Escolar Documentos
Profissional Documentos
Cultura Documentos
Hall Yarborough
Enviado por
Lawrence MbahTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Hall Yarborough
Enviado por
Lawrence MbahDireitos autorais:
Formatos disponíveis
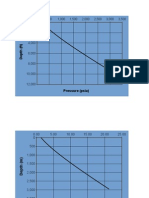
Hall-Yarborough (1973) z-factor calculation
Hall, K.R. & Yarborough, L. (1973): A New Euation of State for Z-factor Calculations,
The Oil and Gas Journal, June 18, 82-92
Molecular weight oxygen, MO 15.9994 g/mole
Molecular weight sulfur, MS 32.07 g/mole
Molecular weight, carbon, MC 12.01 g/mole
Molecular weight, hydrogen, MH 1.01 g/mole
Molecular weight air, MA 28.97 g/mole
Components Molecular weight Mole fraction Tci Tci Pci Pci
g/mole yi oR K psia Mpa
Methan, CH4 16.042 0 343 190 667.8 4.61
Ethan, C2H6 30.07 0 549.8 305 708 4.88
Propan, C3H8 44.10 0 665.7 370 616 4.25
i-Butane, C4H10 58.12 0 734.7 408 529 3.65
n-Butane, C4H10 58.12 0 765 425 551 3.80
i-Pentane C5H12 72.15 0 829 460 491 3.39
n-Pentane C5H12 72.15 0 845 469 489 3.37
Hexane C6H14 86.18 0 913 507 437 3.01
Heptane C7H16 100.21 0 972 540 397 2.74
Hydogen, H2 2.02 0 60 33 187 1.29
Nitrogen, N2 28.01 0 227.4 126 492 3.39
Oxygen, O2 32.00 0 277.8 154 731 5.04
Carbon dioxid, CO2 44.01 0 547.6 304 1071 7.38
Hydrogensulfid, H2S 34.08 0 672.4 373 1306 9.01
Dihydrogenoksid, H2O 18.02 0 1165 647 3199 22.06
Mole fraction 0.0000
Total molecular weight gas 0.00 g/mole
Spesific gravity 0.00
Temperature 0.00
o
C 273.15 K
Pressure 0.000E+00 Pa
Method used (1, 2 or 3) 1
Compressebility factor, z #DIV/0!
Method 1 (Properties from composition, Key's rule)
Pseudo critical temperature
TPc (oil field units) 0.00 oR
TPc (SI units) 0.00 K
Pseudo critical pressure
PPc (oil field units) 0.00 psia
Methods for finding pseudo critical pressure and
pseudo critical temperature
=
i i
y P PPc
=
i i
y T TPc
PPc (SI units) 000.0E+0 Pa
Method 2 (Sutton's correlations)
Pseudo critical temperature
TPc (oil field units) 169.20 oR
TPc (SI units) 93.67 K
Pseudo critical pressure
PPc (oil field units) 756.80 psia
PPc (SI units) 5.2E+6 Pa
Method 3 (Standing's correlation)
Pseudo critical temperature If we have a "dry" gas, SG < 0,75
TPc (oil field units) 168.00 oR
TPc (SI units) 93.00 K
Pseudo critical pressure
PPc (oil field units) 667.00 psia
PPc (SI units) 4.6E+6 Pa
Pseudo reduced properties
TPR #DIV/0!
PPR #DIV/0!
Hall-Yarborough
t #DIV/0!
a #DIV/0!
Reduced-density parameter, y
0
0.01
Continue until f(y) < 1x10^(-5)
Iteration 1
f(y) #DIV/0!
Derivated of f(y), df(y) #DIV/0!
Newton Rapson: Reduced- density parameter, y
1
#DIV/0!
Compressebility factor, z #DIV/0!
Iteration 2
( )
( ) y f
y f
y y
i i
'
1
=
+
( )
( )
( ) ( )( )
t
y t t t t y t t t
y
y y y y
dy
y df
82 , 2 18 , 1 3 2 3 2
4
4 3 2
4 , 42 2 , 242 7 , 90 82 , 2 18 , 2 16 , 9 52 , 19 52 , 29
1
4 4 4 1
+
+ + + +
+ + +
=
( )
( )
( ) ( )
t
pr
y t t t y t t t
y
y y y y
ap y f
82 , 2 18 , 2 3 2 2 3 2
3
4 3 2
4 , 42 2 , 242 7 , 90 58 , 4 76 , 9 76 , 14
1
0
+
+ + +
+ +
+ = =
pr
T
t
1
=
pc
pr
p
p
P =
pc
pr
T
T
T =
2
g
3.6 - 131,0 - 756.8 = PPc
g
2
g g
74,0 - 349.5 + 169.2 = TPc
2
5 , 12 325 168
gHC gHC pcHC
T + =
2
5 , 37 0 , 15 667
gHC gHC pcHC
p + =
y
p
Z
pr
o
=
( ) ( )
2
1 2 , 1
06125 , 0
t
e t a
=
f(y) #DIV/0!
Derivated of f(y), df(y) #DIV/0!
Newton Rapson: Reduced-density parameter y
2
#DIV/0!
Compressebility factor, z #DIV/0!
Iteration 3
fy #DIV/0!
Derivated of f(y), df(y) #DIV/0!
Newton Rapson: Reduced-desity parameter y
3
#DIV/0!
Compressebility factor, z #DIV/0!
Iteration 4
f(y) #DIV/0!
Derivated of f(y), df(y) #DIV/0!
Newton Rapson: Reduced-density parameter y
4
#DIV/0!
Compressebility factor, z #DIV/0!
Iteration 5
f(y) #DIV/0!
Derivated of f(y), df(y) #DIV/0!
Newton Rapson: Reduced-density parameter y
4
#DIV/0!
Compressebility factor, z #DIV/0!
( )
( ) y f
y f
y y
i i
'
1
=
+
Convertion
oC -> K 273.15
atm -> bar 1.01325
cp -> Pas 1.00E-03
bar -> Pa 1.00E+05
m m -> m 1.00E-03
oR -> K 0.56
psig -> bar 0.07
oF -> oR 460
Instructions
1. Insert mole fractions.
2. Insert temperature [C] and pressure [Pa].
3. Choose which pseudo method you will use.
4. Read your z-factor beneath.
(Insert values in cells where the font color is red)
Methods for finding pseudo critical pressure and
pseudo critical temperature
1. Properties from composition, Key's rule (Preferred choice).
Newton-Rapson iteration.
2. Sutton's correlations
3. Standing's correlations
If we have a "dry" gas, SG < 0,75 If we have a "wet" gas, SG 0,75
( )
( )
( ) ( )( )
t
y t t t t y t t t
y
y y y y
dy
y df
82 , 2 18 , 1 3 2 3 2
4
4 3 2
4 , 42 2 , 242 7 , 90 82 , 2 18 , 2 16 , 9 52 , 19 52 , 29
1
4 4 4 1
+
+ + + +
+ + +
=
( )
( )
( ) ( )
t
pr
y t t t y t t t
y
y y y y
ap y f
82 , 2 18 , 2 3 2 2 3 2
3
4 3 2
4 , 42 2 , 242 7 , 90 58 , 4 76 , 9 76 , 14
1
0
+
+ + +
+ +
+ = =
2
g
3.6 - 131,0 - 756.8 = PPc
g
2
g g
74,0 - 349.5 + 169.2 = TPc
2
5 , 12 325 168
gHC gHC pcHC
T + =
2
5 , 37 0 , 15 667
gHC gHC pcHC
p + =
2
5 , 71 330 187
gHC gHC pcHC
T + =
2
1 , 11 7 , 51 706
gHC gHC pcHC
p + =
( ) ( )
2
1 2 , 1
06125 , 0
t
e t a
=
( )
( )
( ) ( )( )
t
y t t t t y t t t
y
y y y y
dy
y df
82 , 2 18 , 1 3 2 3 2
4
4 3 2
4 , 42 2 , 242 7 , 90 82 , 2 18 , 2 16 , 9 52 , 19 52 , 29
1
4 4 4 1
+
+ + + +
+ + +
=
( )
( )
( ) ( )
t
pr
y t t t y t t t
y
y y y y
ap y f
82 , 2 18 , 2 3 2 2 3 2
3
4 3 2
4 , 42 2 , 242 7 , 90 58 , 4 76 , 9 76 , 14
1
0
+
+ + +
+ +
+ = =
Você também pode gostar
- Calculate Bottom Hole Pressure With The Cullender and Smith MethodDocumento2 páginasCalculate Bottom Hole Pressure With The Cullender and Smith Methodprateek_bhoirAinda não há avaliações
- Bottom Hole Nodal GasDocumento7 páginasBottom Hole Nodal Gasarjun2014Ainda não há avaliações
- Lab 5 Reservoir Fluid StudiesDocumento10 páginasLab 5 Reservoir Fluid StudiesIrwan JanuarAinda não há avaliações
- Esp PVT PropertiesDocumento17 páginasEsp PVT Propertieslutfi awnAinda não há avaliações
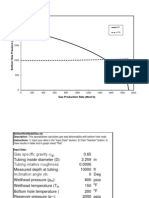
- Gas Production Rate (MSCF/D) : IPR TPRDocumento5 páginasGas Production Rate (MSCF/D) : IPR TPRShakerMahmoodAinda não há avaliações
- Well Head NodalDocumento7 páginasWell Head Nodalmath62210Ainda não há avaliações
- Separator Design: Rev Description Date Prepared by Client ApprovalDocumento18 páginasSeparator Design: Rev Description Date Prepared by Client ApprovalUsɱâñ Måâñ100% (1)
- Ees ExampleDocumento12 páginasEes ExamplesbjAinda não há avaliações
- Bha#44 SMG-X2 ST3 20211009Documento65 páginasBha#44 SMG-X2 ST3 20211009Jose Miguel Vaca CharriasAinda não há avaliações
- Average TZDocumento6 páginasAverage TZvictor javier nuñezAinda não há avaliações
- Gas Down Choke Pressure - Upstream Pressure at Choke For Dry GasesDocumento2 páginasGas Down Choke Pressure - Upstream Pressure at Choke For Dry GasesKALESANG LA BODOAinda não há avaliações
- University of Tripoli Faculty of Engineering Petroleum EngineeringDocumento8 páginasUniversity of Tripoli Faculty of Engineering Petroleum EngineeringRoba SaidAinda não há avaliações
- Mathcad - Gorilla VII Mud Pump CalcsDocumento2 páginasMathcad - Gorilla VII Mud Pump CalcsMohamed SaeedAinda não há avaliações
- Mistflow - Xls Tubing Performance Relationship (TPR) Input Data: InstructionsDocumento1 páginaMistflow - Xls Tubing Performance Relationship (TPR) Input Data: Instructionsmath62210Ainda não há avaliações
- Well Test Analysis - Math + Data + Python CodeDocumento10 páginasWell Test Analysis - Math + Data + Python CodeAllah bakhsh BakhshAinda não há avaliações
- CDAMnemonicsDocumento18 páginasCDAMnemonicsvanthodcAinda não há avaliações
- Hagedorn Brown CorrelationDocumento14 páginasHagedorn Brown CorrelationRoyanAinda não há avaliações
- Pit Volume CalculatorDocumento24 páginasPit Volume CalculatorHunterAinda não há avaliações
- Well Head NodalDocumento7 páginasWell Head NodalMbarouk Shaame MbaroukAinda não há avaliações
- p.2 Basic Drilling Engineering EquationsDocumento2 páginasp.2 Basic Drilling Engineering Equationsnasir.hdip8468Ainda não há avaliações
- Theoretical DeliverabilityDocumento197 páginasTheoretical Deliverabilitymath62210Ainda não há avaliações
- Turnerloading - XLS: Step 1: Update Input Data in Blue Step 2: View ResultsDocumento2 páginasTurnerloading - XLS: Step 1: Update Input Data in Blue Step 2: View Resultsmath62210Ainda não há avaliações
- 3-Phase SeparatorDocumento6 páginas3-Phase SeparatorToniAinda não há avaliações
- Manual Endress-Hauser Proline Promag P 300 ProfibusPADocumento220 páginasManual Endress-Hauser Proline Promag P 300 ProfibusPAJhon Sanchez ChAinda não há avaliações
- Esp PVT-PropertiesDocumento17 páginasEsp PVT-PropertiesJSN179Ainda não há avaliações
- Pump RateDocumento5 páginasPump RateRonald LlerenaAinda não há avaliações
- 15 Survey Calculations - QuartzDocumento2 páginas15 Survey Calculations - QuartzRebarAinda não há avaliações
- Cullender-Smithbhp - Xls Description: This Spreadsheet Calculates Bottom Hole Pressure InstructionsDocumento2 páginasCullender-Smithbhp - Xls Description: This Spreadsheet Calculates Bottom Hole Pressure InstructionsTamer Hesham AhmedAinda não há avaliações
- Drilling Formulas Calculation Shee V1Documento134 páginasDrilling Formulas Calculation Shee V1timz_upAinda não há avaliações
- Step-1: Well Dataset Depth LOT PoDocumento8 páginasStep-1: Well Dataset Depth LOT Ponikhil_barshettiwatAinda não há avaliações
- HIDRAULICA MPJ9-08 24 - 11 - 17 - Hoyo 16'' at 550'Documento1 páginaHIDRAULICA MPJ9-08 24 - 11 - 17 - Hoyo 16'' at 550'alfonzitoAinda não há avaliações
- Air Compressors 1170cfm T4Documento2 páginasAir Compressors 1170cfm T4death666darkAinda não há avaliações
- Hydril 523™ PDFDocumento4 páginasHydril 523™ PDFrenatoAinda não há avaliações
- 9.625 CSG TallyDocumento2 páginas9.625 CSG TallyAdeel Safdar NenseyAinda não há avaliações
- Model A Hydro TripDocumento1 páginaModel A Hydro TripFernando OlaveoAinda não há avaliações
- Gas Viscosity CalculationDocumento2 páginasGas Viscosity CalculationgrabettyAinda não há avaliações
- Bottom Hole Nodal GasDocumento6 páginasBottom Hole Nodal GasJhan GavilanAinda não há avaliações
- Api Casing Design: Design Parameters Loads at A DepthDocumento2 páginasApi Casing Design: Design Parameters Loads at A DepthChak AlGhazelAinda não há avaliações
- Phyllanthus NiruriDocumento6 páginasPhyllanthus NiruriQilah KamarudinAinda não há avaliações
- ADVANTAGE Hydraulics Spreadsheet Report: Case - RomaniaDocumento1 páginaADVANTAGE Hydraulics Spreadsheet Report: Case - RomaniatibismtxAinda não há avaliações
- pR6-40-10-4 HPDocumento2 páginaspR6-40-10-4 HPS.DharanipathyAinda não há avaliações
- Petrowiki Pressure Drop EquationsDocumento14 páginasPetrowiki Pressure Drop Equationsrasnowmah2012Ainda não há avaliações
- Students Industrial Work Experience Scheme (Siwes)Documento46 páginasStudents Industrial Work Experience Scheme (Siwes)Vanessa Ada ElokaAinda não há avaliações
- GasLiftValveDesign SI UnitsDocumento15 páginasGasLiftValveDesign SI UnitsnvnvAinda não há avaliações
- WellheadNodalGas SonicFlowDocumento7 páginasWellheadNodalGas SonicFlowthe_soldier_15_1Ainda não há avaliações
- Tally Bha #10 ActualizadoDocumento6 páginasTally Bha #10 ActualizadoGabriel DánielAinda não há avaliações
- Retention TimeDocumento10 páginasRetention TimealexnomitaAinda não há avaliações
- Tech Drilling FractureGradDocumento43 páginasTech Drilling FractureGradHakan ÖzkaraAinda não há avaliações
- P&a Cmt. Calcu.Documento2 páginasP&a Cmt. Calcu.francisryan4519Ainda não há avaliações
- Bha# 4 Fishing With Spear Assy 1227 Grapple 3032Documento1 páginaBha# 4 Fishing With Spear Assy 1227 Grapple 3032Ivan Dario Benavides BonillaAinda não há avaliações
- Pos North1 - Basic WCR Rev0Documento874 páginasPos North1 - Basic WCR Rev0Felipe RamírezAinda não há avaliações
- Type Curve Analysis: Sameer Bakshi Ee (Reservoir) Ankleshwar AssetDocumento17 páginasType Curve Analysis: Sameer Bakshi Ee (Reservoir) Ankleshwar Assetsameer bakshiAinda não há avaliações
- Underbalance PerforatingDocumento4 páginasUnderbalance Perforatingfrancisryan4519Ainda não há avaliações
- Alat-Alat Lab TeklingDocumento159 páginasAlat-Alat Lab Teklingaufal RiswanAinda não há avaliações
- Hydril Mac IIDocumento4 páginasHydril Mac IIFabian Romero BecerraAinda não há avaliações
- Overview of The Drilling Process and Rig SystemsDocumento3 páginasOverview of The Drilling Process and Rig Systemsعلي عباس جاسم غليم100% (1)
- Calculation of The Total Drilling Cost For The Well 112-87Documento5 páginasCalculation of The Total Drilling Cost For The Well 112-87Shaho Abdulqader Mohamedali100% (1)
- Yokie Suryo Prayogo - ESP DesignDocumento2 páginasYokie Suryo Prayogo - ESP DesignYokie PrayogoAinda não há avaliações
- Solution To HW#1Documento7 páginasSolution To HW#1Elizabeth LeeAinda não há avaliações
- Moran 7ed Therm Eq SP11 - RMDocumento6 páginasMoran 7ed Therm Eq SP11 - RMNathan AndersenAinda não há avaliações
- Spe 936177 G PDFDocumento10 páginasSpe 936177 G PDFsanty222Ainda não há avaliações
- Tatiana: The International Yacht CompanyDocumento23 páginasTatiana: The International Yacht CompanyLawrence MbahAinda não há avaliações
- Water Shutoff - Resin - IGPDocumento10 páginasWater Shutoff - Resin - IGPLawrence MbahAinda não há avaliações
- Completion of Acquisition of 100% Equity Stake in Enyo Retail and Supply LimitedDocumento1 páginaCompletion of Acquisition of 100% Equity Stake in Enyo Retail and Supply LimitedLawrence MbahAinda não há avaliações
- FCMB 20211231Documento1 páginaFCMB 20211231Lawrence MbahAinda não há avaliações
- SPE-188704-MS Coral South FLNG Technology From Screening To Real ApplicationDocumento11 páginasSPE-188704-MS Coral South FLNG Technology From Screening To Real ApplicationLawrence MbahAinda não há avaliações
- 65 Notification of Insider Dealing - Mr. OtedolaDocumento1 página65 Notification of Insider Dealing - Mr. OtedolaLawrence MbahAinda não há avaliações
- AFEX Weekly Commodities Report Nov 15 - Nov 19, 2021Documento5 páginasAFEX Weekly Commodities Report Nov 15 - Nov 19, 2021Lawrence MbahAinda não há avaliações
- SPE-184785-MS Subsea Coiled Tubing Acid Stimulation Operation Using CT Tractor in AngolaDocumento7 páginasSPE-184785-MS Subsea Coiled Tubing Acid Stimulation Operation Using CT Tractor in AngolaLawrence MbahAinda não há avaliações
- SPE-173659-MS Performing Riserless Subsea Operations Utilizing Coiled Tubing in Open WaterDocumento9 páginasSPE-173659-MS Performing Riserless Subsea Operations Utilizing Coiled Tubing in Open WaterLawrence MbahAinda não há avaliações
- SPE-120772-MS Acid Tunneling Stimulation in Oklahoma Limestone Using Coiled TubingDocumento3 páginasSPE-120772-MS Acid Tunneling Stimulation in Oklahoma Limestone Using Coiled TubingLawrence MbahAinda não há avaliações
- SPE 81731 Is Acid Placement Through Coiled Tubing Better Than Bullheading?Documento5 páginasSPE 81731 Is Acid Placement Through Coiled Tubing Better Than Bullheading?Adri SyawalAinda não há avaliações
- Domestic Fund Transfer: Transaction DetailsDocumento1 páginaDomestic Fund Transfer: Transaction DetailsLawrence MbahAinda não há avaliações
- Elon Musks Next MoveDocumento15 páginasElon Musks Next MoveLawrence MbahAinda não há avaliações
- Tutorial 2 - Pentland Field (Full Answer)Documento13 páginasTutorial 2 - Pentland Field (Full Answer)Lawrence MbahAinda não há avaliações
- Tutorial Juniper Field (Decision Tree & Value of Information) Questions (JFQ)Documento2 páginasTutorial Juniper Field (Decision Tree & Value of Information) Questions (JFQ)Lawrence MbahAinda não há avaliações
- Tutorial 5 - Juniper Field (Answer)Documento4 páginasTutorial 5 - Juniper Field (Answer)Lawrence MbahAinda não há avaliações
- Mindfulness: Presented by Joshua Green, M.S. Doctoral Intern at Umaine Counseling CenterDocumento12 páginasMindfulness: Presented by Joshua Green, M.S. Doctoral Intern at Umaine Counseling CenterLawrence MbahAinda não há avaliações
- Tutorial 4 - Lobster Field (Answer)Documento5 páginasTutorial 4 - Lobster Field (Answer)Lawrence MbahAinda não há avaliações
- Tutorial 2 - Pentland Field Cashflow MatrixDocumento3 páginasTutorial 2 - Pentland Field Cashflow MatrixLawrence MbahAinda não há avaliações
- 1 ProductivityDocumento12 páginas1 ProductivityLawrence MbahAinda não há avaliações
- Tutorial Lobster Field (Acceleration Project) Questions (LFQ)Documento1 páginaTutorial Lobster Field (Acceleration Project) Questions (LFQ)Lawrence MbahAinda não há avaliações
- GTL Registrars e Dividend Mandate FormDocumento1 páginaGTL Registrars e Dividend Mandate FormPrecious EsuaAinda não há avaliações
- New Volumetric SDocumento11 páginasNew Volumetric SLawrence MbahAinda não há avaliações
- Reservoir Production FundamentDocumento209 páginasReservoir Production FundamentLawrence MbahAinda não há avaliações
- Greg Goode The Direct PathDocumento40 páginasGreg Goode The Direct PathJack Zap72% (18)
- Causes of Low Well Productivity Wellbore and or Reservoir IssuesDocumento46 páginasCauses of Low Well Productivity Wellbore and or Reservoir IssuesLawrence MbahAinda não há avaliações
- Production OptimizationDocumento36 páginasProduction OptimizationLawrence MbahAinda não há avaliações
- Perforating BasicsDocumento64 páginasPerforating BasicsLawrence Mbah100% (1)
- Casing Leakage MonitoringDocumento6 páginasCasing Leakage MonitoringLawrence MbahAinda não há avaliações
- Math 138 Functional Analysis Notes PDFDocumento159 páginasMath 138 Functional Analysis Notes PDFAidan HolwerdaAinda não há avaliações
- Grade Sheet BlankDocumento50 páginasGrade Sheet BlankCarlo Troy AcelottAinda não há avaliações
- Intro Adobe Photoshop HandoutDocumento13 páginasIntro Adobe Photoshop Handoutoyindamola ayobamiAinda não há avaliações
- PIA B2 - Module 2 (PHYSICS) SubModule 2.2 (Mechanics) FinalDocumento82 páginasPIA B2 - Module 2 (PHYSICS) SubModule 2.2 (Mechanics) Finalsamarrana1234679Ainda não há avaliações
- Ma 2266 - Statistics and Numerical Methods April - May 2011Documento5 páginasMa 2266 - Statistics and Numerical Methods April - May 2011Rahul singhAinda não há avaliações
- Shear Force & Bending Moment TestDocumento11 páginasShear Force & Bending Moment TestKalaiArasanAinda não há avaliações
- Pro ESEDocumento2 páginasPro ESEquadhirababilAinda não há avaliações
- About The MS Regression ModelsDocumento17 páginasAbout The MS Regression ModelsLars LarsonAinda não há avaliações
- Radioddity DMR Programming Tips - (EN+DE)Documento35 páginasRadioddity DMR Programming Tips - (EN+DE)David TutAinda não há avaliações
- PHP Question AnswerDocumento20 páginasPHP Question AnswerManish SharmaAinda não há avaliações
- A Quick Tutorial On RSLogix Emulator 5000Documento9 páginasA Quick Tutorial On RSLogix Emulator 5000slavezerorjAinda não há avaliações
- ProjectDocumento10 páginasProjectabdul basitAinda não há avaliações
- Lift Use CaseDocumento5 páginasLift Use Casedipankar_nath07Ainda não há avaliações
- Differential EquationDocumento17 páginasDifferential EquationAashika DhareAinda não há avaliações
- QuesTeksFerriumC61C64andC6 PDFDocumento23 páginasQuesTeksFerriumC61C64andC6 PDFEmily MillerAinda não há avaliações
- OPTICast BrochureDocumento2 páginasOPTICast BrochureIndra Pratap SengarAinda não há avaliações
- Compaction Factor ExperimentDocumento23 páginasCompaction Factor ExperimentYI HEN ONGAinda não há avaliações
- (Solution Manual) Fundamentals of Electric Circuits 4ed - Sadiku-Pages-774-800Documento35 páginas(Solution Manual) Fundamentals of Electric Circuits 4ed - Sadiku-Pages-774-800Leo AudeAinda não há avaliações
- SIF Corporate-Presentatie 2017Documento35 páginasSIF Corporate-Presentatie 201766apenlullenAinda não há avaliações
- Transformation To An Agile and Virtualized World: Operations Center of The FutureDocumento1 páginaTransformation To An Agile and Virtualized World: Operations Center of The FuturepinardoAinda não há avaliações
- Color Pencil TheoryDocumento2 páginasColor Pencil Theoryapi-246017428Ainda não há avaliações
- (FreeCourseWeb - Com) 1493997599Documento386 páginas(FreeCourseWeb - Com) 1493997599MuruganandamGanesanAinda não há avaliações
- Gaggenau DF 291-760Documento1 páginaGaggenau DF 291-760PurcellMurrayAinda não há avaliações
- Geomechanics: Figure 1 Geomechanics in Oil & Gas Industry (Source: Geomechanics Engineering)Documento2 páginasGeomechanics: Figure 1 Geomechanics in Oil & Gas Industry (Source: Geomechanics Engineering)ابوالحروف العربي ابوالحروفAinda não há avaliações
- OSD PrintoutDocumento18 páginasOSD PrintoutDSAO AmravatiAinda não há avaliações
- Anomaly Detection Principles and Algorithms: Mehrotra, K.G., Mohan, C.K., Huang, HDocumento1 páginaAnomaly Detection Principles and Algorithms: Mehrotra, K.G., Mohan, C.K., Huang, HIndi Wei-Huan HuAinda não há avaliações
- This Study Resource Was: EvaluateDocumento2 páginasThis Study Resource Was: EvaluateMary angel PerjesAinda não há avaliações
- DRK109A&B Touch-Screen Bursting Strength TesterDocumento2 páginasDRK109A&B Touch-Screen Bursting Strength Testermohamadreza1368Ainda não há avaliações
- SM 89Documento36 páginasSM 89Camilo RamosAinda não há avaliações
- 1001451317230Documento12 páginas1001451317230JulioEdgarHanccoZeaAinda não há avaliações