Escolar Documentos
Profissional Documentos
Cultura Documentos
Chapter 7
Enviado por
Kristen TizzanoDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Chapter 7
Enviado por
Kristen TizzanoDireitos autorais:
Formatos disponíveis
Chemistry: A Molecular Approach (Tro) Chapter 7
The Quantu mMechani cal Model of the
1)
A t o m
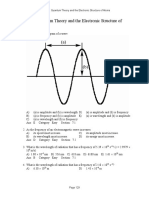
Calculat wavelength (in nm) of a the red light emitted by a neon sign with a frequency of 4.74 1014 Hz. e the A)
633 nm B)
158 nm C)
142 nm D)
704 nm E)
466 nm Answer:
A 2)
Calculat wavelength (in nm) of the blue light emitted by a mercury lamp with a frequency of 6.88 1014 e the Hz. A)
229 nm B)
436 nm C)
206 nm D)
485 nm E)
675 nm Answer:
B 3)
Calculat e the frequency of the red light emitted by a neon sign with a wavelength of 659.9 nm. A)
2.20 1014 s-1 B)
1.98 1014 s-1 C)
4.55 1014 s-1 D)
5.05 1014 s-1 E)
3.32 1014 s-1 Answer:
C 4)
Calculat e the frequency of the green light emitted by a hydrogen atom with a wavelength of 486.1 nm. A)
1.46 1014 s-1 B)
6.86 1014 s-1 C)
4.33 1014 s-1 D)
6.17 1014 s-1 E)
1.62 1014 s-1 Answer:
D 5)
Calculat e the energy of the red light emitted by a neon atom with a wavelength of 703.2 nm. A)
3.54 10B)
19 J
4.27 10C)
19 J
2.34 10D)
19 J
6.45 10E)
19 J
19 J 2.83 10Answer:
6)
Calculat e the energy of the violet light emitted by a hydrogen atom with a wavelength of 410.1 nm. A)
4.85 10B)
19 J
2.06 10C)
19 J
1.23 10D)
19 J
8.13 10E)
19 J
5.27 x 10-19 J Answer:
A 7)
Calculat e the energy of the green light emitted by a mercury lamp with a frequency of 5.49 1014 Hz. A)
2.75 10B)
19 J
3.64 10C)
19 J
5.46 10D)
19 J
1.83 10E)
19 J
19 J 4.68 10Answer:
B 8)
Calculat e the energy of the orange light emitted by a neon sign with a frequency of 4.89 1014 Hz. A)
3.09 10B)
19 J
6.14 10C)
19 J
3.24 10D)
19 J
1.63 10E)
19 J
19 J 5.11 10Answer:
C 9)
Place of electromagnetic radiation in order of increasing wavelength. the followin ultraviolet light gamma rays radio waves g types A)
gamma rays < radio waves < ultraviolet light B)
radio waves < ultraviolet light < gamma rays C)
radio waves < gamma rays < ultraviolet light D)
ultraviolet E)
light < gamma rays < radio waves
gamma rays < ultraviolet light < radio waves Answer:
E 10)
Place of electromagnetic radiation in order of increasing frequency. the followin visible light microwaves X-rays g types A)
microwaves < visible light < X-rays B)
X-rays < C)
visible light < microwaves
microwaves < X-rays < visible light D)
X-rays < E)
microwaves < visible light
visible light < X-rays < microwaves Answer:
11)
Place of electromagnetic radiation in order of decreasing energy. the followin ultraviolet light radio waves microwaves g types A)
radio waves > microwaves > ultraviolet light B)
ultraviolet C)
light > microwaves > radio waves
radio waves > ultraviolet light > microwaves D)
ultraviolet E)
light > radio waves > microwaves
microwaves > radio waves > ultraviolet light Answer:
B 12)
Which
of the following visible colors of light have the largest frequency? A)
green B)
red C)
blue D)
yellow E)
orange Answer:
C 13)
Which
of the following visible colors of light have the longest wavelength? A)
blue B)
green C)
yellow D)
red E)
violet Answer:
D 14)
Which
of the following types of electromagnetic radiation has the shortest wavelength? A)
blue B)
violet C)
orange D)
green E)
yellow Answer:
B 15)
Which
of the following types of electromagnetic radiation has the smallest frequency? A)
yellow B)
blue C)
orange D)
green E)
purple Answer:
C 16)
How many
photons are contained in a burst of yellow light (589 nm) from a sodium lamp that contains 609 kJ of energy? A)
3.37 1019 photons B)
3.06 1030 photons C)
1.81 1024 photons D)
4.03 1028 photons E)
2.48 1025 photons Answer:
17)
How
many photons are contained in a flash of green light (525 nm) that contains 189 kJ of energy? A)
5.67 1023 photons B)
2.01 1024 photons C)
1.25 1031 photons D)
4.99 1023 photons E)
7.99 1030 photons Answer:
D 18)
Which
of the following statements is TRUE? A)
The emission
B)
spectrum of a particular element is always the same and can be used to identify the element.
Part of the proposed that electrons in the hydrogen atom are located in "stationary states" or particular Bohr model orbits around the nucleus. C)
The principle states that we can never know both the exact location and speed of an electron. uncertainty D)
An orbital is the volume in which we are most likely to find an electron. E)
All of the
above are true. Answer:
E 19)
Calculat e the wavelength of an electron (m = 9.11 10-28 g) moving at 3.66 106 m/s. A)
1.99 10B)
10 m
5.03 10C)
10 m
1.81 10D)
10 m
5.52 10-9 m E)
2.76 10-9 m Answer:
A 20)
Calculat e the wavelength of a baseball (m = 155 g) moving at 32.5 m/s. A)
7.60 10B)
36 m
1.32 10C)
34 m
2.15 10D)
32 m
2.68 10E)
34 m
32 m 3.57 10Answer:
B 21)
Determi ne the velocity of a marble (m = 8.66 g) with a wavelength of 3.46 10-33 m. A)
45.2 m/s B)
11.3 m/s C)
22.1 m/s D)
38.8 m/s E)
52.9 m/s Answer:
C 22)
Determi ne the velocity of a medicine ball (m = 10.0 kg) with a wavelength of 1.33 10-35 m. A)
8.81 m/s B)
12.3 m/s C)
2.21 m/s D)
4.98 m/s E)
6.44 m/s Answer:
D 23)
Which of the
following transitions (in a hydrogen atom) represent emission of the longest wavelength photon? A)
n = 1 to n = 2 B)
n = 3 to n = 1 C)
n = 3 to n = 4 D)
n = 4 to n = 2 E)
n = 5 to n = 4 Answer:
E 24)
Which of the
following transitions (in a hydrogen atom) represent absorption of the smallest frequency photon? A)
n = 5 to n = 6 B)
n = 5 to n = 4 C)
n = 4 to n = 1 D)
n = 1 to n = 3 E)
n = 1 to n = 2 Answer:
A 25)
Choose transition (in a hydrogen atom) below that represents the absorption of the shortest wavelength the photon. A)
n = 1 to n = 2 B)
n = 2 to n = 3 C)
n = 4 to n = 5 D)
n = 6 to n = 3 E)
n = 3 to n = 1 Answer:
A 26)
Which
of the following transitions represent the emission of a photon with the largest energy? A)
n = 2 to n = 1 B)
n = 3 to n = 1 C)
n = 6 to n = 3 D)
n = 1 to n = 4 E)
n = 2 to n = 5 Answer:
B 27)
Determi ne the energy change associated with the transition from n=2 to n=5 in the hydrogen atom. A)
-2.18 10- 19 J B)
+6.54 10- 19 J C)
+4.58 10- 19 J D)
-1.53 10- 19 J E)
+3.76 10- 19 J Answer:
C 28)
Determi ne the energy change associated with the transition from n=3 to n=2 in the hydrogen atom. A)
+3.03 10- 19 J B)
-1.82 10- 19 J C)
+5.51 10- 19 J D)
-3.03 10- 19 J E)
+2.69 10- 19 J Answer:
29)
Calculat e the energy change associated with the transition from n=4 to n=1 in the hydrogen atom. A)
+4.89 10- 19 J B)
+1.64 10- 19 J C)
-6.12 10- 19 J D)
+3.55 10- 19 J E)
-2.04 10- 19 J Answer:
E 30)
Calculat e the wavelength of light associated with the transition from n=1 to n=3 in the hydrogen atom. A)
103 nm B)
155 nm C)
646 nm D)
971 nm E)
136 nm Answer:
A 31)
Calculat e the frequency of light associated with the transition from n = 2 to n = 3 in the hydrogen atom. A)
2.19 1014 s-1 B)
5.59 1014 s-1 C)
4.57 1014 s-1 D)
1.79 1014 s-1 E)
3.28 1014 s-1 Answer:
C 32)
It is determine the ionization energy for hydrogen using the Bohr equation. Calculate the ionization possible energy for an atom of hydrogen, making the assumption that ionization is the transition from n=1 to to n=. A)
-2.18 10- 18 J B)
+2.18 10- 18 J C)
+4.59 10- 18 J D)
-4.59 10- 18 J E)
+4.36 x 10- 18 J Answer:
B 33)
It is determine the ionization energy for hydrogen using the Bohr equation. Calculate the ionization possible energy (in kJ) for a mole of hydrogen atoms, making the assumption that ionization is the to transition from n=1 to n=. A)
7.62 103 kJ B)
2.76 103 kJ C)
1.31 103 kJ D)
3.62 103 kJ E)
5.33 103 kJ Answer:
34)
How
much energy (in kJ) is required to ionize 2.78 moles of hydrogen atoms? A)
2.74 103 kJ B)
4.72 103 kJ C)
1.66 103 kJ D)
3.65 103 kJ E)
5.89 103 kJ Answer:
D 35)
Determi end (final) value of n in a hydrogen atom transition, if the electron starts in n = 4 and the atom ne the emits a photon of light with a wavelength of 486 nm. A)
1 B)
5 C)
3 D)
4 E)
2 Answer:
E 36)
Determi end (final) value of n in a hydrogen atom transition, if the electron starts in n = 2 and the atom ne the absorbs a photon of light with a frequency of 4.57 1014 Hz. A)
3 B)
1 C)
4 D)
6 E)
7 Answer:
A 37)
Determi end (final) value of n in a hydrogen atom transition, if the electron starts in n = 1 and the atom ne the absorbs a photon of light with an energy of 2.044 10-18 J. A)
3 B)
4 C)
2 D)
5 E)
6 Answer:
B 38)
Each of following sets of quantum numbers is supposed to specify an orbital. Which of the following sets the of quantum numbers contains an error? A)
n = 2, l = 1 , ml = -1 B)
n = 4, l = 2, ml =0 C)
n = 3, l =3 , ml = -2 D)
n = 1, l = 0, ml =0 E)
n = 3, l = 0, ml =0 Answer:
39)
Each of following sets of quantum numbers is supposed to specify an orbital. Choose the one set of the quantum numbers that does not contain an error. A)
n = 2, l = 2, ml =-1 B)
n = 2, l = 2, ml =0 C)
n = 3, l = 2, ml =-3 D)
n = 4, l = 3, ml =-2 E)
n = 4, l = 2, ml =+4 Answer:
D 40)
Each of following sets of quantum numbers is supposed to specify an orbital. Choose the one set of the quantum numbers that does not contain an error. A)
n = 4, l = 4, ml =0 B)
n = 3, l = 2, ml =+3 C)
n = 4, l = 0, ml =-1 D)
n = 3, l = 1, ml = -2 E)
n = 5, l = 3, ml =-3 Answer:
E 41)
How
many orbitals are contained in the third principal level (n=3) of a given atom? A)
9 B)
3 C)
18 D)
7 E)
5 Answer:
A 42)
How
many sublevels are contained in the second shell (n=2) of a given atom? A)
1 B)
2 C)
9 D)
4 E)
3 Answer:
B 43)
Which
of the following statements are TRUE? A)
The principal quantum number (n) describes the shape of an orbital. B)
The angular momentum quantum number (l) describes the the size and energy associated with an orbital. C)
The D)
magnetic quantum number (ml) describes the orientation of the orbital.
An orbital is the path that an electron follows during its movement in an atom. E)
All of the
above are true. Answer:
C 44)
Which
of the following statements are TRUE? A)
We can B)
sometimes know the exact location and speed of an electron at the same time.
All orbitals in a given atom are roughly the same size. C)
Since electrons
D)
have mass, we must always consider them to have particle properties and never wavelike properties.
Atoms are roughly
E)
spherical because when all of the different shaped orbitals are overlapped, they take on a spherical shape.
All of the
above are true. Answer:
D 45)
Which
of the following occur as the energy of a photon increases? A)
the B)
frequency decreases.
the speed C)
increases.
the D)
deBroglie wavelength increases
the E)
wavelength gets shorter.
None of the above occur as the energy of a photon increases. Answer:
E 46)
Which
of the following occur as the wavelength of a photon increases? A)
the B)
frequency decreases
the energy C)
increases
the speed D)
decreases
Planck's E)
constant decreases
None of the above occur as the wavelength of a photon increases. Answer:
A 47)
How
much energy (in kJ) do 3.0 moles of photons, all with a wavelength of 655 nm, contain? A)
183 kJ B)
303 kJ C)
394 kJ D)
548 kJ E)
254 kJ Answer:
D 48)
What
total energy (in kJ) is contained in 1.0 mol of photons, all with a frequency of 2.75 1014 Hz? A)
182 kJ B)
219 kJ C)
457 kJ D)
326 kJ E)
110 kJ Answer:
E 49)
Determi shortest frequency of light required to remove an electron from a sample of Ti metal, if the ne the binding energy of titanium is 3.14 103 kJ/mol. A)
7.87 x 1015 Hz B)
4.74 x 1015 Hz C)
2.11 x 1015 Hz D)
1.27 x 1015 Hz E)
6.19 x 1015 Hz Answer:
A 50)
Determi longest wavelength of light required to remove an electron from a sample of potassium metal, if ne the the binding energy for an electron in K is 1.76 103 kJ/mol. A)
147 nm B)
68.0 nm C)
113 nm D)
885 nm E)
387 nm Answer:
51)
Which
of the following quantum numbers describes the shape of an orbital? A)
principal B)
quantum number
magnetic C)
quantum number
spin D)
quantum number
shrdinger E)
quantum number
angular
momentum quantum number Answer:
E 52)
Which
of the following quantum numbers describes the orientation of an orbital? A)
magnetic B)
quantum number
principal C)
quantum number
angular D)
momentum quantum number
spin E)
quantum number
schrdinger quantum number Answer:
A 53)
How
many different values of ml are possible in the 4f sublevel? A)
1 B)
7 C)
3 D)
5 E)
2 Answer:
B 54)
How
many different values of l are possible in the third principle level? A)
1 B)
2 C)
3 D)
0 E)
4 Answer:
C 55)
How
many different values of ml are possible in the 3d sublevel? A)
2 B)
1 C)
3 D)
5 E)
7 Answer:
D 56)
If two
electrons in the same atom have the same value of "l", they are A)
in the same level and sublevel. B)
in the same level, but different sublevel. C)
in the same orbital. D)
in different E)
levels and in different shaped orbitals.
None of the above Answer:
57)
In which orbital below would an electron (on average) be farthest from the nucleus? A)
1s B)
4f C)
3s D)
3d E)
2p Answer:
B 58)
In which orbital below would an electron (on average) be closest to the nucleus? A)
2p B)
4s C)
2s D)
5d E)
3p Answer:
C 59)
Sketch
one of the 3p orbitals below. How are they different from the 2p orbitals? Answer:
They are larger in size and contain additional nodes.
60)
How
many orbitals are contained in the n=2 level? Give the l and ml values of each of them. Answer:
Four. The 2s and three 2p orbitals. 2s, l = 0, ml = 0; 2p, l = 1, ml = -1 and l = 1, ml = 0 and l =1, ml = +1.
61)
Why do atoms only emit certain wavelengths of light when they are excited? (Why do line spectra exist?) Answer:
The energies of atoms are quantized. When an electron moves from one energy level to another during emission, a specific wavelength of light (with specific energy) is emitted. The electrons are not allowed "in between" quantized energy levels and thus there is no continuous spectrum observed.
62)
What is the photoelectric effect? Answer:
It is the observation that many metals emit electrons when light of high enough energy is shone on them. This observation brought our classical view of light into question.
63)
Why
don't we observe the deBroglie wavelength of everyday macroscopic objects? Answer:
Due to the large mass of macroscopic objects, the deBroglie wavelength is extremely small. The wavelength is so small that it is impossible to detect compared to the size of the object.
Match the following. 64)
n = 1 to n=2
A) 1 03 nm 6 5 )
n = 3 to n=1
B) 1 22 nm 6 6 )
n = 2 to n=3
C) 7 460 nm 6 7 )
n = 6 to n=5
D) 1 280 nm 6 8 )
n = 5 to n=3
E) 6 57 nm 6 4 )
65)
66)
67)
68)
Você também pode gostar
- Chapter 07tifDocumento38 páginasChapter 07tifAthar RizwanAinda não há avaliações
- Chapter 7 The Quantum-Mechanical Model of The Atom: Principles of Chemistry: A Molecular Approach 2e (Tro)Documento18 páginasChapter 7 The Quantum-Mechanical Model of The Atom: Principles of Chemistry: A Molecular Approach 2e (Tro)rulaalabadi265Ainda não há avaliações
- Chemistry Canadian 2Nd Edition Silberberg Test Bank Full Chapter PDFDocumento36 páginasChemistry Canadian 2Nd Edition Silberberg Test Bank Full Chapter PDFdolores.cook959100% (13)
- Chemistry A Molecular Approach Canadian 2nd Edition Tro Test Bank DownloadDocumento41 páginasChemistry A Molecular Approach Canadian 2nd Edition Tro Test Bank DownloadDaniel Grubbs100% (18)
- Chemistry 11th Edition Chang Test Bank DownloadDocumento21 páginasChemistry 11th Edition Chang Test Bank DownloadMatthew White100% (21)
- C1100e TDDocumento27 páginasC1100e TDghadirtaleb4Ainda não há avaliações
- Practice Test 3 Current PDFDocumento9 páginasPractice Test 3 Current PDFBabeejay2Ainda não há avaliações
- Chang Chemistry - Assessment Chapter 7Documento10 páginasChang Chemistry - Assessment Chapter 7haha_le12Ainda não há avaliações
- Chapter 7 Practice Test AtomStrctrPeriodicTrend GOOD-KEY1Documento5 páginasChapter 7 Practice Test AtomStrctrPeriodicTrend GOOD-KEY1Senthereng MoaisiAinda não há avaliações
- Wolfson Eup3 Ch34 Test BankDocumento17 páginasWolfson Eup3 Ch34 Test BankifghelpdeskAinda não há avaliações
- MCQs For Chapter 7-12 KeyDocumento11 páginasMCQs For Chapter 7-12 KeyismahijAinda não há avaliações
- Electrons & ProtonsDocumento31 páginasElectrons & ProtonsBiprodeep14Ainda não há avaliações
- Review Exam 2Documento9 páginasReview Exam 2justinsong213Ainda não há avaliações
- 11em Chemistry Jee Neet 2 and 3 1708673426Documento7 páginas11em Chemistry Jee Neet 2 and 3 1708673426shanickschoolAinda não há avaliações
- Extra Practice Week 7Documento2 páginasExtra Practice Week 7ShawnAinda não há avaliações
- Atomic Structure: Useful ConstantsDocumento10 páginasAtomic Structure: Useful ConstantsSrinjoy BanerjeeAinda não há avaliações
- Chapter 25 Modern Optics and Matter Waves: Physics For Scientists and Engineers, 2e (Knight)Documento16 páginasChapter 25 Modern Optics and Matter Waves: Physics For Scientists and Engineers, 2e (Knight)Nicole LopezAinda não há avaliações
- Exam1 16Documento12 páginasExam1 16dyrbrmAinda não há avaliações
- Model Question Paper HP Board of School Education Dharamshala Term - II Session: 2021-22 Subject: Physics Class: XII (Regular)Documento5 páginasModel Question Paper HP Board of School Education Dharamshala Term - II Session: 2021-22 Subject: Physics Class: XII (Regular)Jack DourAinda não há avaliações
- Structure of Atom NEETDocumento6 páginasStructure of Atom NEETKavita JainAinda não há avaliações
- Practice Questions For Ch. 7: Identify The Choice That Best Completes The Statement or Answers The QuestionDocumento26 páginasPractice Questions For Ch. 7: Identify The Choice That Best Completes The Statement or Answers The QuestionPaolo PepsAinda não há avaliações
- CT 1 ChemistryDocumento7 páginasCT 1 Chemistrykiruthikpranav147Ainda não há avaliações
- Chapter 2Documento62 páginasChapter 2Satish VermaAinda não há avaliações
- Kcet 2018 Answer Keys by Expert Coaching Classes PDFDocumento41 páginasKcet 2018 Answer Keys by Expert Coaching Classes PDFPrashanth GAinda não há avaliações
- Chemistry Atomic StructureDocumento12 páginasChemistry Atomic Structureraghavendra jAinda não há avaliações
- 6.1 Multiple-Choice and Bimodal QuestionsDocumento18 páginas6.1 Multiple-Choice and Bimodal QuestionsQuốc Thắng NguyễnAinda não há avaliações
- Probles Efecto Compton SolucionDocumento4 páginasProbles Efecto Compton SolucionFrank CamachoAinda não há avaliações
- PhysicsDocumento2 páginasPhysicsSai RameshAinda não há avaliações
- NCERT Solutions For Class 11 Chemistry Chapter 2 Structure of AtomDocumento1 páginaNCERT Solutions For Class 11 Chemistry Chapter 2 Structure of Atomaadit.25520Ainda não há avaliações
- Sjesc 102Documento48 páginasSjesc 102Taksh JoshiAinda não há avaliações
- Physics SolutionDocumento28 páginasPhysics SolutionsharadamastaAinda não há avaliações
- Dual Nature of Radiation and Matter QuestionsDocumento13 páginasDual Nature of Radiation and Matter QuestionsRohit Karandikar0% (1)
- Atomic Structure AnswersDocumento9 páginasAtomic Structure Answerspihu aliAinda não há avaliações
- KIET PhysicsDocumento82 páginasKIET Physicsap cpianAinda não há avaliações
- Question Bank On Ir Spectroscopy-MatDocumento10 páginasQuestion Bank On Ir Spectroscopy-MatRohan Sharma33% (3)
- BSNL Tta Exam PaperDocumento8 páginasBSNL Tta Exam PaperRajnish Chandra Prasad100% (1)
- JEE Main 2020 Question Paper Solutions 7 January MorningDocumento38 páginasJEE Main 2020 Question Paper Solutions 7 January MorningSionna KatiyarAinda não há avaliações
- Physics 104 Long Quiz Sample ADocumento4 páginasPhysics 104 Long Quiz Sample AMico de LeonAinda não há avaliações
- Chapter 2 - Structure of Atom: Page No 65Documento48 páginasChapter 2 - Structure of Atom: Page No 65BEAST王VIPER GamingAinda não há avaliações
- Practice TestDocumento0 páginaPractice TestTooba Sardar100% (1)
- Walker4 ISM Ch32Documento31 páginasWalker4 ISM Ch32Alejandro Romero Mejia100% (1)
- Btech Model QuestionsDocumento22 páginasBtech Model QuestionsAkshayKannanAinda não há avaliações
- Jipmer 2015 Solved QPDocumento67 páginasJipmer 2015 Solved QPS Balagopal Sivaprakasam67% (3)
- Chapter 7Documento18 páginasChapter 7roxy8marie8chanAinda não há avaliações
- Final Exam ReviewModern Physics 1528318833428 TCDocumento24 páginasFinal Exam ReviewModern Physics 1528318833428 TCFrans BorokoAinda não há avaliações
- h λ h λ nh λ λ: t t= t t= tDocumento2 páginash λ h λ nh λ λ: t t= t t= tMuhammad Hassan MaqsoodAinda não há avaliações
- A, A, A B) A, A, A C) A, A, A D) A, A, ADocumento18 páginasA, A, A B) A, A, A C) A, A, A D) A, A, Avenki786Ainda não há avaliações
- HW1 Chem 101 2020Documento6 páginasHW1 Chem 101 2020Muqeem MahmoodAinda não há avaliações
- PhysicsDocumento28 páginasPhysicsSuleman KhanAinda não há avaliações
- Phys102 Final-181 Zero Version Coordinator: Kunwar Saturday, December 15, 2018Documento18 páginasPhys102 Final-181 Zero Version Coordinator: Kunwar Saturday, December 15, 2018Nano SuyatnoAinda não há avaliações
- CHEM101 - Exam 1 - Version A - Final - AnswersDocumento7 páginasCHEM101 - Exam 1 - Version A - Final - AnswersSaudi ArabiaAinda não há avaliações
- Gen Chem Diagnostic TestDocumento11 páginasGen Chem Diagnostic TestNeil CorveraAinda não há avaliações
- 316 Midterm ExamDocumento3 páginas316 Midterm ExamKhaled AbeedAinda não há avaliações
- Chapter 2 Structure of AtomDocumento48 páginasChapter 2 Structure of AtomRehan AhmadAinda não há avaliações
- O level Physics Questions And Answer Practice Papers 2No EverandO level Physics Questions And Answer Practice Papers 2Nota: 5 de 5 estrelas5/5 (1)
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNo EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportAinda não há avaliações
- ATI TEAS Calculation Workbook: 300 Questions to Prepare for the TEAS (2023 Edition)No EverandATI TEAS Calculation Workbook: 300 Questions to Prepare for the TEAS (2023 Edition)Ainda não há avaliações
- Answer Phyiscs Paper 2 Form 5 Midterm 2011Documento6 páginasAnswer Phyiscs Paper 2 Form 5 Midterm 2011Saidin HashimAinda não há avaliações
- MIST MSc-EECE SyllabusDocumento28 páginasMIST MSc-EECE SyllabusSajid100% (1)
- Science10 ActivityDocumento12 páginasScience10 ActivityTitanium NohorAinda não há avaliações
- MCQ AntennaDocumento26 páginasMCQ AntennaRadha Nandhini100% (5)
- Information Brochure Cit Entrance Examination 2019 17Documento70 páginasInformation Brochure Cit Entrance Examination 2019 17Sarash smit kashyapAinda não há avaliações
- An/Trc - 170 TrainingDocumento264 páginasAn/Trc - 170 Trainingkapenrem2003Ainda não há avaliações
- VSWR Measurement, Microwave Engineering, Microwave Measurement, Power Meter, Measurement of Standing Wave PatternsDocumento16 páginasVSWR Measurement, Microwave Engineering, Microwave Measurement, Power Meter, Measurement of Standing Wave PatternsvlsijpAinda não há avaliações
- Dehydration Class LectureDocumento66 páginasDehydration Class Lecturenabil100% (1)
- ABB - KPM - All - Products Eng v1.0 - FINALDocumento12 páginasABB - KPM - All - Products Eng v1.0 - FINALJeff RobertAinda não há avaliações
- Scopus and Wen of Science Journal ListDocumento4 páginasScopus and Wen of Science Journal ListRajendra BhosaleAinda não há avaliações
- Micowave Survey Knowledge-20080226-ADocumento114 páginasMicowave Survey Knowledge-20080226-AFerry Kurniawan100% (4)
- Huawei Transmission For Sale From Power Storm 4SN07121188Documento4 páginasHuawei Transmission For Sale From Power Storm 4SN07121188Ariston AlcantaraAinda não há avaliações
- Antenna 2Documento4 páginasAntenna 2Glaiza Lyn GuilebAinda não há avaliações
- Rethinking The Relationship Between Ew and Emso: Journal of Electromagnetic DominanceDocumento44 páginasRethinking The Relationship Between Ew and Emso: Journal of Electromagnetic DominanceDIckHEAD100% (1)
- MSthesis Raja July2002Documento152 páginasMSthesis Raja July2002Rajasekarakumar VadapooAinda não há avaliações
- Small Cell Backhul The Big PictureDocumento22 páginasSmall Cell Backhul The Big PictureAnonymous 6tuR1hzAinda não há avaliações
- Microwave InspectionDocumento10 páginasMicrowave InspectionZaid TariqAinda não há avaliações
- Grade-10-Module-1-Electromagnetic-Spectrum-Second-EditionDocumento11 páginasGrade-10-Module-1-Electromagnetic-Spectrum-Second-EditionroseAinda não há avaliações
- SabihDocumento10 páginasSabihSabih HaiderAinda não há avaliações
- FYP Titles Offering 2012Documento33 páginasFYP Titles Offering 2012Nurul Amirah JamaluddinAinda não há avaliações
- HX-presentation-main Products Lines VersionDocumento35 páginasHX-presentation-main Products Lines Versionwahyu kurniawanAinda não há avaliações
- Performance of A Low-Density Hypersonic Magneto-Aerodynamic FacilityDocumento21 páginasPerformance of A Low-Density Hypersonic Magneto-Aerodynamic FacilityReshad NawoorAinda não há avaliações
- Mwe Lab Instruction Manual New FinalDocumento120 páginasMwe Lab Instruction Manual New Finalkalyan chakravarthyAinda não há avaliações
- Conical Origami Reconfigurable Antenna Using LabviewDocumento11 páginasConical Origami Reconfigurable Antenna Using LabviewATHIRA V RAinda não há avaliações
- A Compact Microstrip-Fed Patch Antenna With Enhanced Bandwidth and Harmonic SuppressionDocumento8 páginasA Compact Microstrip-Fed Patch Antenna With Enhanced Bandwidth and Harmonic SuppressionBouhafs AbdelkaderAinda não há avaliações
- D-Band Frequency Tripler For Passive Imaging - Final 13th JulyDocumento4 páginasD-Band Frequency Tripler For Passive Imaging - Final 13th JulyTapas SarkarAinda não há avaliações
- Physiotherapy and Electrotherapy EquipmentDocumento41 páginasPhysiotherapy and Electrotherapy EquipmentResidentes PmrAinda não há avaliações
- Rectenna Serves 2.45-Ghz: Wireless Power TransmissionDocumento4 páginasRectenna Serves 2.45-Ghz: Wireless Power TransmissionArnab PattanayakAinda não há avaliações
- Electromagnetic WeaponsDocumento58 páginasElectromagnetic WeaponsutkarshAinda não há avaliações
- Cell Tower Radiation EffectsDocumento4 páginasCell Tower Radiation EffectsNeha Kumar100% (1)