Escolar Documentos
Profissional Documentos
Cultura Documentos
Rowe, L. 2001
Enviado por
Francisco FalconDescrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Rowe, L. 2001
Enviado por
Francisco FalconDireitos autorais:
Formatos disponíveis
International Biodeterioration & Biodegradation 50 (2002) 3340
www.elsevier.com/locate/ibiod
Growth of Bacillus subtilis on polyurethane and the purication and
characterization of a polyurethanase-lipase enzyme
Lori Rowe, Gary T. Howard
Department of Biological Sciences, Southeastern Louisiana University, SLU 10736, Hammond, LA 70402, USA
Received 26 July 2001; received in revised form 20 December 2001; accepted 9 January 2002
Abstract
A soil microorganism capable of degrading polyurethane was isolated from a mesocosm study. This organism was identied as Bacillus
subtilis through 16s rRNA sequencing. The ability of this organism to degrade polyurethane was characterized by the measurement of its
growth kinetics and the purication and characterization of a polyurethane degrading enzyme. The growth kinetics for this microorganism
was obtained on two substrates: Impranil DLN
TM
and tributyrin. The growth of B. subtilis on polyurethane at certain concentrations (1.5
0.18 mg ml
1
) and tributyrin at all concentrations followed simple Monod growth kinetics. LineweaverBurke analysis of the data was
performed to yield a j
max
value of 0.7626 0.04799 doublings h
1
and K
s
value of 0.08559 0.03291 mg ml
1
for Impranil DLN
TM
and
a j
max
value of 0.460030.05407 doublings h
1
and K
s
value of 0.040650.2336 M for tributyrin. At higher concentrations of Impranil
DLN
TM
(9.03.0 mg ml
1
) Monod kinetics were not observed due to the binding of polyurethane to the cell wall of the B. subtilis.
The puried protein as determined by SDS-PAGE has a molecular weight of approximately 28 kDa. Substrate specicity was examined
using -nitrophenyl substrates with varying carbon lengths. The highest substrate specicity was observed for -nitrophenyl-acetate
with an activity of 45 U mg
1
. Additionally, the enzyme is heat labile and not inhibited by phenylmethylsulfonyluoride (PMSF),
adenylmethylsulfonyluoride (AMSF), ethylenediamine-tetra acetic acid (EDTA), or Bromelain. ? 2002 Elsevier Science Ltd. All rights
reserved.
Keywords: Polyurethanase; Lipase; Bacillus
1. Introduction
Plastics are polymers used in a wide variety of industries
and commerce. As the use of plastics increases so does the
amount of plastic being dumped into landlls. Plastics have
reached a level of over 10% by weight and 30% by volume
of solid waste in landlls in the US and Japan. The rate of
depletion of landlls is being greatly impacted by the pres-
ence of these substances. Their persistence in landlls is
adding to the growing water and surface waste litter prob-
lems, which has raised concerns about non-degradable prod-
ucts and promoted increased interest in the development of
products that are degradable or in the development of new
alternative for the reduction of waste (Kawai, 1995).
One process that is being developed for the degrada-
tion of plastics involves bioremediation. Bioremediation
consists of biological remedies for pollution reduction.
Corresponding author. Tel.: +1-504-549-3501; fax: +1-504-549-
3851.
E-mail address: ghoward@selu.edu (G.T. Howard).
Biological means have been in use since the early 1900s
to treat non-refractory wastes such as sewage. The treat-
ment of hazardous and refractory waste (associated with
chemical}industrial activities) through bioremediation is
more recent and still in the developmental stages. In order
to develop bioremediation techniques for hazardous and
refractory waste, such as plastics, knowledge of how these
compounds are metabolized by existing organisms, an in-
vestigation of new organisms, and the development of novel
metabolic capabilities using genetic engineering is needed.
A basic understanding of the biological processes that lead
to chemical degradation will add in the development of new
bioremediation techniques (Shannon and Unterman, 1993).
One type of plastic that has grown in use over the years is
polyurethane. The use of polyurethane has increased because
of its high durability and resistance to degradation. It has
also increased in use because the government is phasing out
the use of chlorinated rubber and other compounds that con-
tain environmentally hazardous volatile organic compounds
(VOC) (Hegedus et al., 1989; Reisch, 1990). Polyurethane
is a xenobiotic substance that refers to a class of poly-
mers that has found widespread applications for over 50
0964-8305/02/$ - see front matter ? 2002 Elsevier Science Ltd. All rights reserved.
PII: S 0964- 8305( 02) 00047- 1
34 L. Rowe, G.T. Howard / International Biodeterioration & Biodegradation 50 (2002) 3340
years. Polyurethanes represent a complex group of synthetic
polymers that can be found in almost every aspect of our
lives. Numerous products are produced from polyurethane,
including rubber adhesives, water blown exible foams,
elastomers, coatings, and bers. Their use ranges from ex-
ible foams in furniture, bedding, and automobile cushions
to thermal insulation on commercial vehicles and ships, to
medical devices used for cosmetic procedures as well as life
saving devices (Ulrich, 1983).
By gaining an understanding of the mechanisms of
polyurethane degradation a more ecient technique for the
biodegradation of polyurethane can be achieved. As the
production and use of polyurethane increases yearly so does
the need for ecient and environmentally friendly tech-
niques for its disposal. In this study, a microorganism was
isolated from a soil sample taken from a mesocosm study
investigating its ora capable of polyurethane degradation.
Several organisms were noted capable of polyurethane
degradation and the colony that showed the highest activity
was isolated for this study. The objectives of the current
research are to identify the organism and to elucidate the
polyurethane-degrading system of this organism.
2. Materials and methods
2.1. Media and culture conditions
LuriaBertani (LB) mediumwas prepared by adding 0.5 g
yeast extract, 1.0 g NaCl, and 1.0 g tryptone to 100 ml dH
2
O.
Yeast extract salts (YES) medium was prepared as previ-
ously described (Crabbe et al., 1994). The soil isolate was
grown at 30
C in either LB or YES media.
2.2. Characterization of polyurethane-degrading soil
bacterium
The organism was isolated into pure culture by streaking
on a LB agar plate. Once a pure colony was isolated, a
gram stain, spore stain, and catalase test were performed.
The isolated colony was sent to the Sequencing Laboratory
of the Biotechnology Center at the University of Illinois at
Urbana-Champaign for 16s rRNA sequencing.
2.3. Determination of bacterial growth kinetics
Once the organism was puried, growth kinetics was
performed. A 5 ml LB-broth culture was inoculated with
the organism and incubated at 30
C with constant shak-
ing for 12 h. YES media was then prepared with the
addition of varying concentrations of Impranil DLN
TM
(Impranil DLN is a polyurethane made from a poly
hexane}neopentyl adipate polyester and hexamethylene di-
isocyanate). The concentrations of Impranil DLN
TM
used
were: 9.0, 6.0, 3.0, 1.5, 0.75, 0.54, 0.375, and 0.18 mg ml
1
.
Each concentration was prepared in triplicate. The media
were then inoculated with 100 l from the LB broth culture.
Cell number was determined using a Coulter Multisizer IIe
instrument (Coulter Scientic Instruments, Inc., Hialeah,
FL) tted with a 30 m aperture. Values for K
s
and j
max
for polyurethane utilization were elucidated. In addition,
K
s
and j
max
values were determined with YES media with
the addition of varying concentrations of tributyrin. The
concentrations of tributyrin used were: 600, 300, 150, and
30 M. Each concentration was run in triplicate.
2.4. Enzyme purication
A 1 l culture of the soil microorganism was grown in
minimal media supplemented with 5 g tryptone and 1%
(wt vol
1
) polyurethane until no visible polyurethane was
present. The culture was centrifuged at 5000 g for 15 min
at 4
C. All remaining manipulations were also conducted at
this temperature. The supernatant was removed and concen-
trated 4-fold by ultraltration on a minitan high-resolution
tangential ow system tted with a 10, 000 mw cuto mem-
brane (Millipore). (NH
4
)
2
SO
4
was added to the supernatant
to give a 60% saturation. The precipitated proteins were col-
lected by centrifugation (10, 000 g, 15 min) and suspended
in 50 mM phosphate buer, pH 7.0. Dialysis was performed
to remove the ammonium sulfate from the protein extract.
To isolate the protein(s) of interest anity chromato-
graphy was employed. The concentrated protein was passed
over a DEAE-cellulose column (314 cm) with a bed
volume of 50 ml, which had been previously equilibrated
with 50 mM phosphate buer, pH 7.0. A linear step-wise
gradient, 0.11.0 M NaCl, was applied and fractions were
collected for each concentration. All fractions were tested
for enzyme activity. The polyurethane-degrading enzyme
was determined to be 95% homogeneity as indicated by
SDS-PAGE.
2.5. Electrophoresis
Sodium dodecyl sulfate (SDS)-PAGE was performed as
described by Laemmli (1970) with a 15% polyacrylamide
resolving gel. Proteins were visualized by Coomassie blue
staining.
2.6. Protein concentration determination
Protein concentration was determined by using the fol-
lowing equation (Kalb and Bernlohr, 1977):
[protein (g ml
1
)] = [(184)(A
230 nm
)
(81.5)(A
260 nm
)](dilution)
2.7. Enzyme assays
Once the protein concentration was determined, substrate
specicities could be determined. Esterase activity was
L. Rowe, G.T. Howard / International Biodeterioration & Biodegradation 50 (2002) 3340 35
determined with j-nitrophenyl-acetate, j-nitrophenyl-
butyrate, j-nitrophenyl-propionate, j-nitrophenyl-caprolate,
and j-nitrophenyl-caprolyate at 8 mM concentrations of
substrate in 50 mM phosphate buer, pH 7.0. One unit of
esterase activity was dened as the amount of enzyme that
produced 1 mol of j-nitrophenol per minute. All enzyme
assays were determined to be linear with respect to time
and protein concentration.
Enzyme activity was calculated for all substrate speci-
cities using the following equation (Deshpande et al.,
1984):
U mg
1
=
(Abs
420 nm
)(vol)
(E
c
)(1)(mg protein)
,
where Abs
420 nm
is the change in absorbance at 420
nm
,
vol is the volume of the assay, E
c
is the molar extinc-
tion coecient of 18.5 ml mol
1
cm
1
for j-nitrophenol
produced, 1 is the change in time in minutes, and
mg protein is the amount of enzyme in milligrams
assayed.
Enzyme inhibition was tested using the same assay
as above with the substrate j-nitrophenyl-propionate and
an enzyme inhibitor. The inhibitor was added just prior
to the addition of the substrate. The inhibitors tested
were 1 mM PMSF, 10 mM PMSF, 1 mM AMSF, 10 mM
EDTA, and 20 l Bromelain inhibitor. The enzyme activ-
ity was also determined after incubation of the enzyme
at 100
C for 10 min to determine heat stability using the
same assay as above with the substrate j-nitrophenyl-
propionate.
In addition, an agarose plate that was previously di-
vided into four quadrants was used to determine lipase
and polyurethane-degrading activities for the puried
protein as described previously (Howard et al., 2001).
In each quadrant, a dierent substrate was tested. The
four substrates used were 1% Impranil DLN
TM
, 1%
Bayhydrol 1100.01% Rodamine B, 1% tributyrin, or
1% triolien0.01% Rodamine B. The plates were incu-
bated at 30
C and observed for zones of clearing under
white light and UV light using a GelDoc light source
(BioRad).
2.8. Recombinant DNA techniques
Chromosomal DNA was isolated by the method of Mar-
mur (1961). The DNA was extracted two times with ether
to remove trace organics (Sambrook et al., 1989). Lambda
Zap II phage libraries were constructed with the use of ei-
ther the restriction endonuclease EcoRI or BamHI as pre-
viously described (Vega et al., 1999). Recombinant phages
were screened by plating in soft agar overlays containing
either Impranil or tributyrin as previously described (Vega
et al., 1999).
3. Results
3.1. Characterization of the soil microorganism
The soil microorganism was found to be a gram-positive
rod that produced swollen central spores. The organism was
also shown to be catalase positive indicating the genus Bacil-
lus. The 16s rRNA sequence was determined and shown to
have a 99% similarity to B. subtilis. The ability of this or-
ganism to degrade Impranil DLN
TM
was tested by growth
on a LB agar plate supplemented with 1% Impranil DLN
TM
.
After incubation at 30
C for 24 h the plate was observed for
zones of clearing (Fig. 1).
3.2. Growth kinetics
A Coulter Multisizer IIe was used to measure the change
in bacterial cell number as the substrate was being utilized.
When grown on 1% Impranil DLN
TM
YES medium, a lag
phase growth was noted for the rst 5 h which was fol-
lowed by logarithmic growth for 8 h, reaching a cell den-
sity of 2.60 10
8
1.17 10
7
. When grown on 3 mM
tributyrin-YES medium, a lag phase growth was noted for
the rst 4 h, which was followed by logarithmic growth for
11 h, reaching a cell density of 1.470 10
8
1.424 10
7
.
Growth kinetic values, K
s
and j
max
, on Impranil DLN
TM
and tributyrin were elucidated by varying the concentra-
tions of the substrates in YES media. For Impranil DLN
TM
the concentrations ranged from 9.00.18 mg ml
1
and for
tributyrin the concentrations ranged from 600 to 30 M.
At higher concentration (9.03.0 mg ml
1
) of Impranil
DLN
TM
typical Monod kinetics were not observed. The
j values dramatically decreased at a concentration of
3.0 mg ml
1
from 1.5 mg ml
1
to 0.466 doublings h
1
from 0.721 doublings h
1
. The j continued to drop at higher
concentrations from 0.466 doublings h
1
at 3.0 mg ml
1
to 0.369 doublings h
1
at 9.0 mg ml
1
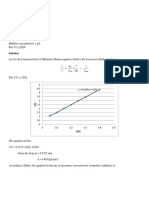
as seen in Fig. 2.
However, utilization of both Impranil DLN
TM
at certain
concentrations (1.50.18 mg ml
1
) and tributyrin at all con-
centrations followed simple Monod Kinetics (Fig. 3a and b,
respectively). Analysis of LineweaverBurke plots of the
data yielded j
max
values of 0.76260.04799 doublings h
1
and K
s
values of 0.08559 0.03291 mg ml
1
for Impranil
DLN
TM
and j
max
values of 0.460030.05407 doublings h
1
and K
s
values of 0.04065 0.2336 M for tributyrin.
3.3. Protein purication and characterization
Bacillus subtilis was observed to degrade Impranil
DLN
TM
in three to four days. Once the culture was clear
with little or no Impranil DLN
TM
remaining, a crude protein
extraction was performed by centrifugation. The super-
natant was claried and concentrated. This fraction was
passed over a DEAE-cellulose ion exchange column. The
protein of interest was eluted in the 0.1 M NaCl}50 mM
36 L. Rowe, G.T. Howard / International Biodeterioration & Biodegradation 50 (2002) 3340
Fig. 1. Growth of B. subtilis on a LB plate supplemented with 1% Impranil DLN
TM
with zones of clearing around the edges of the colony showing
polyurethane degradation.
10 8 6 4 2 0
0. 3
0. 4
0.5
0.6
0.7
0.8
[S] mgml
-1
Fig. 2. Specic growth rate of B. subtilis vs. substrate concentration of
Impranil DLN
TM
ranging from 9.00.18mg ml
1
. The specic growth
rate does not follow simple Monod kinetics.
phosphate buer fraction. A quick assay for enzyme activity
was performed and this fraction was observed to hydrolyze
j-nitrophenyl-caprolate. A sample of this fraction was run
on a 15% SDS-PAGE gel to determine the molecular weight
and purity of the active protein. The molecular weight was
determined to be approximately 28 kDa (Fig. 4).
The enzyme has the capability of hydrolyzing all
j-nitrophenyl substrates tested. As the carbon length in-
creased in the j-nitrophenyl substrates the specicity of the
enzyme for the substrate decreased. The specic activities
for each substrate are listed in Table 1. The enzyme was
not inhibited by any of the inhibitors tested, however the
activity of the enzyme could be decreased signicantly or
completely inhibited by heat (boiling for 10 min at 100
C).
0.00 0.30 0.60 0.90 1.20 1.50 1.80
0.00
0.20
0.40
0.60
0.80
1.00
max
[S] mg
ml
-1
0.00 0.30 0.60 0.90 1.20 1.50 1.80
[S] mM
0. 00
0.10
0.20
0.30
0.40
0.50
0.60
max
(a)
(b)
Fig. 3. Monod plot of B. subtilis grown on (A) YES media supple-
mented with varying concentrations of Impranil DLN
TM
ranging from
1.50.18 mg ml
1
. The j
max
was calculated to be 0.7626 0.04799
doublings h
1
and the K
s
to be 0.08559 0.03291 mg ml
1
. (B) YES
media supplemented with varying concentrations of tributyrin ranging
from 600 to 30 M. The j
max
was calculated to be 0.4603 0.05407
doublings h
1
and the K
s
to be 0.04065 0.2336 M. The data are the
means of three replicates.
L. Rowe, G.T. Howard / International Biodeterioration & Biodegradation 50 (2002) 3340 37
Fig. 4. Commassie blue stain of a SDS-PAGE gel of the puried protein.
Lane 1 is the molecular weight marker Dalton Mark VII and lane 2
is 5 g of the puried protein. The molecular weight of the protein is
approximately 28 kDa.
Lipase and polyurethane-degrading activities were deter-
mined for the puried protein by incubating the protein in
four dierent agarose media: 1% Impranil DLN
TM
, 1% Bay-
hydrol 110 plus Rodamine B, 1% tributyrin, or 1% triolien
plus Rodamine B. After incubation at 30
C, zones of hy-
drolysis could be observed for all substrates used as indi-
cated in Fig. 5. The largest zones of clearing was observed
with 1% Impranil DLN
TM
.
3.4. Screening of the z library for a polyurethanase
recombinant
A genomic library was constructed by ligating 410 kb
of either EcoRI or BamHI DNA fragments of B. subtilis
chromosomal DNA into zZAPII. Screening for recombi-
nants was based on their ability to hydrolyze either Impranil
DLN
TM
or tributyrin. No zones of clearing on any of the
Table 1
Substrate specicity of puried protein
a
Substrate Enzyme activity (U mg
1
)
b
j-Nitrophenyl-acetate 46.06
j-Nitrophenyl-propionate 41.90
j-Nitrophenyl-butyrate 34.17
j-Nitrophenyl-caprolate 21.18
j-Nitrophenyl-capryolate 16.91
a
Protein concentrations were estimated by the method of Kalb and
Bernlohr (1977).
b
Assay were performed at 30
C in 50 nm phosphate butter (pH
7.0) with 12.5 g of protein.
plates were observed. The screening procedure was repeated
three times, resulting in over 20,000 recombinants screened,
and no positive plaques were noted. Because no positive
plaques were found, the gene was not isolated and this part
of the study was not pursued.
4. Discussion
In the present study, the growth of B. subtilis on
polyurethane and tributyrin was determined and a soluble
polyurethane degrading enzyme was puried and charac-
terized. The microorganism was found to be capable of
utilizing a polyester polyurethane, Impranil DLN, as well
as tributyrin as its sole carbon and energy source.
Growth of B. subtilis on Impranil DLN, at certain concen-
trations (1.50.18 mg ml
1
) was similar to that reported for
Comamonas acidovorans (Allen et al., 1999), Pseudomonas
uorescens (Howard and Blake, 1998), and Pseudomonas
chlororaphis (Howard et al., 1999). Growth of these organ-
isms followed simple Monod growth kinetics with j
max
and
K
s
values for B. subtilis (0.7626 0.04799 doublings h
1
and 0.085590.03291 mg ml
1
) being very close to C. aci-
dovorans (0.75 doublings h
1
and 0.38 mg ml
1
) and lower
than those reported for P. uorescens (1.61 doublings h
1
and 0.9 mg ml
1
) and P. chlororaphis (1.31 doublings h
1
and 0.8 mg ml
1
). Growth of B. subtilis on tributyrin at all
concentrations tested followed Monod kinetics. The j
max
was calculated to be 0.46003 0.05407 doublings h
1
and
K
s
values of 0.04065 0.2336 M. The relatively higher
anity of this organism for tributyrin than polyurethane
could be due to tributyrins lack of complexity and smaller
molecular mass as compared to Impranil DLN.
The Monod plot for all concentrations of polyurethane
tested did not follow simple Monod kinetics. At higher con-
centration (9.03.0 mg ml
1
) of Impranil DLN
TM
Monod
kinetics were not observed. The j values dramatically de-
creased at a concentration of 3.0 mg ml
1
from 1.5 mg ml
1
to 0.466 doublings h
1
from 0.721 doublings h
1
. The
j continued to drop at higher concentrations from
0.466 doublings h
1
at 3.0 mg ml
1
to 0.369 doublings h
1
at 9.0 mg ml
1
. This dramatic decrease in j may be ex-
plained by observations in a previous study by Blake et al.
38 L. Rowe, G.T. Howard / International Biodeterioration & Biodegradation 50 (2002) 3340
Fig. 5. Detection of polyurethanase and lipase activities of puried protein by a radial diusion assay. (A) 1% Bayhydrol110}0.01% rhodamine B,
(B) 1% ImpranilT DLN, (C)1% tributyrin, (D) 1% triolein-0.01% rhodamine B. Puried protein (25 l) was pipetted into holes, and the plates were
incubated for 20 h at 30
C. The plate was photographed upon UV irradiation (350 nm).
(1998) that polyurethane was observed to accumulate on
the cell surface of a Bacillus sp. That study revealed that
the growth of the Bacillus sp. on a solid medium resulted
in the visual disappearance of the polyurethane. The com-
plexity of the bacteria-polyurethane interaction was more
apparent when grown on a polyurethane liquid medium.
Incubation of the Bacillus sp. in media supplemented with
polyurethane resulted in the appearance of a chalky pre-
cipitate that appeared to be resistant to further degradation.
Electrophoretic mobility, electrical impedance, and dy-
namic light diraction measurements were performed on
the Bacillus-polyurethane system. The data from the elec-
trophoretic mobility indicated that the Bacillus cells had a
relatively weak negative charge and the polyurethane had a
highly negative charge. Electrical impedance data showed
that on average the Bacillus cell had a volume of around
3.9 m
3
corresponding to a spherical equivalent diameter
of just over 2 m. The majority of the polyurethane parti-
cles were suciently small to be below the detection limit,
0.6 m, for electrical impedance. The relative volumes as
a function of size for polyurethane and Bacillus were de-
termined by static light diraction methods. The results
from the static light diraction methods veried that of the
electrical impedance results.
The above methods were then used to examine the for-
mation of a complex between Bacillus and polyurethane.
The electrophoretic mobility data showed that the peaks
that were associated with the free polyurethane and the
free Bacillus were replaced by a single peak that possessed
the size and charge properties anticipated for a complex
of the large Bacillus with the strongly negatively charge
polyurethane. This evidence was corroborated with elec-
trical impedance measurements that showed there was an
increase in the total volume of the Bacillus cells as a
function of time as they were mixed with an excess of
polyurethane. Evidence that the increase in cell size oc-
curred at the expense of the polyurethane came from light
diraction measurements. Further evidence that the Bacil-
lus cell forms a complex with polyurethane was obtained
through microscopic observations. These observations
showed that the majority of the cells in the presence of
polyurethane were coated with small particles of various
dimensions.
This evidence indicates that two populations exist in
polyurethane cultures: one that is coated with polyurethane
and one that is not. At lower concentrations of polyurethane,
it may be that the two populations of bacteria are de-
pendent on dierent sources of nutrition. The rst popu-
lation is coated with polyurethane and the polyurethane
is metabolized into small, soluble metabolites, which are
released into the medium. The second population, which
is not covered in polyurethane, uses the small, soluble
metabolites produced by the rst population to grow. At
higher concentrations of polyurethane all the cells present
in the media may be coated with polyurethane. The more
cells coated with polyurethane the more polyurethane
that is degraded and the more metabolites available
for growth. This would result in polyurethane-coated
cells, which are not free in solution and therefore not
detectable.
The puried polyurethanase enzyme from B. subtilis
has a molecular mass of 28 kDa, displayed esterase and
L. Rowe, G.T. Howard / International Biodeterioration & Biodegradation 50 (2002) 3340 39
lipase activity but not protease activity. In addition, the en-
zyme was not inhibited by PMSF, and was heat labile after
10 min at 100 C. The molecular weight is very similar to the
polyurethanase enzyme isolated from P. uorescens, which
had a molecular weight of 29 kDa, however this enzyme
from P. uorescens displayed protease activity (Howard
and Blake, 1998). Also, it was similar in molecular weight
to a polyurethanase enzyme from P. chlororaphis that had
a molecular weight of 31 kDa and displayed only esterase
activity (Ruiz et al., 1999). A polyurethanase enzyme has
also been isolated from Curvularia sengalensis that had a
molecular weight of 28 kDa and displayed esterase activity
(Crabbe et al., 1994). However, these enzymes are heat
stable and inhibited by PMSF.
Bacteria produce several dierent classes of lipases:
carboxylesterases (EC 3.1.1.1) which hydrolyze small
ester-containing molecules at least partly soluble in water,
true lipases (EC 3.1.1.3) which display maximum activity
towards water-insoluble long-chain triglycerides, and vari-
ous types of phospholipases. The true lipases (Family I li-
pases) are characterized by a conserved serine residue with
an active site that is involved in catalysis. The serine residue
usually appears in the conserved pentapeptide GlyXaa
SerXaaGly (Arpigny and Jaeger, 1999). Reaction of this
serine residue is inhibited by PMSF. Of the polyurethanase
enzymes that have been sequenced the serine hydrolase has
been conserved and follow the pattern that PMSF inhibits
the serine residue. Various Bacillus lipases are known that
also show the conserved serine residue, with the exception
that the rst glycine is replaced by alanine: AlaXaaSer
XaaGly. Two Bacillus species, B. subtilis and B. pumilus,
have been shown to be the smallest true lipases known
(Family VII lipases) and show very little sequence similar-
ity (approximately 15%) to other Bacillus species (Arpigny
and Jaeger, 1999). The esterase activity from this group of
lipases is active against phenyl carbamate hydrolysing the
central carbamate bond. In addition, the esterase was found
to eciently hydrolyse j-nitrobenzyl esters; however, this
protein has not been further characterized. The enzyme from
B. subtilis in this study is not inhibited by PMSF and there-
fore it is expected that the serine residue is not conserved.
Another unique aspect of the polyurethanase en-
zyme isolated from B. subtilis is its specic activity
for j-nitrophenyl-acetate. The specic activity of the
polyurethanase enzyme isolated from B. subtilis on
j-nitrophenyl-acetate was found to be 46.06 U ml
1
. This
is approximately 220 times higher in activity than that
noted for the other polyurethanase enzymes isolated.
The polyurethane-degrading enzyme isolated from B.
subtilis is similar to the enzymes previously isolated from
other organisms in some ways, while it diers in others.
The K
s
and j
max
values as well as the molecular mass is
shared with some of the enzymes isolated from the other
organism, but while it is not inhibited by PMSF and is heat
labile suggests that this enzyme may be a member of a
dierent family of lipases.
There are several reasons why the gene encoding a
polyurethanase was not isolated. First is use of restric-
tion enzymes. In this study, only two restriction enzymes
were used (EcoRI and BamHI). In the creation of a
shot-gun library, the chromosomal DNA is cut with a
restriction enzyme. The exact location of the restriction
enzyme recognition sites is not known. There is a pos-
sibility that the restriction enzyme cuts within the open
reading frame of the polyurethanase gene. If the re-
striction enzyme cuts within the open reading frame of
the gene, a functional enzyme will never be produced
from the fragments, and therefore cannot be detected
when screening the library. This is the simplest expla-
nation as to why the gene could not be isolated and
could easily be remedied with the use other restriction
enzymes.
Another reason that the gene could not be isolated in-
volves problems associated with trying to clone from a gram
(+) organism (B. subtilis) to a gram () organism (E. coli).
There are several problems associated with trying to clone
from gram (+) to a gram (). The rst is the secretion of
extra-cellular proteins. The cell membrane of a gram (+)
organism consists of one membrane, while the cell mem-
brane of a gram () organism consists of two membranes.
Each type of cell has a unique transport system to transport
proteins outside the membrane. The gram () host, E. coli,
may not have the appropriate transport proteins to transport
the gram (+), B. subtilis, protein outside of the cell. This
problem was addressed using the bacteriophage z. Chromo-
somal DNA fragments were inserted into the bacteriophage
z and then used to infect E. coli. The bacteriophage lysis the
cell, the proteins that were made are released from the cell,
and therefore transport of the protein out of the cell does not
have to occur. Therefore, this explanation is unlikely.
The third possibility for why the gene was not isolated
is that the gene could be located within a plasmid. The
chromosomal DNA was isolated and used to create the li-
brary, which does not include plasmid DNA not associated
with the chromosomal DNA. Not all specimen of B. sub-
tilis found have the ability to degrade polyurethane, which
would lead to the belief that the ability of this organism to
degrade polyurethane may have occurred through the ac-
quisition of a plasmid. In order to rectify this problem the
plasmid DNA would need to be isolated and screened for
the polyurethanase gene.
The nal reason why the gene might not be detected
is that the gene may have been isolated, but requires
post-translational chaperone proteins in order to become
a functional protein. In a study by Quyen et al. (1999)
it was observed that the overexpression of Pseudomonas
genes in E. coli was relatively easy, however the proteins
in the bacterial cytoplasm were found to be enzymati-
cally inactive. The inactivity of these enzymes is due to
the complex process of folding and secretion of these
lipase enzymes. The lipase enzymes require a special-
ized helper protein (Lip B) and the involvement of outer
40 L. Rowe, G.T. Howard / International Biodeterioration & Biodegradation 50 (2002) 3340
membrane secretion machinery proteins (Xcp proteins) to
mediate the secretion of these enzymes. It is possible that
the lipase gene was isolated from B. subtilis, but that the
expression of the functional enzyme requires additional
proteins.
Future research with B. subtilis will include attempts
to isolate the polyurethanase gene by reverse genetics,
determination of the sequence, and to identify any se-
quence similarities to other polyurethanase genes. In ad-
dition, further enzymatic studies are needed to further
classify the type of enzyme activity responsible for the
degradation of polyurethane. The purication and char-
acterization of the polyurethanase is important for future
research aimed at the development of new bioremediation
techniques.
Acknowledgements
This work was supported by a faculty development grant
fromSoutheastern Louisiana University, grant number 1264.
References
Allen, A., Hilliard, N., Howard, G.T., 1999. Purication and
characterization of a soluble polyurethane degrading enzyme
from Comamonos acidovorans. International Biodeterioration &
Biodegradation 43, 3741.
Arpigny, J.L., Jaeger, K.E., 1999. Bacterial lipolytic enzymes:
classication and properties. Biochemistry Journal 343, 177183.
Blake, R.C., Norton, W.N., Howard, G.T., 1998. Adherence and growth of
a Bacillus species on an insoluble polyester polyurethane. International
Biodeterioration & Biodegradation 42, 6373.
Crabbe, J.R., Campbell, J.R., Thompson, L., Walls, S.L., Schultz, W.W.,
1994. Biodegradation of a colloidal ester-based polyurethane by soil
fungi. International Biodeterioration & Biodegradation 33, 103113.
Deshpande, M.V., Eriksson, K.E., Peterson, L.G., 1984. An assay
for selective determination of exo-1,4-B-glucanases in a mixture of
cellulolytic enzymes. Analytical Biochemistry 138, 481487.
Hegedus, C.K., Pulley, D.F., Spadafora, S.J., Eng, A.T., Hirst, D.J., 1989.
A review of organic coating technology for U.S. naval aircraft. Journal
of Coatings Technology 61, 3142.
Howard, G.T., Blake, R.C., 1998. Growth of Pseudomonas uorescens on
a polyester-based polyurethane and the purication and characterization
of a polyurethanase-protease enzyme. International Biodeterioration &
Biodegradation 42, 2330.
Howard, G.T., Ruiz, C., Hilliard, N., 1999. Growth of Pseudomonas
chlororaphis on a polyester-polyurethane and the purication and
characterization of a polyurethanase-esterase enzyme. International
Biodeterioration & Biodegradation 43, 712.
Howard, G.T., Vicknair, J., Mackie, R.I., 2001. Sensitive plate assay for
screening and detection of bacterial polyurethanase activity. Letters in
Applied Microbiology 32, 211214.
Kalb, V.F., Bernlohr, R.W., 1977. A new spectrophotometric assay for
protein in cell extracts. Analytical Biochemistry 82, 362371.
Kawai, F., 1995. Breakdown of plastics and polymers by microorganisms.
Advances in Biochemical Engineering and Biotechnology 52, 151194.
Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly
of the head of bacteriophage T4. Nature (London) 227, 680685.
Marmur, J., 1961. A procedure for the isolation of deoxyribonucleic acid
from microorganisms. Journal of Molecular Biology 3, 208218.
Quyen, D.T., Schmidt-Dannert, C., Schmid, R.D., 1999. High-level
formation of active Pseudomonas cepacia lipase after heterologous
expression of the encoding gene and its modied chaperone
in Escherichia coli and rapid in vitro refolding. Applied and
Environmental Microbiology 65, 787794.
Reisch, M.S., 1990. Marine paint makers strive to meet environmental
concerns. Chemical and Engineering News 17, 3968.
Ruiz, C., Main, T., Hilliard, N., Howard, G.T., 1999. Purication and
characterization of two polyurethanase enzymes from Pseudomonas
chlororaphis. International Biodeterioration and Biodegradation 43,
4347.
Sambrook, J., Fritsch, E., Maniatis, T., 1989. Molecular Cloning: A
Laboratory Manual, 2nd Edition. Cold Spring Harbor Laboratory Press,
Cold Spring Harbor, NY.
Shannon, M.J.R., Unterman, R., 1993. Evaluating bioremediation:
Distinguishing fact from ction. Annual Reviews of Microbiology 47,
715738.
Ulrich, H., 1983. Polyurethane. In Modern Plastics Encyclopedia, Vol.
60(10a). McGraw-Hill, New York, NY, pp. 7684.
Vega, R.T., Main, T., Howard, G.T., 1999. Cloning and
expression in Escherichia coli of a polyurethane-degrading enzyme
from Pseudomonas uorescens. International Biodeterioration &
Biodegradation 43, 4955.
Você também pode gostar
- Catalytic Bleaching of Cotton - Thesis - Topalovic PDFDocumento195 páginasCatalytic Bleaching of Cotton - Thesis - Topalovic PDFeduardoaffre100% (1)
- Microbial Bioremediation: An Eco-Friendly Approach to Environmental Clean-UpTITLEDocumento30 páginasMicrobial Bioremediation: An Eco-Friendly Approach to Environmental Clean-UpTITLEMdhe asif alamAinda não há avaliações
- Practice Kinetics ProblemsDocumento1 páginaPractice Kinetics ProblemsClint Charles P. Brutas100% (1)
- AFLPDocumento38 páginasAFLPLeandro Silva100% (1)
- Numerical Methods For The Life Scientist PDFDocumento160 páginasNumerical Methods For The Life Scientist PDFAnonymous Zsi5ODm2PY100% (1)
- Lecture 08 - DNA Fingerprinting-1Documento76 páginasLecture 08 - DNA Fingerprinting-1Alkhair SangcopanAinda não há avaliações
- 2018 Book ENZYMESCatalysisKineticsAndMec PDFDocumento560 páginas2018 Book ENZYMESCatalysisKineticsAndMec PDFZainab K UjjainwalaAinda não há avaliações
- Downstream ProcessingDocumento5 páginasDownstream ProcessingLuis Hernandez AlvarezAinda não há avaliações
- USP Seminar - Fundamentals of Bioassay Practices 2014 PDFDocumento140 páginasUSP Seminar - Fundamentals of Bioassay Practices 2014 PDFnsk79in@gmail.comAinda não há avaliações
- Bio FilmsDocumento33 páginasBio FilmsALisaAinda não há avaliações
- Isolation and Structure Elucidation of Flavonoid From Leaves Extract of Balanites Aegyptiaca DelilDocumento4 páginasIsolation and Structure Elucidation of Flavonoid From Leaves Extract of Balanites Aegyptiaca Delilinternational journal of modern chemistry and applied scienceAinda não há avaliações
- Hohne DSCDocumento304 páginasHohne DSCHari Shankar100% (1)
- Modeling of The Bacterial Growth Curve.Documento7 páginasModeling of The Bacterial Growth Curve.Adrian Bermudez LoeraAinda não há avaliações
- Molecular Mechanisms of Insecticide ResistanceDocumento11 páginasMolecular Mechanisms of Insecticide ResistancejoharijalinasAinda não há avaliações
- Batangas State University ChE Course on Enzyme KineticsDocumento53 páginasBatangas State University ChE Course on Enzyme KineticsNasser Gemina PantaoAinda não há avaliações
- 44 - Mapping The Bonanza Geographies of Mining & Neoliberal Reform - BridgeDocumento17 páginas44 - Mapping The Bonanza Geographies of Mining & Neoliberal Reform - Bridgefedenacif6788Ainda não há avaliações
- Downstream ProcessDocumento40 páginasDownstream ProcessVamsi KrishnaAinda não há avaliações
- Downstream ProcessingDocumento21 páginasDownstream ProcessingNasir Ahmed YusufAinda não há avaliações
- Analysis of Variance - NominalDocumento33 páginasAnalysis of Variance - Nominalsalman672003Ainda não há avaliações
- Microalgae Cultivation in PBR. Independent Study and Familiarization With Topic.Documento25 páginasMicroalgae Cultivation in PBR. Independent Study and Familiarization With Topic.Núria ValAinda não há avaliações
- Relationships Between A Project Management Methodology and Project Succe...Documento16 páginasRelationships Between A Project Management Methodology and Project Succe...Carlos Andrés Erazo MuñozAinda não há avaliações
- Biochemistry Kinetics Problems and GraphsDocumento6 páginasBiochemistry Kinetics Problems and GraphsDr-Dalya Shakir0% (1)
- Jespersen Lone 201710 PHD PDFDocumento173 páginasJespersen Lone 201710 PHD PDFDasayu ConsultingAinda não há avaliações
- Bacterial degradation of PCBs by isolates from contaminated sitesDocumento8 páginasBacterial degradation of PCBs by isolates from contaminated sitesNabila Agnasia DesmaraAinda não há avaliações
- Polychlorinated Biphenyls and Their BiodegradationDocumento15 páginasPolychlorinated Biphenyls and Their BiodegradationTrinh Quang ThanhAinda não há avaliações
- Degradation of Polychlorinated Biphenyls (PCBS) by Sthaphylococcus Xylosus in Liquid Media and Meat MixtureDocumento8 páginasDegradation of Polychlorinated Biphenyls (PCBS) by Sthaphylococcus Xylosus in Liquid Media and Meat MixtureCarlos CamargoAinda não há avaliações
- Read Me GenAlEx 6.41Documento10 páginasRead Me GenAlEx 6.41ParkAinda não há avaliações
- Usp 1035 - Biological Indicators For SterilizationDocumento4 páginasUsp 1035 - Biological Indicators For SterilizationoktaAinda não há avaliações
- Seroprevalence of Brucellosis in Goats Around HargeisaDocumento50 páginasSeroprevalence of Brucellosis in Goats Around HargeisaAbdiAinda não há avaliações
- Coller Protocol BookDocumento120 páginasColler Protocol BookEAPAinda não há avaliações
- SDS PageDocumento5 páginasSDS Pageamit545Ainda não há avaliações
- QF-PCR March 09Documento10 páginasQF-PCR March 09Phạm Quốc ToảnAinda não há avaliações
- Violations of OLSDocumento64 páginasViolations of OLSOisín Ó CionaoithAinda não há avaliações
- Art WALTON Quality Work LifeDocumento10 páginasArt WALTON Quality Work Liferosegoncalves27Ainda não há avaliações
- The Role of Microorganisms in Bioremediation-A ReviewDocumento9 páginasThe Role of Microorganisms in Bioremediation-A ReviewKashmeera Geethanjali AnirudhanAinda não há avaliações
- 2D Gel Electrophoresis Training: Proteomics and Mass Spectrometry FacilityDocumento12 páginas2D Gel Electrophoresis Training: Proteomics and Mass Spectrometry FacilityEli HeberAinda não há avaliações
- Algorithms for Sequence AlignmentDocumento37 páginasAlgorithms for Sequence AlignmentKrishnaAinda não há avaliações
- Conversion RPM G CentrifugaDocumento1 páginaConversion RPM G CentrifugaEsaú E RodriguezAinda não há avaliações
- Géstion Des DéchetsDocumento11 páginasGéstion Des DéchetsNoura AzaouagAinda não há avaliações
- By Alphonse Meyer, José Deiana, Alain Bernard - Cours de Microbiologie Générale PDFDocumento452 páginasBy Alphonse Meyer, José Deiana, Alain Bernard - Cours de Microbiologie Générale PDFBella ImèneAinda não há avaliações
- Analytical Methods For Therapeutic Drug Monitoring and ToxicologyDocumento34 páginasAnalytical Methods For Therapeutic Drug Monitoring and ToxicologyAbdul ManafAinda não há avaliações
- Tbars (Tca Method)Documento15 páginasTbars (Tca Method)Ho Yoke MeiAinda não há avaliações
- HFJHDocumento7 páginasHFJHMelly Fitriany SyamAinda não há avaliações
- Embryonic Stem Cells - Basic Biology To Bioengineering - 2011Documento490 páginasEmbryonic Stem Cells - Basic Biology To Bioengineering - 2011zamfirescu68Ainda não há avaliações
- Organophosphorus Insecticide Induced Hemorrhagic Pancreatitis - A Case ReportDocumento3 páginasOrganophosphorus Insecticide Induced Hemorrhagic Pancreatitis - A Case ReportIOSRjournalAinda não há avaliações
- Microbial Bioremediation and Bioreactor Design PDFDocumento20 páginasMicrobial Bioremediation and Bioreactor Design PDFLISETH JOHANA CHACON MOLINAAinda não há avaliações
- Systematic Bacteriology: The and The Deeply Branching and PhototrophicDocumento18 páginasSystematic Bacteriology: The and The Deeply Branching and PhototrophicMuhammad JamalAinda não há avaliações
- Transgenic CropsDocumento13 páginasTransgenic Cropsrana shahid100% (1)
- Tbars AssayDocumento13 páginasTbars AssaySHIVAGOVINDANAinda não há avaliações
- NEW Annex 7 Qualification of Mass Spectrometers PDFDocumento11 páginasNEW Annex 7 Qualification of Mass Spectrometers PDFParkhomyukAinda não há avaliações
- Amara SamiraDocumento35 páginasAmara SamiraHassinaAinda não há avaliações
- 1 Surfactant Chemistry and General Phase BehaviourDocumento10 páginas1 Surfactant Chemistry and General Phase BehaviourAnonymous cgKtuWzAinda não há avaliações
- Multiplication in Vitro Du Palmier Dattier (Phoenix Dactylifera L.) Par Bourgeonnement - Khalid SETTINIDocumento30 páginasMultiplication in Vitro Du Palmier Dattier (Phoenix Dactylifera L.) Par Bourgeonnement - Khalid SETTINIstudio saidAinda não há avaliações
- BioremediationDocumento23 páginasBioremediationAmit SharmaAinda não há avaliações
- 1 Ahamd Sarki GumelDocumento11 páginas1 Ahamd Sarki GumelAhmad Mohammed GumelAinda não há avaliações
- Heart Disease Prediction by Support Vector Machine ClassifierDocumento44 páginasHeart Disease Prediction by Support Vector Machine ClassifierBDE AK UM6PAinda não há avaliações
- Guidelines for biostimulant registrationDocumento6 páginasGuidelines for biostimulant registrationbabji dudekulaAinda não há avaliações
- Ethanol From Molasses ToolDocumento31 páginasEthanol From Molasses ToolfarahnazAinda não há avaliações
- Protocol BO16348FDocumento116 páginasProtocol BO16348FCarmina KvietkauskasAinda não há avaliações
- Detection of Integrons in Multidrug Resistant Wound IsolatesDocumento7 páginasDetection of Integrons in Multidrug Resistant Wound IsolatesEditor IJTSRDAinda não há avaliações
- TCA Precipitation ProtocolDocumento1 páginaTCA Precipitation ProtocolMuhammad ShabirAinda não há avaliações
- Enjeux européens et mondiaux de la protection des données personnellesNo EverandEnjeux européens et mondiaux de la protection des données personnellesAinda não há avaliações
- PositiveThinkingCase Rio Tinto HSEDocumento12 páginasPositiveThinkingCase Rio Tinto HSEJLChiaAinda não há avaliações
- Factors Determining Immunogenicity and ToleranceDocumento33 páginasFactors Determining Immunogenicity and ToleranceVegan IstaAinda não há avaliações
- Appl. Environ. Microbiol. 2011 Russell 6076 84Documento9 páginasAppl. Environ. Microbiol. 2011 Russell 6076 84Anonymous o6nw0jMgnAinda não há avaliações
- Biodegradation of Polyester Polyurethane by Endophytic FungiDocumento9 páginasBiodegradation of Polyester Polyurethane by Endophytic FungiJuan Pablo Unfried HuertasAinda não há avaliações
- JB 000981Documento10 páginasJB 000981Francisco FalconAinda não há avaliações
- Lecture 4Documento33 páginasLecture 4Francisco FalconAinda não há avaliações
- Central Difference Approximation of DerivativesDocumento13 páginasCentral Difference Approximation of DerivativesFrancisco FalconAinda não há avaliações
- 2003 - Biodiesel Production From Waste Cooking Oil - ProcessDocumento16 páginas2003 - Biodiesel Production From Waste Cooking Oil - ProcessKaoutar SefriouiAinda não há avaliações
- Course Outline BIO091 20202021 and Lesson N AssessmentDocumento13 páginasCourse Outline BIO091 20202021 and Lesson N AssessmentkenyuutaAinda não há avaliações
- Porcine Heart Lactate DehydrogenaseDocumento7 páginasPorcine Heart Lactate DehydrogenaseOuwehandAinda não há avaliações
- Biotransformation and Bioconversion of Phenolic CompoundsDocumento7 páginasBiotransformation and Bioconversion of Phenolic CompoundsDark-ObsessionsAinda não há avaliações
- Allosteric regulation overviewDocumento7 páginasAllosteric regulation overviewANIS NURALYSA AFFENDIAinda não há avaliações
- MSC Orgchem Syllabus PVSL 2017 18 For Website PDFDocumento41 páginasMSC Orgchem Syllabus PVSL 2017 18 For Website PDFLucky SiddiqueAinda não há avaliações
- Prolyl Endopeptidase From Aspergillus Niger Immobilized On A Food-GradeDocumento7 páginasProlyl Endopeptidase From Aspergillus Niger Immobilized On A Food-GradeCris LopesAinda não há avaliações
- Hydrometallurgy: Hajime Miki, Michael NicolDocumento5 páginasHydrometallurgy: Hajime Miki, Michael NicolOscar Salazar MorenoAinda não há avaliações
- Xu2010 PDFDocumento10 páginasXu2010 PDFIndah WulansariAinda não há avaliações
- Han, Levenspiel - 1988 - Extended Monod Kinetics For Substrate, Product, and Cell Inhibition-AnnotatedDocumento8 páginasHan, Levenspiel - 1988 - Extended Monod Kinetics For Substrate, Product, and Cell Inhibition-AnnotatedMarisol Muñoz PonceAinda não há avaliações
- Sylabus For Enzyme TechnologyDocumento2 páginasSylabus For Enzyme TechnologyBalew GetaAinda não há avaliações
- 1 s2.0 S000634951830208X Main PDFDocumento11 páginas1 s2.0 S000634951830208X Main PDFBrayan JimenezAinda não há avaliações
- Comprehensive Exam Syll Rev 2010 IITDDocumento5 páginasComprehensive Exam Syll Rev 2010 IITDswjaiswalAinda não há avaliações
- China Paper Supporting DataDocumento84 páginasChina Paper Supporting DataRAVI KUMAR AkulaAinda não há avaliações
- Enzyme Kinetics ExplainedDocumento43 páginasEnzyme Kinetics ExplainedBlessings Chawinga100% (1)
- Unit 18 Enzyme KineticsDocumento12 páginasUnit 18 Enzyme KineticsBethelAinda não há avaliações
- 09931275D L265 UV Lab Software Users GuideDocumento127 páginas09931275D L265 UV Lab Software Users GuideAbdul KalimAinda não há avaliações
- Problems in Biochemical EngineeringDocumento22 páginasProblems in Biochemical EngineeringAdu GilbertAinda não há avaliações
- ContinueDocumento3 páginasContinueJúÑi ØrAinda não há avaliações
- Book & Media Reviews: by Paul L. HoustonDocumento1 páginaBook & Media Reviews: by Paul L. HoustonKarthikAinda não há avaliações
- LSM1101 Enzyme2Documento40 páginasLSM1101 Enzyme2givena2ndchanceAinda não há avaliações
- Biological Reaction Engineering - DunnDocumento521 páginasBiological Reaction Engineering - DunnCarlos Royo PascualAinda não há avaliações
- Palaganas Hw2 Che 197Documento5 páginasPalaganas Hw2 Che 197Elah Palaganas100% (1)
- Chemical Kinetics: Practice ExamplesDocumento31 páginasChemical Kinetics: Practice ExamplesJudith Del Valle MorejonAinda não há avaliações