Escolar Documentos
Profissional Documentos
Cultura Documentos
Organic Chemistry Review
Enviado por
Lind MondanoDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Organic Chemistry Review
Enviado por
Lind MondanoDireitos autorais:
Formatos disponíveis
Organic chemistry review
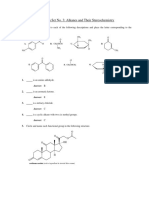
1. Draw structures for the following compounds: a) 2-methylbutane b) 3,3-dichlorohexane c) 4-ethyl-3,3,4-trimethyldecane d) ethanamide e) cis-1,3-dimethylcyclohexane f) g) h) i) j) 2-methyl-4-octanol propanal methyl ethanoate methyl propyl ether 2-pentanone
2. Write the correct name for each of the following structures: O H3C a b c H H
H C C C H C C H
H3C CH3
H3C
CH3
H 3C
CH3
H3 C
CH2 N CH3
CH2 CH3
d i ii
HO
3. Draw the polymers that result from: a) the reaction of i) and ii) b) the reaction of ii) and iii) c) the addition of ethene molecules d) the addition of 3-hexene molecules
HO
O C
CH 2 CH 2OH
O C OH
iii
H2 N CH NH2 CH3
4. What is the difference between a saturated and unsaturated hydrocarbon? Which is benzene? 5. Identify each pair as structural isomers, geometric isomers, or neither a) cyclopentane, pentane f Br Br H Cl b) 1,1-dichloroethene, trans-1,2-dichloroethene C C C C c) hexanoic acid, propyl propanoate H Cl H H d) cis-1,2-dichlorocyclopentane, trans-1,2-dichlorocyclopentane e) cis-1,2-dichlorocyclopentane, trans-1,3-dichlorocyclopentane 6. C2H6O exists as two structural isomers. Draw each isomer and name each. Predict the relative boiling point and solubility in water of the two isomers. Explain. 7. Indicate if the functional group contains a cabonyl, carboxyl or hydroxyl group: alcohol ketone ester carboxylic acid carboxyl group carbonyl group hydroxyl group 8. In each case indicate how the following molecule can be made by drawing the reaction, naming all products and reactants, and naming the type of reaction that is occurring. a) 1,2-dichlorocyclopentane (from a hydrocarbon) b) 1,1-dichlorocyclopentane (from a hydrocarbon) c) A condensation reaction to make butyl methanoate d) The reactants from c) (not using hydrogenation) 9. Using Br2(aq), how can you easily distinguish between pentane, 1-pentene, and 1-pentyne? 10. What is wrong with the following names? a) 3-methyl-2-butyne b) 1,2-dichlorocyclobutane c) 3-methyloctene d) 2,3-dimethyl-4-ethylnonane e) 1-butanone f) 2-ethyl-2-methylhexane
Additional practice (pg. 1049-1053): 24.5, 7, 11, 12, 13, 14, 17, 19, 32, 36, 37
1.
H3C
CH CH2 CH3 CH3
d
H3C
O C NH2
H CH3 H H H
O CH3
j
O CH3
CH3 CH2 CH
H 3C
Cl CH2 CH2 CH3
CH3 H H H H H H
h
H3 C
O C O CH3
CH3 CH2 C Cl
H 3C
H3 C CH3 CH2 C H3 C
CH3 C CH2 CH2 CH2 CH2 CH2 CH3
CH3 CH CH2 CH CH2 CH2 CH2 CH3 CH3 OH
CH2 CH3
2. a) 1,3-cyclopentadiene b) 4-ethyl-2,7-dimethyl-2,5-nonadiene c) ethyl propanoate d) diethylmethylamine
O O CH2 CH2O
O NH CH NH C CH3
3. a)
HO CH2CH2O
O C
O C
O C O CH2CH2O
O NH CH NH C CH3
O C
O C OH
O C
O C
O C OH
b)
H 2N
CH NH C CH3
H 3C c)
H3C CH CH CH CH CH CH CH CH CH3 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3
CH3
d)
4. 5. 6.
Saturated = only single bonds. Unsaturated = presence of multiple bonds (e.g. benzene) a) neither b) structural c) structural d) geometric e) structural f) geometric
CH 3 CH 2OH dimethyl ether ethanol ethanol would have a higher boiling point and would be more soluble in water because of the polar OH bond (the C-O bonds in the ether are less polar).
H 3C O CH3
7.
alcohol carboxyl group carbonyl group hydroxyl group
8.
ketone
ester
carboxylic acid
9. 10.
a) via chlorination/halogenation reaction (cyclopentene + Cl2) b) via substitution reaction (thus, low yield of desired product) (cyclopentane + Cl2 with catalyst and heat) c) esterification (1-butanol + methanoic acid with acid catalyst) d) 1-butanol can be formed via hydrolysis from 1-butene + H2O with acid catalyst (2-butanol will also form, so yield will not be 100%). Methanoic acid can be formed via the oxidation of methanal. Br2 will not react with pentane. Br2 will discolour in 1-pentene. Twice as much Br2 will discolour in 1-pentyne. a) The third carbon has too many atoms bonded to it b) Should have cis or trans in name c) The position of the double bond in octene is not indicated (e.g., might be 3-methyl-1-octene) d) ethyl should come before methyl (should be 4-ethyl-2,3-dimethylnonane) e) This is an aldehyde not a ketone (should be butanal) f) The longest carbon chain has not been used in the naming of this molecule (should be 3,3dimethylheptane)
Você também pode gostar
- Quarter 1 - Module 1Documento31 páginasQuarter 1 - Module 1Roger Santos Peña75% (4)
- Organic ReviewerDocumento4 páginasOrganic ReviewerRanie MagpocAinda não há avaliações
- Lab Manual Organic Analytical ChemistryDocumento30 páginasLab Manual Organic Analytical ChemistryKayrol29100% (1)
- Organic Chemistry - Worksheet 1Documento10 páginasOrganic Chemistry - Worksheet 1Prakas PalanychamyAinda não há avaliações
- Analytical NotesDocumento25 páginasAnalytical NotesRyan BoodramlallAinda não há avaliações
- Organic Chemistry Board Exam Questions PDFDocumento10 páginasOrganic Chemistry Board Exam Questions PDFDonPedrewAinda não há avaliações
- Alkanes Alkenes AlkynesDocumento10 páginasAlkanes Alkenes AlkynesPanda Boy100% (2)
- Lab Manual 2016 (Analytical and Organic Chemistry)Documento30 páginasLab Manual 2016 (Analytical and Organic Chemistry)Arif HilmiAinda não há avaliações
- Atoms, Molecules, and IonsDocumento44 páginasAtoms, Molecules, and Ionsholley_kennethAinda não há avaliações
- Toeic: Check Your English Vocabulary ForDocumento41 páginasToeic: Check Your English Vocabulary ForEva Ibáñez RamosAinda não há avaliações
- Organic Chemistry ReviewerDocumento22 páginasOrganic Chemistry ReviewerKaren Kate LozadaAinda não há avaliações
- Organic Chemistry ReviewerDocumento10 páginasOrganic Chemistry ReviewerJan Chester ChanAinda não há avaliações
- Exam3 Practice ExamDocumento4 páginasExam3 Practice ExamMatthew FernandezAinda não há avaliações
- Acid-Base Practice Problems-Answers PDFDocumento5 páginasAcid-Base Practice Problems-Answers PDFSuci PrameswariAinda não há avaliações
- Illustrating An Experiment, Outcome, Sample Space and EventDocumento9 páginasIllustrating An Experiment, Outcome, Sample Space and EventMarielle MunarAinda não há avaliações
- Inorganic Chemistry Exam 20100621Documento2 páginasInorganic Chemistry Exam 20100621曾鈞浩Ainda não há avaliações
- Reviewer Organic Chemistry ReviewerDocumento4 páginasReviewer Organic Chemistry Reviewerash cortesAinda não há avaliações
- Practical Organic Chemistry III ExamDocumento3 páginasPractical Organic Chemistry III ExamTesfahun100% (1)
- Organic Chem (Online Review)Documento211 páginasOrganic Chem (Online Review)Spencer Thomas100% (1)
- BiochemistryDocumento7 páginasBiochemistryAbdelwahab AliAinda não há avaliações
- Organic Chemistry Alkenes WorksheetDocumento2 páginasOrganic Chemistry Alkenes Worksheetoc100% (1)
- General and Analytical ChemistryDocumento8 páginasGeneral and Analytical ChemistryHeherson CabreraAinda não há avaliações
- Interlocking Block TechnologyDocumento15 páginasInterlocking Block TechnologyChaula Trivedi100% (5)
- Design of Penstock: Reference Code:IS 11639 (Part 2)Documento4 páginasDesign of Penstock: Reference Code:IS 11639 (Part 2)sunchitk100% (3)
- Ch29 Brown Organic Chemistry SolutionsDocumento8 páginasCh29 Brown Organic Chemistry SolutionsGabby Tanaka100% (1)
- Cisco UCS Adapter TroubleshootingDocumento90 páginasCisco UCS Adapter TroubleshootingShahulAinda não há avaliações
- MCQ Inorganic Chemistry Part 1Documento6 páginasMCQ Inorganic Chemistry Part 1zubairmaj341767% (15)
- Organic Chemistry TestDocumento8 páginasOrganic Chemistry Testzafarchem_iqbalAinda não há avaliações
- Organic Chemistry 2 Practice Exam 1Documento15 páginasOrganic Chemistry 2 Practice Exam 1KaybidoAinda não há avaliações
- Chapter 1 Fundamentals of Organic ChemistryDocumento5 páginasChapter 1 Fundamentals of Organic ChemistryOchem90Ainda não há avaliações
- OrganicChemistryChapter5 PDFDocumento19 páginasOrganicChemistryChapter5 PDFJuliet Tatiana CumbeAinda não há avaliações
- Organic As Test P-2Documento9 páginasOrganic As Test P-2zafarchem_iqbalAinda não há avaliações
- Multiple Choices Questions: K K K K (CO)Documento14 páginasMultiple Choices Questions: K K K K (CO)MutasimAinda não há avaliações
- Separation of Organic Compounds Using Liquid-Liquid ExtractionDocumento8 páginasSeparation of Organic Compounds Using Liquid-Liquid ExtractionShyam BhaktaAinda não há avaliações
- Icp Tup Chem Tech Review Acids and BasesDocumento7 páginasIcp Tup Chem Tech Review Acids and BasesAimee MangubatAinda não há avaliações
- ANALYTICAL CHEMISTRY ReviewerDocumento6 páginasANALYTICAL CHEMISTRY ReviewerJeff Nieva CardelAinda não há avaliações
- Organic Chem Lab Final ExamDocumento7 páginasOrganic Chem Lab Final ExammvmbappleAinda não há avaliações
- Unit 2 Organic ChemistryDocumento18 páginasUnit 2 Organic Chemistrydeepashashikumar10100% (1)
- Substituent Effects - Organic ChemistryDocumento8 páginasSubstituent Effects - Organic ChemistrytracyymendozaAinda não há avaliações
- Inorganic Chemistry II (100 Items)Documento11 páginasInorganic Chemistry II (100 Items)maria jeusa matiasAinda não há avaliações
- Chemical Engineering Board Problems October 1977Documento2 páginasChemical Engineering Board Problems October 1977Nikki EbañezAinda não há avaliações
- Organic Chemistry 2 - Syllabus - USTHDocumento3 páginasOrganic Chemistry 2 - Syllabus - USTHMinh MinhAinda não há avaliações
- Mock Exam 2-AnswersDocumento8 páginasMock Exam 2-AnswersKhaledEl-MaghallawyAinda não há avaliações
- Lab Safety RulesDocumento6 páginasLab Safety RulesnettextsAinda não há avaliações
- Spectroscopic Methods in Organic ChemistryDocumento7 páginasSpectroscopic Methods in Organic ChemistryAkpemi Musa100% (1)
- Organic Chemistry IIDocumento7 páginasOrganic Chemistry IIRoberto SIlvaAinda não há avaliações
- Organic Chemistry Test 1 MemorandumDocumento7 páginasOrganic Chemistry Test 1 MemorandumSandile SynthaxError Mabika0% (1)
- Review 402Documento50 páginasReview 402Serdar CharyyevAinda não há avaliações
- Organic Chemistry TestDocumento1 páginaOrganic Chemistry Testron971Ainda não há avaliações
- History of Organic ChemistryDocumento16 páginasHistory of Organic ChemistrypugandjAinda não há avaliações
- Organic Chemistry 2021Documento76 páginasOrganic Chemistry 2021Arah Mae BonillaAinda não há avaliações
- MCQS ORGANIC ChemistryDocumento6 páginasMCQS ORGANIC Chemistrymalikimran28Ainda não há avaliações
- ORGANIC CHEMISTRY (Gen-Misc. and Alkanes,-Enes-ynes) (106 Items)Documento10 páginasORGANIC CHEMISTRY (Gen-Misc. and Alkanes,-Enes-ynes) (106 Items)Marlon PeterosAinda não há avaliações
- Dilution Problem Solving PDFDocumento8 páginasDilution Problem Solving PDFลูซี้หมี ขวัญใจ ปัญญาสรกุลAinda não há avaliações
- Inorganic Special Examination 2015 20161Documento2 páginasInorganic Special Examination 2015 20161Rodriguez RommelAinda não há avaliações
- Pharmaceutical Organic ChemistryDocumento2 páginasPharmaceutical Organic ChemistryPankaj KushwahAinda não há avaliações
- Alcohols, Phenols and Ethers - MCQs Test - 1Documento3 páginasAlcohols, Phenols and Ethers - MCQs Test - 1Prasant Kumar100% (1)
- CHEM 200 - Organic Chemistry (Laboratory)Documento7 páginasCHEM 200 - Organic Chemistry (Laboratory)yeeeyyyAinda não há avaliações
- Problem Set 3 - Alkanes and StereochemDocumento6 páginasProblem Set 3 - Alkanes and StereochemKatrina Louise GonzalesAinda não há avaliações
- Organic ChemistryDocumento60 páginasOrganic ChemistryPavani PrabhakarAinda não há avaliações
- Chapter 10 PDFDocumento10 páginasChapter 10 PDFKelsi Kyla PeraltaAinda não há avaliações
- Practice Exam 2 Organic Chemistry 237 UWDocumento7 páginasPractice Exam 2 Organic Chemistry 237 UWNgoc Minh NgoAinda não há avaliações
- Assignment 4 (Spectroscopy) : CH CH CH CCH O CH CH CH CH ODocumento1 páginaAssignment 4 (Spectroscopy) : CH CH CH CCH O CH CH CH CH OIbrahim MuhamadAinda não há avaliações
- Things To Review Na Lumabas Sa Chem Tech Board ExamDocumento1 páginaThings To Review Na Lumabas Sa Chem Tech Board ExamposterAinda não há avaliações
- Fundamentals of Public Health ManagementDocumento3 páginasFundamentals of Public Health ManagementHPMA globalAinda não há avaliações
- Micro Lab Midterm Study GuideDocumento15 páginasMicro Lab Midterm Study GuideYvette Salomé NievesAinda não há avaliações
- C2 - Conveyors Diagram: Peso de Faja Longitud de CargaDocumento1 páginaC2 - Conveyors Diagram: Peso de Faja Longitud de CargaIvan CruzAinda não há avaliações
- Tutorial 5 SolvedDocumento3 páginasTutorial 5 SolvedAshutoshKumarAinda não há avaliações
- Safety Procedures in Using Hand Tools and EquipmentDocumento12 páginasSafety Procedures in Using Hand Tools and EquipmentJan IcejimenezAinda não há avaliações
- Perancangan Crushing Plant Batu Andesit Di PT Nurmuda Cahaya Desa Batujajar Timur Kecamatan Batujajar Kabupaten Bandung Barat Provinsi Jawa BaratDocumento8 páginasPerancangan Crushing Plant Batu Andesit Di PT Nurmuda Cahaya Desa Batujajar Timur Kecamatan Batujajar Kabupaten Bandung Barat Provinsi Jawa BaratSutan AdityaAinda não há avaliações
- Brand Strategy - in B2BDocumento6 páginasBrand Strategy - in B2BKrishan SahuAinda não há avaliações
- Fast Track Design and Construction of Bridges in IndiaDocumento10 páginasFast Track Design and Construction of Bridges in IndiaSa ReddiAinda não há avaliações
- Mosfet Irfz44Documento8 páginasMosfet Irfz44huynhsang1979Ainda não há avaliações
- EPAS 11 - Q1 - W1 - Mod1Documento45 páginasEPAS 11 - Q1 - W1 - Mod1Alberto A. FugenAinda não há avaliações
- Dwnload Full Principles of Economics 7th Edition Frank Solutions Manual PDFDocumento35 páginasDwnload Full Principles of Economics 7th Edition Frank Solutions Manual PDFmirthafoucault100% (8)
- The cardioprotective effect of astaxanthin against isoprenaline-induced myocardial injury in rats: involvement of TLR4/NF-κB signaling pathwayDocumento7 páginasThe cardioprotective effect of astaxanthin against isoprenaline-induced myocardial injury in rats: involvement of TLR4/NF-κB signaling pathwayMennatallah AliAinda não há avaliações
- Music CG 2016Documento95 páginasMusic CG 2016chesterkevinAinda não há avaliações
- YIC Chapter 1 (2) MKTDocumento63 páginasYIC Chapter 1 (2) MKTMebre WelduAinda não há avaliações
- Systems Analysis and Design in A Changing World, Fourth EditionDocumento41 páginasSystems Analysis and Design in A Changing World, Fourth EditionKoko Dwika PutraAinda não há avaliações
- Bachelor of Arts in Theology: Christian Apologetics/ Seventh-Day Adventist Contemporary IssuesDocumento13 páginasBachelor of Arts in Theology: Christian Apologetics/ Seventh-Day Adventist Contemporary IssuesRamel LigueAinda não há avaliações
- 1 - 2020-CAP Surveys CatalogDocumento356 páginas1 - 2020-CAP Surveys CatalogCristiane AokiAinda não há avaliações
- Julia Dito ResumeDocumento3 páginasJulia Dito Resumeapi-253713289Ainda não há avaliações
- WAQF Podium Design Presentation 16 April 2018Documento23 páginasWAQF Podium Design Presentation 16 April 2018hoodqy99Ainda não há avaliações
- Winter CrocFest 2017 at St. Augustine Alligator Farm - Final ReportDocumento6 páginasWinter CrocFest 2017 at St. Augustine Alligator Farm - Final ReportColette AdamsAinda não há avaliações
- Book 1518450482Documento14 páginasBook 1518450482rajer13Ainda não há avaliações
- Applied Economics 2Documento8 páginasApplied Economics 2Sayra HidalgoAinda não há avaliações
- Summary of Bill of Quantities ChurchDocumento52 páginasSummary of Bill of Quantities ChurchBiniamAinda não há avaliações
- Topic 3Documento21 páginasTopic 3Ivan SimonAinda não há avaliações