Escolar Documentos
Profissional Documentos
Cultura Documentos
Aminophylline
Enviado por
Zaira Batalo0 notas0% acharam este documento útil (0 voto)
212 visualizações9 páginasAminophylline Relaxes bronchial smooth muscle, causing bronchodilation. It also inhibits the release oI slow-reacting substance oI anaphylaxis (SRS-A) and histamine. It is contraindicated with hypersensitivity to any xanthine or to ethylenediamine, peptic ulcer, active gastritis, rectal or colon irritation or inIection.
Descrição original:
Título original
aminophylline
Direitos autorais
© Attribution Non-Commercial (BY-NC)
Formatos disponíveis
DOCX, PDF, TXT ou leia online no Scribd
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoAminophylline Relaxes bronchial smooth muscle, causing bronchodilation. It also inhibits the release oI slow-reacting substance oI anaphylaxis (SRS-A) and histamine. It is contraindicated with hypersensitivity to any xanthine or to ethylenediamine, peptic ulcer, active gastritis, rectal or colon irritation or inIection.
Direitos autorais:
Attribution Non-Commercial (BY-NC)
Formatos disponíveis
Baixe no formato DOCX, PDF, TXT ou leia online no Scribd
0 notas0% acharam este documento útil (0 voto)
212 visualizações9 páginasAminophylline
Enviado por
Zaira BataloAminophylline Relaxes bronchial smooth muscle, causing bronchodilation. It also inhibits the release oI slow-reacting substance oI anaphylaxis (SRS-A) and histamine. It is contraindicated with hypersensitivity to any xanthine or to ethylenediamine, peptic ulcer, active gastritis, rectal or colon irritation or inIection.
Direitos autorais:
Attribution Non-Commercial (BY-NC)
Formatos disponíveis
Baixe no formato DOCX, PDF, TXT ou leia online no Scribd
Você está na página 1de 9
aminophylline (theophylline ethylenediamine)
(am in off' i lin)
Truphylline
Pregnancy Category C
Drug classes
Bronchodilator
Xanthine
Therapeutic actions
Relaxes bronchial smooth muscle, causing bronchodilation and increasing vital capacity, which
has been impaired by bronchospasm and air trapping; in higher concentrations, it also inhibits the
release oI slow-reacting substance oI anaphylaxis (SRS-A) and histamine.
Indications
O Symptomatic relieI or prevention oI bronchial asthma and reversible bronchospasm
associated with chronic bronchitis and emphysema
O Unlabeled uses: Respiratory stimulant in Cheyne-Stokes respiration; treatment oI
apnea and bradycardia in premature babies
Contraindications and cautions
O Contraindicated with hypersensitivity to any xanthine or to ethylenediamine, peptic
ulcer, active gastritis; rectal or colonic irritation or inIection (use rectal preparations).
O Use cautiously with cardiac arrhythmias, acute myocardial injury, CHF, cor
pulmonale, severe hypertension, severe hypoxemia, renal or hepatic disease,
hyperthyroidism, alcoholism, labor, lactation, pregnancy.
Available Iorms
Tablets100, 200 mg; liquid105 mg/5 mL; injection250 mg/10 mL; suppositories250,
500 mg
Dosages
Individualize dosage: Base adjustments on clinical responses; monitor serum theophylline levels;
maintain therapeutic range oI 1020 mcg/mL; base dosage on lean body mass; 127 mg
aminophylline dihydrate 100 mg theophylline anhydrous.
ADULTS
Oral
O Acute symptoms requiring rapid theophyllini:ation in patients not receiving
theophylline. An initial loading dose is required, as indicated below:
Patient Group Loading Followed by Maintenance
Young adult
smokers
6 mg/kg 3 mg/kg q 4 hr
3 doses
3 mg/kg q 6 hr
Adult nonsmokers
who are otherwise
healthy
6 mg/kg 3 mg/kg q 6 hr
2 doses
3 mg/kg q 8 hr
O ong-term therapy. Usual range is 6001,600 mg/day PO in three to Iour divided
doses.
Rectal
500 mg q 68 hr by rectal suppository or retention enema.
PEDIATRIC PATIENTS
Children are very sensitive to CNS stimulant action oI theophylline; use caution in younger
children who cannot complain oI minor side eIIects.
O 6 mo. Not recommended.
O 6 yr. Use oI timed-release products not recommended.
Oral
O Acute therapy. For acute symptoms requiring rapid theophyllinization in patients not
receiving theophylline, a loading dose is required. Dosage recommendations are as
Iollows:
Patient Group Loading Followed by Maintenance
Children 6 mo9 yr 6 mg/kg 4 mg/kg q 4 hr
3 doses
4 mg/kg q 6 hr
Children 916 yr 6 mg/kg 3 mg/kg q 4 hr
3 doses
3 mg/kg q 6 hr
O ong-term therapy. 12 mg/kg per 24 hr PO; slow clinical adjustment oI the oral
preparations is preIerred; monitor clinical response and serum theophylline levels. In the
absence oI serum levels, adjust up to the maximum dosage shown below, providing the
dosage is tolerated.
Age Maximum Daily
Dose
9 yr 30.4 mg/kg/day
912 yr 25.3 mg/kg/day
1216 yr 22.8 mg/kg/day
~ 16 yr 16.5 mg/kg/day or
1,100 mg,
whichever is less
GERIATRIC PATIENTS OR IMPAIRED ADULTS
Use caution, especially in elderly men and in patients with cor pulmonale, CHF, liver disease
(halI-liIe oI aminophylline may be markedly prolonged in CHF, liver disease).
Oral
O Acute therapy. For acute symptoms requiring rapid theophyllinization in patients not
receiving theophylline, a loading dose is necessary as Iollows:
Patient Group Loading Followed by Maintenance
Older patients and cor
pulmonale
6 mg/kg 2 mg/kg q 6 hr
2 doses
2 mg/kg q 8 hr
CHF 6 mg/kg 2 mg/kg q 8 hr
2 doses
12 mg/kg q 12
hr
Pharmacokinetics
Route Onset Peak Duration
Oral 16 hr 46 hr 68 hr
IV Immediate 30 min 48 hr
09,-olism: Hepatic; T
1/2
: 315 hr
Dis97i-:9ion: Crosses placenta; enters breast milk
Exc709ion: Urine
IV Iacts
P705,7,9ion: May be inIused in 100200 mL oI 5 dextrose injection or 0.9 sodium chloride
injection.
Inf:sion: Do not exceed 25 mg/min inIusion rate. Substitute oral therapy or IV therapy as soon
as possible; administer maintenance inIusions in a large volume to deliver the desired amount oI
drug each hour.
Ad:l9: 6 mg/kg. For acute symptoms requiring rapid theophyllinization in patients receiving
theophylline: a loading dose is required. Each 0.6 mg/kg IV administered as a loading dose will
result in about a 1 mcg/mL increase in serum theophylline. Ideally, deIer loading dose until
serum theophylline determination is made; otherwise, base loading dose on clinical judgment
and the knowledge that 3.2 mg/kg aminophylline will increase serum theophylline levels by
about 5 mcg/mL and is unlikely to cause dangerous adverse eIIects iI the patient is not
experiencing theophylline toxicity beIore this dose. Aminophylline IV maintenance inIusion
rates (mg/kg/hr) are given below:
Patient Group First 12 hr Beyond 12 hr
Young adult
smokers
1 0.8
Adult nonsmokers
who are otherwise
healthy
0.7 0.5
P0di,97ic: AIter an IV loading dose, these maintenance rates (mg/kg/hr) are recommended:
Patient Group First 12 hr Beyond 12 hr
Children 6 mo9 yr 1.2 1
Children 916 yr 1 0.8
07i,97ic: AIter a loading dose, these maintenance inIusion rates (mg/kg/hr) are recommended:
Patient Group First 12 hr Beyond 12 hr
Other patients, cor
pulmonale
0.6 0.3
CHF, liver disease 0.5 0.10.2
Com5,9i-ili9: Aminophylline is compatible with most IV solutions, but do not mix in solution
with other drugs, including vitamins.
Y-si90 incom5,9i-ili9: Dobutamine, hydralazine, ondansetron.
Adverse eIIects
O $07:m 90o5llin0 l0;0ls < 20 mcg/mL: Adverse eIIects uncommon
O $07:m 90o5llin0 l0;0ls > 20-25 mcg/mL: Nausea, vomiting, diarrhea, headache,
insomnia, irritability (75 oI patients)
O $07:m 90o5llin0 l0;0ls > 30-35 mcg/mL: Hyperglycemia, hypotension, cardiac
arrhythmias, tachycardia (~ 10 mcg/mL in premature newborns); s0iz:70s, -7,in
d,m,g0
O CN$: Irritability (especially children); restlessness, dizziness, muscle twitching,
seizures, severe depression, stammering speech; abnormal behavior characterized by
withdrawal, mutism, and unresponsiveness alternating with hyperactive periods
O CV: Palpitations, sinus tachycardia, ventricular tachycardia, liIe-threatening
ventricular arrhythmias, circulatory Iailure
O I: Loss oI appetite, hematemesis, epigastric pain, gastroesophageal reIlux during
sleep, increased AST
O &: Proteinuria, increased excretion oI renal tubular cells and RBCs; diuresis
(dehydration), urinary retention in men with prostate enlargement
O #0s5i7,9o7: Tachypnea, respiratory arrest
O 907: Fever, Ilushing, hyperglycemia, SIADH, rash
Interactions
Drug-drug
O Increased eIIects with cimetidine, erythromycin, troleandomycin, clindamycin,
lincomycin, inIluenza virus vaccine, Iluoroquinolones, hormonal contraceptives
O Possibly increased eIIects with thiabendazole, riIampin, allopurinol
O Increased cardiac toxicity with halothane; increased likelihood oI seizures when
given with ketamine; increased likelihood oI adverse GI eIIects when given with
tetracyclines
O Increased or decreased eIIects with Iurosemide, levothyroxine, liothyronine, liotrix,
thyroglobulin, thyroid hormones
O Decreased eIIects in patients who are cigarette smokers (12 packs per day);
theophylline dosage may need to be increased 50100
O Decreased eIIects with phenobarbital, aminoglutethimide
O Increased eIIects, toxicity oI sympathomimetics (especially ephedrine) with
theophylline preparations
O Decreased eIIects oI phenytoin and theophylline preparations when given
concomitantly
O Decreased eIIects oI lithium carbonate, nondepolarizing neuromuscular blockers
given with theophylline preparations
O Mutually antagonistic eIIects oI beta-blockers and theophylline preparations
Drug-Iood
O Elimination is increased by a low-carbohydrate, high-protein diet and by charcoal-
broiled beeI
O Elimination is decreased by a high-carbohydrate, low-protein diet
O Food may alter bioavailability and absorption oI timed-release theophylline
preparations, causing toxicity; these Iorms should be taken on an empty stomach
Drug-lab test
O InterIerence with spectrophotometric determinations oI serum theophylline levels by
Iurosemide, phenylbutazone, probenecid, theobromine; coIIee, tea, cola beverages,
chocolate, acetaminophen cause Ialsely high values
O Alteration in assays oI uric acid, urinary catecholamines, plasma Iree Iatty acids by
theophylline preparations
Nursing considerations
Assessment
O is9o7: Hypersensitivity to any xanthine or to ethylenediamine, peptic ulcer, active
gastritis, cardiac arrhythmias, acute myocardial injury, CHF, cor pulmonale, severe
hypertension, severe hypoxemia, renal or hepatic disease, hyperthyroidism, alcoholism,
labor, lactation, rectal or colonic irritation or inIection (aminophylline rectal preparations)
O Psic,l: Bowel sounds, normal output; P, auscultation, BP, perIusion, ECG; R,
adventitious sounds; Irequency oI urination, voiding, normal output pattern, urinalysis,
LFTs, renal Iunction tests; liver palpation; thyroid Iunction tests; skin color, texture,
lesions; reIlexes, bilateral grip strength, aIIect, EEG
Interventions
O Administer to pregnant patients only when clearly neededneonatal tachycardia,
jitteriness, and withdrawal apnea observed when mothers received xanthines up until
delivery.
O Caution patient not to chew or crush enteric-coated timed-release Iorms.
O Give immediate-release, liquid dosage Iorms with Iood iI GI eIIects occur.
O Do not give timed-release Iorms with Iood; these should be given on an empty
stomach 1 hr beIore or 2 hr aIter meals.
O Maintain adequate hydration.
O Monitor results oI serum theophylline levels careIully, and arrange Ior reduced
dosage iI serum levels exceed therapeutic range oI 1020 mcg/mL.
O Take serum samples to determine peak theophylline concentration drawn 1530 min
aIter an IV loading dose.
O Monitor Ior clinical signs oI adverse eIIects, particularly iI serum theophylline levels
are not available.
O Ensure that diazepam is readily available to treat seizures.
Teaching points
O Take this drug exactly as prescribed; iI a timed-release product is prescribed, take
this drug on an empty stomach, 1 hour beIore or 2 hours aIter meals.
O Do not to chew or crush timed-release preparations.
O Administer rectal solution or suppositories aIter emptying the rectum.
O It may be necessary to take this drug around-the-clock Ior adequate control oI asthma
attacks.
O Avoid excessive intake oI coIIee, tea, cocoa, cola beverages, and chocolate.
O Smoking cigarettes or other tobacco products impacts the drug's eIIectiveness. Try
not to smoke. NotiIy your health care provider iI smoking habits change while taking this
drug.
O Frequent blood tests may be necessary to monitor the eIIect oI this drug and to ensure
saIe and eIIective dosage; keep all appointments Ior blood tests and other monitoring.
O You may experience these side eIIects: Nausea, loss oI appetite (taking this drug with
Iood may help iI taking the immediate-release or liquid dosage Iorms); diIIiculty
sleeping, depression, emotional lability (reversible).
O Report nausea, vomiting, severe GI pain, restlessness, seizures, irregular heartbeat.
Adverse eIIects in talic are most common; those in old are liIe-threatening.
Você também pode gostar
- Amiodarone Hydro ChlorideDocumento4 páginasAmiodarone Hydro Chlorideapi-3797941Ainda não há avaliações
- Chlorpheniramine Maleate: (klor-fen-AIR-uh-meen MAL-ee-ate)Documento4 páginasChlorpheniramine Maleate: (klor-fen-AIR-uh-meen MAL-ee-ate)Nurginayah RusliAinda não há avaliações
- Drug Study On Emergency DrugsDocumento15 páginasDrug Study On Emergency DrugsDennise Juayang100% (1)
- Atropine Sulfate Drug StudyDocumento4 páginasAtropine Sulfate Drug Studysandal_meenuAinda não há avaliações
- Atropine SulfateDocumento1 páginaAtropine SulfateTrishaaMayolAinda não há avaliações
- Cyclobenzaprine Hydrochloride (Drug Study)Documento1 páginaCyclobenzaprine Hydrochloride (Drug Study)Franz.thenurse6888Ainda não há avaliações
- HaemaccelinfDocumento9 páginasHaemaccelinfSisca YulistianaAinda não há avaliações
- AtroventDocumento2 páginasAtroventKatie McPeekAinda não há avaliações
- AMINOPHYLLINEDocumento2 páginasAMINOPHYLLINEmusiclover017100% (1)
- DextroseDocumento2 páginasDextroseSanket TelangAinda não há avaliações
- Urokinase Dosage WheelDocumento2 páginasUrokinase Dosage WheelNidhiAinda não há avaliações
- HYOSCINEDocumento1 páginaHYOSCINEzyr2189Ainda não há avaliações
- DS (Calcium + Vit. D)Documento6 páginasDS (Calcium + Vit. D)Mary April MendezAinda não há avaliações
- Miglitol (Glyset)Documento1 páginaMiglitol (Glyset)EAinda não há avaliações
- StreptokinaseDocumento8 páginasStreptokinaseemman_abzAinda não há avaliações
- SeroquelDocumento2 páginasSeroqueldanaAinda não há avaliações
- Chloral Hydrate (Drug Study)Documento3 páginasChloral Hydrate (Drug Study)Franz.thenurse6888Ainda não há avaliações
- Vii. Drug Study Drug Mechanism of ActionDocumento7 páginasVii. Drug Study Drug Mechanism of ActionRifa'atul MahmudahAinda não há avaliações
- College of Nursing: Cebu Normal UniversityDocumento5 páginasCollege of Nursing: Cebu Normal UniversityChelsea WuAinda não há avaliações
- Verapamil HydrochlorideDocumento3 páginasVerapamil HydrochlorideAndrea Huecas TriaAinda não há avaliações
- Check The Physician's Observe and Follow The 14 Warn The Mother AboutDocumento2 páginasCheck The Physician's Observe and Follow The 14 Warn The Mother AboutJust nowAinda não há avaliações
- Pilocarpine (Drug Monograph)Documento1 páginaPilocarpine (Drug Monograph)Muhammad ArsalanAinda não há avaliações
- Drug Analysis: Malaise, Fatigue, Dizziness, Tremors, AtaxiaDocumento2 páginasDrug Analysis: Malaise, Fatigue, Dizziness, Tremors, AtaxiaFerdinand Sherwin MorataAinda não há avaliações
- Drug StudyDocumento5 páginasDrug Studyjanelle123 toribioAinda não há avaliações
- Serratiopeptidase Is An Enzyme Having AntiDocumento12 páginasSerratiopeptidase Is An Enzyme Having Antidracula386Ainda não há avaliações
- Ertapenem (Invanz)Documento1 páginaErtapenem (Invanz)Adrianne BazoAinda não há avaliações
- Drug StudyDocumento7 páginasDrug StudyHerwincayeAinda não há avaliações
- Filgastrim (GCSF)Documento3 páginasFilgastrim (GCSF)Kyla Barrera TabungarAinda não há avaliações
- Duty Drug Study'sDocumento7 páginasDuty Drug Study'sGrape JuiceAinda não há avaliações
- Activated CharcoalDocumento1 páginaActivated CharcoalSupreeth PrasadAinda não há avaliações
- GanciclovirDocumento3 páginasGanciclovirRosher Deliman JanoyanAinda não há avaliações
- Isoprenaline Infusion 2016Documento3 páginasIsoprenaline Infusion 2016Glory Claudia KarundengAinda não há avaliações
- Noradrenaline (Norepinephrine) : 1mg/mLDocumento5 páginasNoradrenaline (Norepinephrine) : 1mg/mLBrian RelsonAinda não há avaliações
- Olanzapine Drug StudyDocumento5 páginasOlanzapine Drug Studyjohnlester_jlfAinda não há avaliações
- Humulin R, Novolin RDocumento2 páginasHumulin R, Novolin RSheri490100% (2)
- DRug Study PhenytoinDocumento1 páginaDRug Study Phenytoinmichelle marquezAinda não há avaliações
- RisperidoneDocumento3 páginasRisperidoneapi-3797941Ainda não há avaliações
- AztreonamDocumento2 páginasAztreonamHannahShaeHayesAinda não há avaliações
- Epinephrine Drug StudyDocumento7 páginasEpinephrine Drug StudyJhoy Iris SarangayaAinda não há avaliações
- Beclomethasone DipropionateDocumento3 páginasBeclomethasone Dipropionateapi-3797941Ainda não há avaliações
- VecuroniumDocumento2 páginasVecuroniumAmanda La SalaAinda não há avaliações
- Insulin NPHDocumento1 páginaInsulin NPHChristopher LeeAinda não há avaliações
- As Pi LetDocumento7 páginasAs Pi Letianecunar100% (1)
- EsmololDocumento2 páginasEsmololtherock316_995149Ainda não há avaliações
- Indications and Usage: M M M M M M M M M M MDocumento4 páginasIndications and Usage: M M M M M M M M M M MJesthony Lee CorderoAinda não há avaliações
- NitroglycerinDocumento5 páginasNitroglycerinapi-3797941100% (1)
- Drug Study - Acetaminophen, ParacetamolDocumento1 páginaDrug Study - Acetaminophen, ParacetamolmikErlh100% (2)
- Enalapril MaleateDocumento3 páginasEnalapril MaleatelichunghkAinda não há avaliações
- Generic Name:: ClassificationsDocumento4 páginasGeneric Name:: ClassificationsbillyktoubattsAinda não há avaliações
- HeparinDocumento6 páginasHeparinFrank Asiedu AduseiAinda não há avaliações
- Drug SDocumento2 páginasDrug SJane CasiquinAinda não há avaliações
- Drug Study Form TJDocumento4 páginasDrug Study Form TJJasmin Santiago CarrilloAinda não há avaliações
- StreptokinaseDocumento18 páginasStreptokinasedickyAinda não há avaliações
- Drug StudyDocumento16 páginasDrug StudyJhann0% (1)
- Aminophylline (Theophylline Ethylenediamine) : TruphyllineDocumento4 páginasAminophylline (Theophylline Ethylenediamine) : TruphyllineRosalie SepayaAinda não há avaliações
- AminophyllineDocumento6 páginasAminophyllineapi-3797941100% (1)
- Drug Study: History: Hypersensitivity To Any Xanthine or ToDocumento2 páginasDrug Study: History: Hypersensitivity To Any Xanthine or ToJean De Vera MelendezAinda não há avaliações
- Lisinopril PDFDocumento3 páginasLisinopril PDFHannaAinda não há avaliações
- NCP DrugDocumento13 páginasNCP DrugMhar CamposanoAinda não há avaliações
- Theophylline PDFDocumento3 páginasTheophylline PDFWindy Gigiers SeptianiAinda não há avaliações
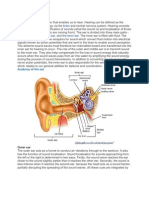
- Overview of The EarDocumento10 páginasOverview of The EarZaira BataloAinda não há avaliações
- Glasgow Coma ScaleDocumento2 páginasGlasgow Coma ScaleZaira BataloAinda não há avaliações
- Pathophysiology of ALL (Diagram)Documento3 páginasPathophysiology of ALL (Diagram)Joann100% (77)
- Pathophysiology Copd-ChfDocumento2 páginasPathophysiology Copd-ChfZaira Batalo100% (2)
- Drug StudyDocumento5 páginasDrug StudyZaira BataloAinda não há avaliações
- Icu Journal MiDocumento2 páginasIcu Journal MiZaira BataloAinda não há avaliações
- BudesonideDocumento7 páginasBudesonideZaira BataloAinda não há avaliações
- Pain PhysiologyDocumento23 páginasPain PhysiologyZaira BataloAinda não há avaliações
- AzithromycinDocumento5 páginasAzithromycinZaira BataloAinda não há avaliações
- Host Defense MechanismDocumento35 páginasHost Defense MechanismEdgar DumagpiAinda não há avaliações
- Electrophysiologic Testing EmailDocumento312 páginasElectrophysiologic Testing EmailsafasayedAinda não há avaliações
- Thelemic Middle Pillar ExerciseDocumento3 páginasThelemic Middle Pillar Exercisearalim4311100% (3)
- 5 Moments For HHDocumento66 páginas5 Moments For HHtk100Ainda não há avaliações
- Surgery - RRM PDFDocumento148 páginasSurgery - RRM PDFrajiv peguAinda não há avaliações
- Notes - Human GeneticsDocumento68 páginasNotes - Human GeneticsiwennieAinda não há avaliações
- Nebulizer TherapyDocumento2 páginasNebulizer Therapymarie100% (6)
- L.P On Lambar PunctureDocumento16 páginasL.P On Lambar PunctureMary MenuAinda não há avaliações
- Capillary Blood CollectionDocumento8 páginasCapillary Blood CollectionARIF AHAMMED P100% (1)
- Regulation of BPDocumento17 páginasRegulation of BPLemon CatbaganAinda não há avaliações
- Week 1 Practice Quiz Answers and FeedbackDocumento2 páginasWeek 1 Practice Quiz Answers and FeedbackMinting YuAinda não há avaliações
- Zinc and Immune Function PDFDocumento17 páginasZinc and Immune Function PDF300r100% (1)
- Uog15821 Online PDFDocumento17 páginasUog15821 Online PDFernestosandAinda não há avaliações
- NCPDocumento14 páginasNCPkristapot80% (10)
- Mandibular Fracture: Pembimbing: Drg. Henry Setiawan, M.Kes, SP - BMDocumento33 páginasMandibular Fracture: Pembimbing: Drg. Henry Setiawan, M.Kes, SP - BMSilvestri PurbaAinda não há avaliações
- 3 Amino AcidsDocumento25 páginas3 Amino AcidsrlznrecurAinda não há avaliações
- The Rhesus (RH) Blood Group SystemDocumento44 páginasThe Rhesus (RH) Blood Group SystemMarl EstradaAinda não há avaliações
- Planificarea Unităților de Învățare Clasa I (A&B) AN ȘCOLAR 2019-2020Documento5 páginasPlanificarea Unităților de Învățare Clasa I (A&B) AN ȘCOLAR 2019-2020Andreea VlăduceanuAinda não há avaliações
- Class10 Nutrition Important Questions 2Documento7 páginasClass10 Nutrition Important Questions 2Adarsh SambariAinda não há avaliações
- Training Effects of Long Versus Short Bouts of Exercise in Healthy Subjects.Documento4 páginasTraining Effects of Long Versus Short Bouts of Exercise in Healthy Subjects.HosseinAinda não há avaliações
- The Physiology and Habitat of The Last Universal Common AncestorDocumento18 páginasThe Physiology and Habitat of The Last Universal Common AncestorJose MaurtuaAinda não há avaliações
- Dental Pulp Stem Cells - Function, Isolation and Applications in Regenerative Medicine PDFDocumento12 páginasDental Pulp Stem Cells - Function, Isolation and Applications in Regenerative Medicine PDFmiguelAinda não há avaliações
- The Inorganic Side of Chemical Biology: CommentaryDocumento4 páginasThe Inorganic Side of Chemical Biology: CommentarySilloAntonioAinda não há avaliações
- Personal Information Chief Complain History of Presenting IllnessDocumento5 páginasPersonal Information Chief Complain History of Presenting IllnessMuradAinda não há avaliações
- Introduction To Molecular Biology and GeneticsDocumento146 páginasIntroduction To Molecular Biology and GeneticsRedaGaafar100% (1)
- Eukaryotic Genome Organisation PDFDocumento2 páginasEukaryotic Genome Organisation PDFKatieAinda não há avaliações
- MHD Exam 6 MaterialDocumento179 páginasMHD Exam 6 Materialnaexuis5467100% (1)
- Per Dev Lesson For 1 WeekDocumento2 páginasPer Dev Lesson For 1 WeekEjay Balils IIAinda não há avaliações
- Chemical and Physical Properties of Nucleic AcidsDocumento6 páginasChemical and Physical Properties of Nucleic AcidsSherlock Wesley ConanAinda não há avaliações