATORY IDENTIFICATION Non-fastidious Hemolysis on horse and sheep blood agar double zone of beta hemolysis (See Figure 5, Color Plates, page 4) Cream to white colonies (See Figure 6, Color Plates, page 4) Lecithinaes positive Mannitol negative Policarpio. Rabino. Sagun. Sistoso. Sobremisana. Sorbito.
Source Incubation period Manifestation Duration Toxin Responsible
Rice < 6 hours Severe vomiting 8-10 hours Heat-stable toxin
Meat/Vegetables > 6 hours Diarrhea 20-36 hours Heat-labile enterotoxin
OTHER MANIFESTATIONS Local skin and wound infections, periodontitis, ocular infections and invasive disease including bacteremia, catheter-associated infections, endocarditis, osteomyelitis, pneumonia, brain abscesses, and meningitis Ocular involvement such as panophthalmitis, endophthalmitis and keratitis INVASIVE RISK FACTORS Intravenous drug use, indwelling catheters or implanted devices, neutropenia or immunosuppression, or preterm birth Bacillus cereus endophthalmitis occurs after penetrating ocular trauma and intravenous drug use PATHOGENESIS Heat-stable toxin o Associated with spore germination o Present in food contaminated with Bacillus cereus o Vomiting within 1-5 hours after ingestion Heat-labile enterotoxin MICROBIOLOGY Lecture 14 (2-3)| Page 1 of 4
o Acquired after ingestion o Produced by Bacillus cereus in the intestine o Diarrhea within 10-15 hours after ingestion DIAGNOSIS Isolation of the organism in a concentration of > 105 colony forming unit (CFU) per gram of incriminated food Organism in feces or vomitus of ill people is not a definitive evidence of infection unless isolates from several ill patients are demonstrated to be the same serotype or unless stool cultures from a matched control group test negative Toxin testing is not widely available Phage typing, DNA hybridization, plasmid analysis, enzyme electrophoresis are used as epidemiologic tools in foodborne outbreaks Those with risk factors, isolation of the organism from wounds, blood or other sterile body fluids is significant TREATMENT For foodborne illness, supportive treatment only since illness is short-lived and self-limiting Removal of infected foreign bodies For invasive disease, give antibiotics o Drug of choice is vancomycin o Alternative drugs include clindamycin, carbapenems, ciprofloxacin PREVENTION Hygienic preparation of food Cooked food should be stored in the refrigerator and reheated thoroughly before serving Bacillus anthracis From the Greek word anthrakis which means coal and referring to the most common form of the disease, cutaneous anthrax, in which large black skin lesions are formed Major disease threat to herbivores (cattle, sheep, horses, hogs, goats) Humans are infected by: 1. Direct contact with diseased animal, hides, wool, brushes, or bone meal 2. Inhalation of infected dust (Woolsorter's disease) 3. Ingestion of infected meat
Spores formed in culture, soil, and dead animal tissues but not in live animals Spores remain viable in soil for decades Encapsulates in 5% CO2 atmosphere Only bacterium with a protein capsule (D-glutamate)
CULTURE CHARACTERISTICS Round, cut glass appearance Hemolysis is uncommon (while among saprophytic bacilli, hemolysis is common) See Figure 9, Color Plates, page 4 GROWTH CHARACTERISTICS Animal products as hides, bristles, hair, wool, bone contaminated with anthrax spores can be sterilized only by autoclaving EPIDEMIOLOGY Incidence o A zoonotic disease that occurs in rural regions in the world Transmission o Spores remain viable in soil for decades Source of infection for livestock through ingestion o Human infection is through contact with infected animal or animal products o Outbreaks of gastrointestinal anthrax is associated with ingestion of undercooked or raw meat o Can be used as a biological weapon because 1. Spores are highly stable 2. Spores can infect via the respiratory route resulting in inhalational disease that is lethal 3. Spores can also be introduced into food products or water supplies INCUBATION PERIOD For all forms of anthrax, less than 2 weeks In inhalational anthrax, because of spore dormancy, there is slow clearance from the lungs and incubation period may be prolonged to as long as several months FORMS OF ANTHRAX 1. Cutaneous 2. Pulmonary 3. Gastrointestinal 1. Cutaneous anthrax Accounts for more than 95% of human cases Spores enter the broken skin, germinate and rapidly proliferate at the site of entry Vesicular papule at the site of infection Blue-black edema Rupture of papule will reveal a black eschar (malignant pustule) See Figure 10, Color Plates, page 4 2. Inhalational Anthrax Spore inhalation and phagocytosis Naturally occurring inhalational anthrax was historically a disease associated with wool sorters at industrial mills A result of the deposition of spore-bearing particles of 1 to 5 m into the alveolar spaces Disease immediately follows germination Germination, replication, and infection of hilar lymph nodes Chest X-ray shows lobulated mediastinal widening, which may have infiltrates (See Figure 11, Color Plates, page 4) Abrupt onset of respiratory failure (1-2 days later) Hemorrhage, edema, and necrosis are the results of bacterial toxins released during replication. The first stage of illness is flu-like with fever, myalgia, dyspnea, cough, headache, vomiting, chills, abdominal pain, and chest pain. Sudden fever, dyspnea, diaphoresis, and shock characterize the second stage of illness. In the second stage of illness, cyanosis and hypotension result in a rapid death Death usually results 2-3 days after the onset of symptoms Chest radiographs showing a widened mediastinum are evidence of hemorrhagic mediastinitis and pleural effusions MICROBIOLOGY Lecture 14 (2-3)| Page 2 of 4
Figure 1. How Bacillus anthracis affects humans HISTORICAL BACKGROUND The first disease to fulfill Kochs postulate in 1876 The first bacterial disease for which immunization was available (1881) Review Kochs postulates RECENT CASES OF ANTHRAX IN THE U.S (2001) Two postings of contaminated letters (4 were found) 22 cases (plus 1 laboratory acquired case) 11 inhalational and 11 skin cases 5 deaths 32,000 were given prophylactic antibiotics Extensive contamination of postal system Thousands of false alarms and hoaxes MORPHOLOGY & PHYSIOLOGY Gram-positive, aerobic, non-motile, spore-forming bacilli with square ends tending to form long chains Bamboo canelike (See Figures 7 and 8, Color Plates, page 4) Policarpio. Rabino. Sagun. Sistoso. Sobremisana. Sorbito.
3. Gastrointestinal Anthrax Not seen in the United States Ingestion of infected meat Proliferation within the gastrointestinal tract Fever, acute abdomen, vomiting, bloody diarrhea Presence of intestinal eschar (similar to cutaneous lesion) Spreads to mesenteric lymph nodes, septicemia, shock and death PATHOGENESIS (VIRULENCE FACTORS) Capsule o Coded as pX02 plasmid o Polypeptide of D-glutamic acid o Encoded by a plasmid o Antiphagocytic o Not a good immunogen o Antibodies to the capsule are not protective against the disease Exotoxins o Coded as pX01 plasmid o A plasmid-encoded, heat-labile, heterogeneous protein complex made up of 3 components 1. Edema factor (EF) 2. Lethal factor (LF) 3. Protective antigen (PA) o In vivo, these three factors act synergistically (for toxic effects). The protective antigen binds to surface receptors on eukaryotic cells and is subsequently cleaved by a cellular protease. The larger C-terminal piece of PA remains bound to the receptor and then binds either EF or LF, which enters the cell by endocytosis. Edema Factor, when inside the cells binds calmodulindependent and acts as adenylate cyclase.
o o o o
Large gram-positive rods Nonmotile Nonhemolytic Rapid screening assay (PCR and antigen-detection based) for use on cultures and directly on clinical specimens Confirmatory criteria for identification of Bacillus anthracis Capsule production and visualization and lysis by gamma-phage Direct fluorescent antibody assays (DFA) for capsule antigen and cell wall-associated polysaccharide
TREATMENT Prompt therapy Protect against the multiplication of the organism Protect against the effects of the anthrax toxin, if there is septicemia Ciprofloxacin o The antibiotic of choice for victims of terrorism or warfare o 400 mg IV q12h Doxycycline o 100 mg IV q 12h Pen G o Endemic anthrax o 2M U IV q2h o 4M U IV q4h Postexposure prophylaxis o Ciprofloxacin 500mg po q 12h o Doxycycline 100 mg po q12h o Vaccine x 3 doses at 0, 2 and 4 weeks o Starting antibiotics within 24 hours after aerosol exposure is expected to provide significant protection Duration of 60 days with or without vaccine Most effective when combined with vaccination Antibiotics are still indicated even when fully immunized Long-term antibiotics necessary because of spore persistence in lung/lymph node tissue PREVENTION Antibody to the toxin complex is neutralizing and protective Two vaccines available for humans (1) and animals (1) o Immunize cattle and other herbivores o Immunize humans at risk SUMMARY Presumptive indentification
Clinical specimen (blood, CSF, etc.)
Figure 2. Pathogenesis of Edema Factor o Lethal factor's mechanism of action involves activation of macrophages and production of cytokines which cause necrosis, fever, shock and death. Individually, the three proteins have no known toxic activity. Antibodies to protective antigens prevent PA binding to cells stop EF and LF entry.
Gram stain Figures 7 and 8
Isolate on SBA Figure 12
Figure 3. Pathogenesis of Lethal Factor DIAGNOSIS Presumptive o History and clinical findings o Gram positive bacilli on blood smears Definitive o Culture and identification o After incubation on a blood agar plate for 1224 hours at 3537oC, well-isolated colonies are 25 mm in diameter and heavily inoculated areas may show growth in 68 hours o Gray-white, flat or slightly convex colonies are irregularly round, with edges that slightly undulate, and have ground glass appearance o Often have comma-shaped protrusions from colony edge Medusa head colonies (See Figure 9, Color Plates, page 4) Laboratory Criteria for Identification o From clinical samples, such as blood, cerebrospinal fluid (CSF), skin lesion (eschar), or oropharyngeal ulcer, microscopy and gram stain will show encapsulated gram-positive rods o From growth on sheep blood agar Policarpio. Rabino. Sagun. Sistoso. Sobremisana. Sorbito.
Colony morphology Hemolysis, Motility, Spores Figure 13 Gram stain
Confirmatory identification
Isolate
Phage lysis Figure 14
Capsule
DFA
Horse blood (MFadyean Stain) Figure 15
Bicarbonate Media (MFadyean stain India ink stain) Figure 16
Capsule antigen Cell wall
Figure 17
MICROBIOLOGY Lecture 14 (2-3)| Page 3 of 4
COLOR PLATES
Figure 10. Cutaneous anthrax. Rupture will reveal a black eschar (malignant pustule).
Figure 4. Gram stain of Bacillus sp. Large, gram-positive bacilli with squared-off ends occurring singly and in chains. Some species demonstrate endospores, which may be located centrally or terminally along the bacilli.
Figure 11. Mediastinal Widening and Pleural Effusion on Chest X-Ray in Inhalational Anthrax
Figure 5. Bacillus cereus with double zone of beta hemolysis
Figure 12. Isolate on 5% sheep blood agar
Figure 13. Motility of Bacillus anthracis Figure 6. Colonies of Bacillus cereus on 5% sheep blood agar. Cream to white colonies and beta hemolysis.
Figure 14. Phage lysis
Figure 7. Gram stain of Bacillus anthracis
Figure 15. Bacillus anthracis with MFadyean Stain
Figure 8. Gram-positive Bacillus anthracis in the CSF
Figure 16. Bacillus anthracis on Bicarbonate Media
Figure 17. Capsule antigen cell wall of Bacillus anthracis Figure 9. Medusa head colonies of Bacillus anthracis Policarpio. Rabino. Sagun. Sistoso. Sobremisana. Sorbito. MICROBIOLOGY Lecture 14 (2-3)| Page 4 of 4
Policarpio. Rabino. Sagun. Sistoso. Sobremisana. Sorbito.
MICROBIOLOGY Lecture 14 (2-3)| Page 5 of 4
Você também pode gostar
- 1.2 - Bacillus AnthracisDocumento26 páginas1.2 - Bacillus Anthracissajad abasAinda não há avaliações
- Bacillus AnthracisDocumento44 páginasBacillus AnthracisBalaji KrishnanAinda não há avaliações
- Death by FoodDocumento7 páginasDeath by FoodSuryanti SultanAinda não há avaliações
- Food Borne Disease Presentation FinalDocumento25 páginasFood Borne Disease Presentation FinalSagar WaghmareAinda não há avaliações
- Anthrax and Ts PreventionDocumento60 páginasAnthrax and Ts PreventionZahid Qamar100% (1)
- Gram Positive BacilliDocumento6 páginasGram Positive BacilliSteve ShirmpAinda não há avaliações
- Clostridia and Bacillus Lecture NotesDocumento37 páginasClostridia and Bacillus Lecture NotesPrincewill SeiyefaAinda não há avaliações
- FST-602 Food Safety Lecture on Bacterial Food Intoxication by Bacillus cereusDocumento17 páginasFST-602 Food Safety Lecture on Bacterial Food Intoxication by Bacillus cereusmuqaddasAinda não há avaliações
- Clinically Important BacilliDocumento9 páginasClinically Important BacilliJhomz EllaiAinda não há avaliações
- Food Poisoning and IntoxicationsDocumento26 páginasFood Poisoning and IntoxicationslakshmijayasriAinda não há avaliações
- Leptospirosis, Plague, Salmonellosis and Anthrax DiseasesDocumento9 páginasLeptospirosis, Plague, Salmonellosis and Anthrax DiseasesGetom NgukirAinda não há avaliações
- FST-602 Food Safety Bacterial Food Infections (Part-3) Lecture # 5Documento18 páginasFST-602 Food Safety Bacterial Food Infections (Part-3) Lecture # 5muqaddasAinda não há avaliações
- Bacillus Spp. Micropara - MjBrionesDocumento28 páginasBacillus Spp. Micropara - MjBrionesMj BrionesAinda não há avaliações
- FOOD POISONING SYMPTOMS AND CAUSESDocumento68 páginasFOOD POISONING SYMPTOMS AND CAUSESredityoAinda não há avaliações
- Faculty of Medicine Hashemite University DR Mohammad Al-Tamimi, MD, Master Biomed, PHDDocumento46 páginasFaculty of Medicine Hashemite University DR Mohammad Al-Tamimi, MD, Master Biomed, PHDDaniel AtiehAinda não há avaliações
- FST-602 Food Safety Bacterial Food Infections (Part-1) Lecture # 3Documento20 páginasFST-602 Food Safety Bacterial Food Infections (Part-1) Lecture # 3muqaddasAinda não há avaliações
- Diagnostic Microbiology - : University of Santo Tomas - Medical TechnologyDocumento6 páginasDiagnostic Microbiology - : University of Santo Tomas - Medical TechnologyWynlor AbarcaAinda não há avaliações
- Food Poisoning Causes, Symptoms and PreventionDocumento49 páginasFood Poisoning Causes, Symptoms and PreventionMazinAinda não há avaliações
- Micro CH 6 BacteriaDocumento58 páginasMicro CH 6 BacteriaBernadette Joyce PascualAinda não há avaliações
- Food Poisoning: Dr. SabirDocumento29 páginasFood Poisoning: Dr. SabirciciliaAinda não há avaliações
- Presumptive ID of B. anthracisDocumento16 páginasPresumptive ID of B. anthracisMyro DarlandanAinda não há avaliações
- Handout 13 Biotechnology in Promoting Law and Order PDFDocumento36 páginasHandout 13 Biotechnology in Promoting Law and Order PDFMiccah Angela Parreño FraynaAinda não há avaliações
- Food PoisoningDocumento55 páginasFood PoisoningAnusikta PandaAinda não há avaliações
- Coccidiosis in PoultryDocumento26 páginasCoccidiosis in PoultryMunir ullahAinda não há avaliações
- Birao Sas 13 Microbiology and ParasitologyDocumento9 páginasBirao Sas 13 Microbiology and ParasitologyFrancis Jacob Dejecacion GarcesAinda não há avaliações
- Nervous System InfectionDocumento50 páginasNervous System InfectionputiAinda não há avaliações
- ClostridumDocumento30 páginasClostridumFrancesca VargasAinda não há avaliações
- What Is Bacillus AnthracisDocumento6 páginasWhat Is Bacillus AnthracisFrancis AjeroAinda não há avaliações
- Bacillus and Biological Warfare: Dr. Samah Binte Latif M - Phil (Part-1), Microbiology Dhaka Medical CollegeDocumento67 páginasBacillus and Biological Warfare: Dr. Samah Binte Latif M - Phil (Part-1), Microbiology Dhaka Medical CollegeTania.dmp20Ainda não há avaliações
- LegionellaDocumento17 páginasLegionellaShubham Pareshkumar KadiwalaAinda não há avaliações
- Food Borne Diseases Associated With Foods of Animal OriginDocumento60 páginasFood Borne Diseases Associated With Foods of Animal OriginWan SyarifuddinAinda não há avaliações
- Food PoisoningDocumento7 páginasFood PoisoningSushanta DasAinda não há avaliações
- Microbio AssignmentDocumento3 páginasMicrobio AssignmentEia LaughAinda não há avaliações
- UII Gram Pos Spore-FormfefefeDocumento30 páginasUII Gram Pos Spore-FormfefefeDito TrunogatiAinda não há avaliações
- L15 SalmonellaDocumento19 páginasL15 SalmonellaMohammed RedhaAinda não há avaliações
- Communicable DiseasesDocumento164 páginasCommunicable DiseasesJasmin Jacob33% (3)
- Antrax LectureDocumento49 páginasAntrax LecturefraolAinda não há avaliações
- Microbio AssignmentDocumento3 páginasMicrobio AssignmentEia LaughAinda não há avaliações
- Intoxication of FoodDocumento19 páginasIntoxication of FoodabinayaAinda não há avaliações
- AnthraxDocumento11 páginasAnthraxrobbyrbbyAinda não há avaliações
- Week 7 Short Answer QuestionsDocumento3 páginasWeek 7 Short Answer Questionslewiskatisha16Ainda não há avaliações
- Infection of Clostridium SP in Animals - Sonya Yolanda HT (2202101010061)Documento14 páginasInfection of Clostridium SP in Animals - Sonya Yolanda HT (2202101010061)Sonya YolandaAinda não há avaliações
- Daren G. Linatoc, RN, RM, LPT, Man, MaedDocumento36 páginasDaren G. Linatoc, RN, RM, LPT, Man, MaedMike Faustino SolangonAinda não há avaliações
- Food and Waterborne DiseasesDocumento103 páginasFood and Waterborne DiseasesnatrubuclathrmacomAinda não há avaliações
- University of Santo Tomas - Medical Technology Diagnostic MicrobiologyDocumento5 páginasUniversity of Santo Tomas - Medical Technology Diagnostic Microbiologythedarkwing100% (1)
- Food PoisoningDocumento42 páginasFood Poisoningalif67% (3)
- BACILLUSDocumento11 páginasBACILLUSFatima AbasovaAinda não há avaliações
- 07.food PoisoningDocumento34 páginas07.food PoisoningI Made Yoga Satria AnggaraAinda não há avaliações
- Final Written ReportDocumento5 páginasFinal Written ReportJillianne MoyanoAinda não há avaliações
- FOOD INFECTIONDocumento20 páginasFOOD INFECTIONtesnaAinda não há avaliações
- Pertemuan Ke-3 GASTRODocumento36 páginasPertemuan Ke-3 GASTROwidya melianitaAinda não há avaliações
- Bacterial Agents:: o Staphylococcus AureusDocumento2 páginasBacterial Agents:: o Staphylococcus Aureussabrina_teng_2Ainda não há avaliações
- Microbial Diseases of The Digestive SystemDocumento34 páginasMicrobial Diseases of The Digestive SystemMa. Antonietta PennisAinda não há avaliações
- COXIELLADocumento6 páginasCOXIELLAFatima AbasovaAinda não há avaliações
- Anthrax ReaserchDocumento17 páginasAnthrax Reaserchsozakarem35Ainda não há avaliações
- Research About BacteriasDocumento13 páginasResearch About BacteriasDawn RallosAinda não há avaliações
- Bacterial Food PoisoningDocumento40 páginasBacterial Food Poisoningdigracia manatigaAinda não há avaliações
- Anthrax 140129091950 Phpapp02Documento28 páginasAnthrax 140129091950 Phpapp02Palwasha KhanAinda não há avaliações
- Microbiology in Everyday Life - VIIDocumento14 páginasMicrobiology in Everyday Life - VIIRawal Rafiq LeghariAinda não há avaliações
- 911 Pigeon Disease & Treatment Protocols!No Everand911 Pigeon Disease & Treatment Protocols!Nota: 4 de 5 estrelas4/5 (1)
- Quality Test of ParenteralsDocumento12 páginasQuality Test of ParenteralsAlishba MushtaqAinda não há avaliações
- Child Abuse Lesson PlanDocumento23 páginasChild Abuse Lesson PlanSAYMABANU83% (6)
- Pomegranate One Fruit That Cures Hundred AilmentsDocumento8 páginasPomegranate One Fruit That Cures Hundred AilmentsAsad ImranAinda não há avaliações
- AdenotonsilitisDocumento35 páginasAdenotonsilitisAriSugiharto100% (2)
- Case Report OrthoDocumento31 páginasCase Report OrthoRohani TaminAinda não há avaliações
- Angina PectorisDocumento3 páginasAngina PectorisKhalid Mahmud Arifin100% (2)
- Orange Peel MSDSDocumento4 páginasOrange Peel MSDSarvind kaushikAinda não há avaliações
- ArdsDocumento29 páginasArdsAmani KayedAinda não há avaliações
- Epididymal CystDocumento6 páginasEpididymal CystShree Kumar MishraAinda não há avaliações
- Singapore's Merchant Shipping Regulations on Medical StoresDocumento36 páginasSingapore's Merchant Shipping Regulations on Medical StoresLoka Radhakrishna NarasaiahAinda não há avaliações
- RWSSConsolidatedDocumento104 páginasRWSSConsolidatedToto TataAinda não há avaliações
- Article - Importance of Fruits, Nuts, and Vegetables in Human Nutrition and HealthDocumento3 páginasArticle - Importance of Fruits, Nuts, and Vegetables in Human Nutrition and HealthAlena JosephAinda não há avaliações
- Ark AngelDocumento172 páginasArk AngelCrina Serban67% (3)
- Host Modulation in Periodontics PDFDocumento12 páginasHost Modulation in Periodontics PDFCindyJessica100% (1)
- Lect 3 Trauma CounsellingDocumento28 páginasLect 3 Trauma Counsellingumibrahim75% (8)
- Humanistic and Experiential Theories of Psychotherapy PDFDocumento32 páginasHumanistic and Experiential Theories of Psychotherapy PDFpsicandreiaAinda não há avaliações
- H.4 Borderline PowerPoint Revised 2015Documento31 páginasH.4 Borderline PowerPoint Revised 2015Ptrc Lbr LpAinda não há avaliações
- Pharmaceutical Excipients-Boolket For Pharmacy StudentsDocumento33 páginasPharmaceutical Excipients-Boolket For Pharmacy StudentsRaja Bhai89% (9)
- AquaNereda Brochure 1017 WebDocumento4 páginasAquaNereda Brochure 1017 WebdmnAinda não há avaliações
- WestPacific ICD-10 26AUG15v3Documento2 páginasWestPacific ICD-10 26AUG15v3NczthAinda não há avaliações
- Women's Sexual Dysfunction Revised and Expanded DefinitionsDocumento7 páginasWomen's Sexual Dysfunction Revised and Expanded DefinitionsjoaomartinelliAinda não há avaliações
- Diagnosing rare facial tumorsDocumento1 páginaDiagnosing rare facial tumorsTayyaba RafiqAinda não há avaliações
- Stevia Global MarketDocumento12 páginasStevia Global MarketPratibha Singh50% (2)
- Ebola Virus Disease: Causes, Symptoms and TreatmentDocumento15 páginasEbola Virus Disease: Causes, Symptoms and TreatmentAlvin AlmazanAinda não há avaliações
- Department Check List Final 3Documento27 páginasDepartment Check List Final 3BOOKREADER_NOWAinda não há avaliações
- Chapter 4 - P4 P5 The Role of Complementary TherapiesDocumento8 páginasChapter 4 - P4 P5 The Role of Complementary TherapiesdesbestAinda não há avaliações
- Family & Medical Leave PolicyDocumento5 páginasFamily & Medical Leave PolicyGeorge RizkAinda não há avaliações
- Ankle Dislocations Health Conference 2010Documento16 páginasAnkle Dislocations Health Conference 2010Aswan IsmailAinda não há avaliações
- Operating TheaterDocumento5 páginasOperating Theateraksinu100% (1)
- Beldner 2020Documento10 páginasBeldner 2020Salsabila HMAinda não há avaliações