Escolar Documentos
Profissional Documentos
Cultura Documentos
EEG Default Mode Network in The Human Brain
Enviado por
Mdcarmen AranaDescrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
EEG Default Mode Network in The Human Brain
Enviado por
Mdcarmen AranaDireitos autorais:
Formatos disponíveis
www.elsevier.
com/locate/ynimg NeuroImage 41 (2008) 561 574
EEG default mode network in the human brain: Spectral regional field powers
Andrew C.N. Chen, Weijia Feng, Huixuan Zhao, Yanling Yin, and Peipei Wang
Center for Higher Brain Functions, Capital Medical University, Beijing 100069, China Received 17 September 2007; revised 12 December 2007; accepted 19 December 2007 Available online 15 January 2008
Eyes-closed (EC) and eyes-open (EO) are essential behaviors in mammalians, including man. At resting EC-EO state, brain activity in the default mode devoid of task-demand has recently been established in fMRI. However, the corresponding comprehensive electrophysiological conditions are little known even though EEG has been recorded in humans for nearly 80 years. In this study, we examined the spatial characteristics of spectral distribution in EEG field powers, i.e., sitting quietly with an EC and EO resting state of 3 min each, measured with high-density 128-ch EEG recording and FFT signal analyses in 15 right-handed healthy college females. Region of interest was set at a threshold at 90% of the spectral effective value to delimit the dominant spatial field power of effective energy in brain activity. Low-frequency delta (0.53.5 Hz) EEG field power was distributed at the prefrontal area with great expansion of spatial field and enhancement of field power (t = 2.72, p b 0.02) from the EC to the EO state. Theta (47 Hz) EEG field power was distributed over the fronto-central area and leaned forward from EC to the EO state but with drastic reduction in field power (t = 4.04, p b 0.01). The middle-frequency alpha-1 (7.5 9.5 Hz) and alpha-2 (1012 Hz) EEG powers exhibited bilateral distribution over the posterior areas with an anterior field in lower alpha-1. Both showed significantly reduction of field powers (respectively, W = 120, p b 0.001 for alpha-1; t = 4.12, p b 0.001 for alpha-2) from EC to the EO state. Beta-1 (1323 Hz) exhibited a similar spatial region over the posterior area as in alpha-2 and showed reduction of field power (t = 4.42, p b 0.001) from EC to the EO state. In contrast, high-frequency beta-2 and gamma band exhibited similar, mainly prefrontal distribution in field power, and exhibited no change from EC to the EO state. Corresponding correlation analyses indicated significant group association between EC and EO only in the field powers of delta (r = 0.95, p b 0.001) and theta (r = 0.77, p b 0.001) band. In addition, the great inter-individual variability (90 folds in alpha-1, 62 folds in alpha-2) in regional field power was largely observed in the EC state (10 folds) than the EO state in subjects. To summarize, our study depicts a network of spectral EEG activities simultaneously operative at well defined regional fields in the EC state, varying specifically between EC and EO states. In contrast to transient EEG spectral rhythmic dynamics, current study of long-lasting (e.g. 3 min)
spectral field powers can characterize state features in EEG. The EEG default mode network (EEG-DMN) of spectral field powers at rest in the respective EC or EO state is valued to serve as the basal electrophysiological condition in human brain. In health, this EEGDMN is deemed essential for evaluation of brain functions without task demands for gender difference, developmental change in age span, and brain response to task activation. It is expected to define brain dysfunction in disease at resting state and with consequences for sensory, affective and cognitive alteration in the human brain. 2008 Published by Elsevier Inc. Keywords: EEG field power; 90% effective power; Regional distribution; Default mode network; EC-EO state; Individual differences
Introduction EEG and Brain Function/Dysfunction EEG reflects vital brain activities, in fetal (Preissl et al., 2004) and from neonate (Vanhatalo and Kaila, 2004) to aging (Cummins and Finnigan, 2007) in health and in disease. The function of EEG in the sleep/awake state (Chornet-Lurbe et al., 2002), perception (e.g. Basar et al., 2006; Andrade and Bhattacharya, 2003), attention (Vuilleumier and Driver, 2007), memory (Klimesch et al., 2006a; Wilding and Herron, 2006), emotion (Cacioppo, 2004), thinking (Srinivasan, 2007), as well as dysfunction in epilepsy (Benbadis, 2006), autism (Kagan-Kushnir et al., 2005), dyslexia (Arns et al., 2007), AD/HD (Barry et al., 2007; Hobbs et al., 2007), anxiety (Smit et al., 2007), depression (Hunter et al., 2007), MCI (van der Hiele et al., 2007; Buchem et al., 2007), dementia (Babiloni et al., 2006; Rossini et al., 2006), schizophrenia (Prichep, 2007), coma (Husain, 2006), vegetative state (Guerit, 2005), and even death (Sediri et al., 2007) is largely documented. In fact, EEG dynamics is closely impacted in man's whole life. In additional to genetic factors (Begleiter and Porjesz, 2006), one of the main effects on the EEG is the state of eyes-closed (EC) and eyes-open (EO) differentiation at rest. In the course of one's life, we often consciously or unconsciously lapse into an eyes-closed state, sometimes transiently
Corresponding author. E-mail address: ac@cpums.edu.cn (A.C.N. Chen). Available online on ScienceDirect (www.sciencedirect.com). 1053-8119/$ - see front matter 2008 Published by Elsevier Inc. doi:10.1016/j.neuroimage.2007.12.064
562
A.C.N. Chen et al. / NeuroImage 41 (2008) 561574
within a second, briefly in minutes or even in fraction of hour not counting napping-sleep. Yet the majority of time in the remaining hours we live in an eyes-open state. In state of eyes-open, the brain is bombarded with tremendous bits of visual stimuli. At the resting eyes-closed state, it was argued recently that the brain is in a default mode when devoid of any external task (Raichle et al., 2001). The Default Mode Brain State defines a baseline state in the human brain lying quietly with eyes-closed (Raichle et al., 2001, Raichle and Synder, 2007) and is of great interest in that element for the default-mode network (DMN) or connectivity is readily measured from PET (Raichle et al., 2001; Geday et al., 2007) and fMRI (e.g. Damoiseaux et al., 2006). Measurement in health can be related to: (a) stable oxygen extraction function of organized resting brain function and suspended upon specific goal-directed behaviors (Raichle et al., 2001); (b) both medial prefrontal cortex and anterior cingular cortex are embedded in an organized network for initiation of the functional self (Gusnard et al., 2001); (c) dorsal mPFC increased in internal feeling judgment and ventral mPFC reduced in external cognitive judgment (Gusnard et al., 2001); (d) network connectivity of ventral anterior cortex and post cingulate cortex enhanced during resting state (Greicius et al., 2003), or the ventral medial prefrontal cortex and posterior cingulate cortex exhibiting largest activation at rest and deactivation upon tasks (Greicius and Menon, 2004); (e) slow oscillation between internal introspection and external extrospection of brain state at rest (Fransson, 2005; Bellec et al., 2006), or slow respiratory activity (Birn et al., 2006); (f) intrinsic visual, auditory, somatosensory and olfactory sensory cortical activation in silence at rest (Hunter et al., 2006; Wilson et al., 2007); (g) attention and memory processing (Fransson, 2006; Esposito et al., 2006); (h) age-related DMN (Grady et al., 2006; Damoiseaux et al., in press); (i) essential patterns in the DMN (Damoiseaux et al., 2006); and (j) light-sleep, even in the absence of conscious awareness during light sleep (Horovitz et al., 2007). However, a different opinion on the nature of fMRI-DFN is also recently raised (Fransson, 2006). Subsequently, the fMRI-DMN alternations in brain malfunctions are now demonstrated in fragile X syndrome (Menon et al., 2004), attention disorder (Helps et al., in press; Castellanos et al., 2008), epilepsy (Laufs et al., 2007), Tourette's syndrome (Marsh et al., 2007), anxiety disorder (Zhao et al., 2007), bipolar disorder (Calhoun et al., in press), major depression (Greicius et al., 2007), schizophrenia (Harrison et al., 2007; Garrity et al., 2007; Calhoun et al., in press; Zhao et al., 2007), mild cognitive impairment (Rombouts et al., 2005) and Alzheimer's disease (Wang et al., 2006; Rombouts et al., in press; Sorg et al., 2007). In neuroimaging, most of the study conditions comparing the fMRI-DFN are relied on the EC but little work on EO state. Issues When one closes his/her eyes, one never ceases feeling and thinking, unless under adequate anesthesia, in a dysfunctional mode of coma, vegetative state or death. From the literature on the default mode of brain function in fMRI, the distinction of the eyes-closed condition is well studied but the eyes-open resting condition has been infrequently made. In contrast since Hans Berger, our knowledge of EC and EO in nearly 80 years of studying EEG is limited to a few transient classical alpha block (Pollen and Trachtenberg, 1972) or contemporary alpha desynchronization (Pfurtscheller et al., 1996; Klimesch et al., 2007; Neuper et al., 2006) studies. EEG has largely been dealt with as a set of isolated frequency fluctuations
in the temporal waveform domain, much less up to now as a set of spectral field energy in the spatial domain. Awealth of information is perhaps under-exploited regarding the spectral energy changes at regional brain areas, other than the alpha-block studies, largely at the visual field of the occipital brain upon EC to EO state. Study aims Taking into consideration the aforementioned, this study was aimed at examining (a) the full spatial distribution of the spectral field powers at the basic EC state, (b) how these regional spectral field powers varies from the EC to EO state, (c) the individual characteristics of regional spectral field powers in both states, and (d) if some associated networks of regional spectral field powers exist in either EC or the EO state. Materials and methods Subjects Fifteen healthy female volunteers (age: 2026 years old) from the University community participated in the study. Subjects were excluded from the study if they had any physical/psychological problem or were using medication. Written informed consent was obtained from each subject in accordance with the Helsinki Declaration, and the study was approved by the local ethical committee. Experimental design Each subject was asked to sit in an armchair, throughout the duration of the experiment, in a quiet room (temperature: 2224 C) at shaded daylight. Each subject was asked to keep the eyes-closed for 3 min and eyes-open for another 3 min, in that order, while EEG was recorded. Each subject was not given any instruction but asked to stay fully relaxed. EEG recordings and pre-treatment of EEG A high-density 128-ch EEG recording, using Ag/AgCl electrodes and two channels of electro-oculogram (EOG) were continuously gathered for 3 min during the EC and EO states, respectively with the A.N.T. EEG System (A.N.T. Enschede, Netherlands). The electrodes were mounted according to the 10/5 montage system (Oostenveld and Praamstra, 2001). The 3D 128-ch montage and nomenclature is depicted in Fig. 1. Bilateral EOG was recorded from the horizontal and vertical sites to monitor blinking or eye movements. The portion of EOG contamination of each scalp trace was removed in the off-line analysis. All the EEG channels were recorded in an averaged reference but were offline bilaterally re-referenced (averaged A1 + A2). EEG data were sampled at 512 Hz and electrode impedance was kept lower than 5 k. EEG data were filtered with 0.5100 Hz bandpass filter off-line and were subjected to epoching (2 s each), linear-detrend, and artefact rejection pre-processing. The artifact rejection methods consisted of exclusion in epoch with large amplitude (over 80 V), DC bias, blinks, and slow eye movement coincident with EOG. Bad electrodes were replaced with the extrapolated virtue values from the neighboring electrodes. After rejection of EOG contamination and non-specific artifacts, each set of EEG data (2-s epoch) was subjected to Fast-Fourier Transform (FFT) analysis to obtain the absolute EEG band power (v2) at each electrode in the
A.C.N. Chen et al. / NeuroImage 41 (2008) 561574
563
Fig. 1. EEG recording electrode sites and nomenclatures. Superior-anterior view (left panel) and superior-posterior view (right panel) perspectives of the 128-ch EEG electrode nomenclature in 10/5 montage.
following 7 bands: Delta (0.53.5 Hz), theta (47 Hz), Alpha-1 (7.59.5 Hz), Alpha-2 (1012 Hz), Beta-1 (1323 Hz), Beta-2 (24 34 Hz) and Gamma (3545 Hz). These broad bands were defined by the conventional IFCN guideline (Nuwer et al., 1999) but not according to individuals' spectral characteristics (cf. Doppelmayr et al., 1998; Klimsch, 1999).
For each EC and EO state, each 3-min period EEG was analyzed in 2 sec epochs, resulting in 90 epochs. Out of them, around 7080 valid epochs at the EC state and 6080 epochs at the EO state across the 15 subjects, on average, were subjected to further analyses. For each EC and EO state the valid epochs were averaged after procedure of fast Fourier Transform (FFT) performed by ASA 3.0 software (A.N.T.
Fig. 2. Topographic spectral mapping of low-frequency delta and theta field powers in EC vs. EO states. Delta field power was distributed over the prefrontal area and the theta field power at the fronto-central area. Increase and expansion of delta field power, but marked reduction of theta field power were respectively shown from EC to EO state.
564
A.C.N. Chen et al. / NeuroImage 41 (2008) 561574
Fig. 3. Left panels: t-tests of EC and EO field power showed significant increase of delta, but radical reduction of theta field power from EC to EO. Right panels: Correlation analyses indicated significant associations of the values between EC and EO states for delta and theta. Greater inter-individual variability was noted in EC than in EO state for both field powers. Subjects were ordinally listed from the EC state.
Enschede, Netherlands). The grand averages of FFT maps of different stages in topographic maps (256 hues) of the mean amplitude of the surface EEG power were calculated on a 3D quasi-realistic cortical model by a spline interpolating function (Tandonnet et al., 2005; Babiloni et al., 2006; Perrin et al., 1987). For each of the 7 EEG bands, a scalp field power spectral map was interpolated from the 128-ch EEG and the site/electrode of maximal power was isolated with its value calculated. Through automatic scaling, the range of maximum and minimum values in EEG spectral power was established. Region of Interest (ROI) The total field power (sum of the electrode spectral power) in each spectral EEG bands within a region of interest (ROI) was
Table 1 Magnitudes of the individual differences at the EC and EO state (field power in V2) EC max/min = ratio Delta Theta Alpha-1 Alpha-2 Beta-1 Beta-2 Gamma 845/151 = 5.91 29.3/6.7 = 4.40 230/2.3 = 90.60 62.7/1.0 = 62.70 20.3/4.1 = 5.00 22.9/1.9 = 12.16 33.7/0.9 = 36.18 EO max/min = ratio 582/90 = 10.86 12.3/5.2 = 2.00 20.5/2.1 = 9.54 20.5/2.1 = 9.54 11.6/3.7 = 3.15 5.6/2.5 = 7.62 29.4/1.3 = 21.42 EC/EO 5.91/10.86 = 0.54 4.4/2.0 = 2.20 90.6/9.54 = 10.34 62.7/8.12 = 7.10 5.0/3.15 = 1.59 12.16/7.62 = 1.59 36.18/21.42 = 1.69
calculated, respectively in each of the 7 broad bands at the EC and EO state, as dependent measure. The ROI was spatially delimited by the area exhibiting the band power value in electrodes exceeding a critical value that equaled to 90% of the effective value (90% EV), termed effective energy. As in the mapping of brain activity by PETfMRI, EEG activity in this study is based on the threshold criterion. From the threshold criterion, the effective size of spatial area and magnitude of activity can be defined and measured. The measured area in this study was the region of interest, while the measured magnitude was the dominant EEG power. From each spectral EEG band, separate spectral EEG power was calculated based on the sum of the measured power of all electrodes in the ROI. In the study, the 90% EV (effective value) was the sum of all EEG powers from the electrodes in the ROI, above 90% of the effective value. The EV was defined by the 5th upper percentile ranking value of the 128 electrodes, from the zero to maximum value of all the 128 EEG sites. Often, the maximal value has been used in the calibration range. However, we choose to use the 5th percentile ranking value, instead of the maximum value (the EEG site of the highest rank), for the sake of preventing the outlier electrode value, which might occur. In this manner, the use of 5th percentile ranking value for EV was more conservative and would effectively exclude the probable outlier maximum electrode (such as from a bad electrode site with a spurious effect on the EEG). Thus, we defined the ROI field power as the dominant spectral EEG field power. In this study, the 90% EV of spectral EEG well delimited the dominant regional field power respectively for delta, theta, alpha-1, alpha-2, beta-1, beta-2 and
A.C.N. Chen et al. / NeuroImage 41 (2008) 561574
565
Fig. 4. Topographic spectral mapping of the middle-frequency alpha-1 and alpha-2 field powers. The alpha-1 field power was distributed mainly at the posterior area with an anterior extension, while the upper alpha-2 field power was restricted at the posterior area. Both showed marked reduction in the field power from EC to EO state.
gamma broad bands in one of the two states. A grand average of the resting EC and EO states was computed then the statistical effects of the state change were conducted on the effective spectral field power at the regional areas in each of the 7 bands. For statistical comparison between states or across conditions, the ROI territory that contains the largest electrode array was selected as a template. As a general principle, the sum of the electrode values within the ROI in each subject was calculated for statistical analysis. In this manner, the dependent variable of interest was the field power of array of electrodes, but not a power value of a single electrode. In this study, the spatial template of delta field power was applied by the EO state since it exhibited higher 90% EV than that of the EC state, while the rest of spectral powers were applied by the EC state as these values were higher at the EC than EO state. The dominant 90% EV of the spectral field power in an EEG delimited a spectral regional area with 10-spacings set in each spectral field power range: below 10% in blue and above 90% in red between the range of zero and the top 90% EV (see the calibration bars in Figs. 2, 4, 6). Statistical analysis Statistical comparisons were performed on the scalp EEG field power by paired t-test for comparison of EC and EO states. We elected to use the raw values, i.e. without logarithmic transformation of the EEG values (e.g. van Albada and Robinson, 2007), to rely on the original recording for straightforward interpretation of EEG values. In our past experience, log transformation of EEG
values cannot completely eliminate the problem of statistical normality in the distribution of EEG values. Thus, if the data sets were confirmed to normality distribution, dependent t-test of means and the Pearsons correlation test on the association of spectral field powers between these two states were used. If not, the Wilcoxon test for nonparametric comparison of ranks and Spearman correlation of ranks were employed to evaluate the probability significance. In all tests, p b 0.05 was accepted as significant. The statistical program SigmaStat 3.04v (SPSS Inc, Chicago, USA) was used for statistical procedures. Results Low-frequency EEG field power Spatial Mapping In Fig. 1, the dominant delta field power was found to be at the prefrontal area for both the EC and the EO states. In comparison, theta power was distributed at the fronto-central area, centered over the vertex for both (EC and EO) states. Effect of the change of states From the EC to the EO state, the expansion of the delta field power in the left fronto-lateral region was noted, but reduction of theta field power in amplitude and space at the fronto-central area was clearly shown. These effects were verified and presented in Fig. 3. The t-tests independently demonstrated a marked enhance-
566
A.C.N. Chen et al. / NeuroImage 41 (2008) 561574
ment of the delta power (EC: mean 354.9 V2, EO: mean 407.4 V2; t = -2.72, p b 0.2), while a great reduction in the theta power (EC: mean 14.7 V2, EO: mean 9.1 V2; t = 4.04, p b 0.001) was found. Individual's profile The individuals varied greatly in EEG powers, largely in the resting EC than EO state. The right panels in Fig. 3 depict the magnitude of the variability reached as high as 5.91 folds in the delta power between the maximum and minimum values in the subjects at the EC state, but only 0.54 fold at the EO state (see Fig. 3, left panel; Table 1). For the theta field power, the ratios were 4.4 folds at the EC and 2.00 at the EO state (see Fig. 3, left panel; Table 1). All of the comparisons of the inter-individual variability are listed in the Table 1. Nevertheless, the measured field powers also displayed a set of significant correlations between EC and EO states in both delta and theta powers (r = 0.95, p b 0.001 and r = 0.77, p b 0.001, respectively).
power was restricted at the posterior area only with a clear contour at the EC state. At the EO state, both alpha-1 and alpha-2 field powers were not discernible in the regional topography under the same scaling at the EC state. Effect of the states From the EC to the EO state, the magnitude and spatial extent of the alpha-1 field power was greatly reduced (Fig. 5). The t-tests independently indicated a marked reduction of the alpha field power (EC: mean 54.9, median 41.6 V2, EO: mean 6.7, median 4.3 V2; W = 120, p b 0.001), while a similar large reduction of the upper alpha-2 field power was also evident (EC: mean 23.6 V2, EO: mean 5.6 V2; t = 4.12, b 001). Individuals profile Again, the great inter-individual variability in EEG powers of alpha-1 and alpha-2 was observed to be limited to the resting EC state unlike the EO state. From the Fig. 5 right panels, the magnitude of the variability reached to peak values of 90.6 folds in the alpha-1 field power between the maximum and minimum values in the subjects at the EC state, but 9.54 folds the EO state. Similar large values, a 62.7 folds increase in EC state and a 8.12 folds in the EO state for the upper alpha-2 field power were noted (see Table 1). No correlation was shown in either alpha-1 or alpha-2 between the EC and the EO states.
Middle-frequency EEG field power Spatial Mapping In Fig. 4, the dominant alpha-1 field power was found to be at the posterior area along with a frontal field extension much eminently at the EC than the EO state. In comparison, the upper alpha-2 field
Fig. 5. Left panels: W-test (Wilcoxon rank test) and t-test of EC and EO power comparison showed, respectively, significant decrease of both alpha-1 and alpha-2 field power from EC to EO. Right panels: Correlation analyses indicated non-significant (ns) association of the values between EC and EO states for alpha-1 and alpha-2 field powers. However, marked greater inter-individual variability was shown respectively at EC than EO state for both field powers. Subjects were ordinally listed from the EC state.
A.C.N. Chen et al. / NeuroImage 41 (2008) 561574
567
Fig. 6. Topographic spectral mapping of high-frequency beta-1 distributed at posterior area, beta-2 and gamma field powers (both distributed at the midline frontal and prefrontal areas) in EC vs. EO state. Large topographic change in the beta-1 field power was discerned.
High-Frequency EEG Field Power Spatial mapping In Fig. 6, the beta-1 field power was found to be over the posterior area as in the upper alpha-2 field power and exhibited reduction from the EC to the EO state. In comparison, the high-frequency beta-2 and gamma field powers were limited mainly at the prefrontal area. Effect of the states The observed statistical significant difference in beta-1 field power between the EC and EO states is shown in the top-left panel of Fig. 7. The t-test independently indicated a reduction of the beta-1 field power (EC: mean 12.9 V2, EO: mean 6.8 V2; t = 4.12, p b 0.001), while no significant effects were noted in both the beta-2 and gamma field powers.
Profile of the individuals The individual distribution profile of the beta-1 field power was greatly dissimilar and distinguished the beta-1 from those of the beta-2 and gamma field powers. All correlations between the EC and the EO states in these high-frequency beta-1, beta-2 and gamma field powers were proved not significant (right panels, Fig. 7). Spectral co-habilitation and correlations among the Field Powers at each EC and EO State At each of the EC or EO state, the dominant regional EEG field powers are shown to be a distinct set of spectral activities distributed in the head space. In Fig. 8, a composite model in each EC and EO state was respectively made from two viewing perspectives, superior-anterior (sa) and superior-posterior (sp) to
568
A.C.N. Chen et al. / NeuroImage 41 (2008) 561574
Fig. 7. Left panels: t-test of EC and EO field power comparison showed a significantly decrease of beta-1, but W-tests (Wilcoxon rank test) of EC and EO power comparisons showed no significant (ns) effects of beta-2 and gamma field powers from EC to EO state. Right panels: Correlation analyses indicated nonsignificant (ns) association of the values between EC and EO states for all the high-frequency spectral field powers. Great inter-individual variability in each spectral field power was noted. Subjects were ordinally listed from the EC state.
illustrate the coexistence of these spectral activities in space. This Fig. 8 was computerized by a virtual brain model in respective the EC and the EO state of 25 young male subjects studied in Denmark (data not published before). Again, the study data revealed a dramatic inter-individual variability in human EEG magnitude. Table 1 lists the maximum and minimum values of regional 7 spectral field powers. In 15 subjects a high end of 96.0 folds of alpha-1 and low end 4.4 of theta in the group at EC state was revealed. The ratios of maximum/minimum manifested to a lesser differentiation at the EO state, ranging from 0.86 of the delta field power to 8.12 folds of alpha-1 (drastically different from that in the EC state) in the EO state. The magnitude of the individual differences, comparing the EC and EO states is
presented in the rightist column of Table 1. The ratios in variability between the EC and EO states for alpha-1 and alpha-2 were 10.34 and 7.10 folds respectively, demonstrating markedly greater variability in the group at the EC than in the EO state for alpha-1 and alpha-2 field powers. Table 2a,b list the calculated cross-correlations among the 7 spectral field powers, respectively in EC and EO states. At the EC state, correlation analyses indicated that only the high frequency beta2 and gamma powers at the prefrontal head region were correlated (r = 0.92, p b 0.001). No other spatial EEG band powers in the EC state showed significant correlation (Table 2a). At the EO state, in contrast, the prefrontal delta field power exhibited significant correlation with the posterior beta-1 (r = 0.86, p b 0.001) and prefrontal
A.C.N. Chen et al. / NeuroImage 41 (2008) 561574
569
Fig. 8. Constellation of spectral field powers measured in a 3-min period depicts the regional distribution of the respective seven frequency band powers on the brain topography, at EC vs. EO state, viewed from superior-anterior (s. a.) and superior-posterior (s. p.) perspectives. The resting EEG DFN was composed from data gathered in 25 healthy college young males from Aalborg, Denmark, while the author Chen was directing the Human Brain Mapping Laboratory. Data were not published previously.
gamma field powers (r = 0.554, p = 0.036). In addition, the frontocentral theta field power was correlated with the anterior-posterior alpha-1 field power (r = 0.52, p = 0.046), while the prefrontal beta-2 and gamma field powers were significantly correlated (Table 2b). Discussion EEG DMN The main study findings indicate that the resting EEG in eyesclosed state devoid of stimulation or task is composed of a defined set of regional spectral activities in the classic 7 broad bands, termed EEG default mode network (EEG DMN). The distribution maps of spatial regional field powers are consistent with a previous observation using Danish subjects (Chen et al., 2006). This spatial distribution of the field EEG powers has not fully been appreciated. After completing the current work, an important off-press publication indicated that bold fMRI can be correlated with EEG powers in six widely distributed resting state networks, characterized by EEG features involved in combination with different brain rhythms
(Mantini et al., 2007). In EEG studies, it is necessary to define not only the spectral information but also the spatial characteristics of the EEG of subjective experience. Extending from current work in a preliminary report (Feng and Chen, submitted for publication), the relations of regional EEG field powers in listening to music compared to lecture (speech, 3 min eyes-closed) and variety of emotional ratings are effectively revealed, as to validate the nature of EEG DMN and brain functions. Dominant EEG Spectral Field Power and Function The spatial nature of the field EEG power may reflect some underlying co-activity in structure-function relations. For example, the eminent prefrontal delta EEG field power (see Fig. 2) could indicate some prefrontal metabolic/functional significance. Though it is tempted to categorize it as some residue of blinking/eyesmovement generated in the prefrontal subcortical site, two facts argue against it. The likelihood of a blinking artifact on the prefrontal delta EEG was diminished since our artifact algorithm was set at a threshold to eliminate all epochs exceeding 80 V such as
570
A.C.N. Chen et al. / NeuroImage 41 (2008) 561574
Table 2a EC state: cross correlations of the regional spectral field powers (correlation values in bold, p-values in italics; significant values in shaded field)
the blinking on the EEG (150 V). This argument was also not tenable as nearly no blinking episode was found in the EEG record during the EC state (only one blink across the 15 subjects). This conjecture may be viable with the EO state, which exhibited high frequency of blinking (mean: 37.4/min). However, no correlation of the blinking rate and the prefrontal EEG field power could be established (r = 0.344, p = 0.21). Recently, it has been shown that EEG is associated with cerebral metabolic rate, as it may explain 65% of the variance in the delta power (Boord et al., 2007). Also, the delta (1.54 Hz) power correlates with brain atrophy in Alzheimer's disease patients (Fernandez et al., 2005) as well as the amplitude of delta (24 Hz) sources with the prefrontal white matter across the mild cognitive impairment and Alzheimer's patients (Babiloni et al., 2006). At resting state, a positive correlation of medial frontal cortical metabolism with delta EEG power has been established (Alper et al., 2006). In the theta EEG power, 53% of the variance over age span is reported to be explained by cerebral metabolic rate (Boord et al., 2007). Our finding of the theta EEG field power distributed eminently in the fronto-central area (see Fig. 2) is consistent with the known frontal midline theta EEG activity (Inanaga, 1998). It is generated in the medial prefrontal cortex and/or anterior cingulate cortex in monkey (Tsujimoto et al., 2006) and in man (Cahn and Polich, 2006). The regional preeminent theta EEG has been related to infant arousal (Grigg-Damberger et al., 2007), attention (Sauseng et al., 2007), perception (Basar et al., 2006), memory (Onton et al., 2005), emotion (Guntekin and Basar, 2007), music (Feng and Chen, submitted for publication), and cognitive aging (Brickman et al., 2005). In the middle-frequency alpha EEG, we observed a frontal extension along with a more prominent posterior distribution in the lower alpha-1 field power (Fig. 4) either in congruent or at variance with the resting anterior alpha asymmetry in emotion (Cacioppo, 2004). The spectral field power in lower alpha-1 differentiated the upper alpha-2 field power with no anterior extension and only the similar posterior portion is of interest. The lower and upper alpha in EEG literature (Klimesch, 1997; Klimesch et al., 2006b) has been described in function but little is known on the spatial differentiation. Recently, the neural source of alpha EEG is revealed in the brain region of distinct cortical areas, such that the sleep spindle alphaEEG is likely from thalamus, the mu-rhythm in motor function is from central sulcus, and visual alpha EEG in perceptual function is from occipital lobes (Goncalves et al., 2006).
Correlations of the averaged power of alpha EEG (812 Hz) and bold fMRI signals can be found in occipital, parietal and even frontal lobes (Goncalves et al., 2006) but with wide individual variability at rest (de Munck et al., 2007). Nevertheless, the regional territory of the eminent alpha-1 and alpha-2 field powers reside more on the parietal area than occipital area (see Fig. 4). This observed alpha-1 and alpha-2 field powers in territory may lead to reconsider the important aspect of frontal and parietal alpha EEG in source and function, extending from the conventional occipital alpha EEG. In high frequency beta-1, beta-2 and gamma EEG field powers, the observed beta-1 field power in regional distribution, similar to that of alpha-2 at the posterior scalp area (Fig. 6), suggests that beta-1 as a spectral lingering of alpha-2 activity and belongs to the same family. It is proposed to call beta-2 as the high-frequency alpha-3, a plausibility that further remains to be proven. The great similarity in distribution at the bilateral temporal area and the prefrontal area in the beta-2 and gamma EEG field powers (Fig. 6) and their strong correlation at both the resting EC and EO states (Table 2a,b) renders these beta-2 and gamma EEG in the same family. In the past, little spatial information of the beta and gamma EEG field in human brain has been reported in literature. Due to their tiny EEG power, in the range less than 3 V in amplitude, the distribution of these activities at some discrete field opens to new challenge for understanding the structurefunction relations in beta-gamma EEG, thought to reflect binding of perception in brain function (Bertrand and Tallon-Baudry, 2000). The transient beta activity in motor function is associated with fMRI bold activation of the sensorimotor cortex (Parkes et al., 2006), while the transient gamma activity in visual perception with bold activation of the calcarine sulcus (Hoogenboom et al., 2006). Of special interest is the site of eminent gamma field power at the dorso-lateral frontal areas in the left hemisphere, which is noted in the differentiation of music from speech perception (Feng and Chen, submitted for publication). We now are in the process to validate the cortical and subcortical structures of the observed regional scalp EEG field powers described in this study, by current source imaging of LORETA on spectral EEG field powers in Talairach standard brain modeling. State changes The spectral topographic distributions showing little changes attest the stability of the underlying structures associated with the EEG field powers. Instead, the major changes largely in reduction of the field power, except the increase of prefrontal delta, are the
Table 2b EO state: cross correlations of the regional spectral field powers (correlation values in bold, p-values in italics; significant values in shaded field)
A.C.N. Chen et al. / NeuroImage 41 (2008) 561574
571
main manifestation of the EC to EO state. Such results of strong modulation at various spectral EEG field power greatly expand our understanding of the limited classical alpha-block in EEG from EC to EO (Pollen and Trachtenberg, 1972) or modern alpha desynchronization (Rihs et al., 2007) from the state transaction. In fact, the observed changes in spectral EEG field powers at different regional distribution attests to the complex and consorted EEG changes from EC to EO at rest. Since no task or instruction was given to the subjects at the resting states, these changes may occur intrinsically from the EC to the EO state both in terms of physiological visual information processing and psychological terms of visual perception in seeing. This EC and EO differential states have recently not explicitly elucidated in the fMRI literature as the default mode of brain function, largely defined by the lack of explicit task demands on the brain in subjects in the EC state, lying at rest (Raichle et al., 2001). Several reports of the brain state in the default mode from fMRI studies include self-referential mental activity (Gusnard et al., 2001), exteroceptive and interoceptive deployment of attention (Nagai et al., 2004), mind-readiness alert (Fransson 2005), and inner-speech (Hunter et al., 2006). Our observed spectral EEG field powers in the regional distribution between the EC and EO states, if is corresponding to the above hypothesis, remain to be examined. Inter-individual variability in EEG field powers and the inter-state consistency Two aspects of the inter-subject variability deserve special attention. Firstly, the large differences in alpha field powers noted, as much as 90 folds in alpha-1 and 60 folds in alpha-2 in the group of 15 subjects, are in magnitudes higher than expected. Secondly, these inter-individual difference ratios are 10 folds higher at the resting EC than EO state. Though there is some knowledge that a 1015% of the normal healthy subjects have little or nearly no alpha activity, the magnitude of such differences comes as a surprise. These results warrant consideration as a practical issue and call for re-consideration in statistical parametric comparisons of means. It seems that non-parametric Wilcox-test of ranks can be justified and similarly non-parametric Spearman correlation test is appropriate for evaluating statistical features. In the current study, little spectral EEG field powers (except delta and theta) showing significant correlations may indicate two divergent networks between the EC and EO state. The robust correlations of high-frequency beta-2 and gamma field powers in both states do suggest some invariant /function of these activities at the prefrontal area, regardless of the state. Implication Methodologically, our approach in the delimiting the dominant regional distribution of spectral EEG field power is an important extension of the conventional quantitative EEG, qEEG, mapping (Duffy et al., 1994). The emphasis is on the field potential, in a discrete area, over conventional use of selective electrode spectral power in an interpolated map. Using high-density 128-ch montage, the spectral dominant EEG field power can be extracted in region of interest (ROI) for robust comparisons and is based on the effective-energy concept. In current study, we selected the 90% EV (see Methods) as a sensible threshold to delimit a reference cluster of field power for comparison of states. This work further entrusts the steady-state comparison of experience episode in the brain, expressed in EEG state. In general, the EEG DMN is aimed
at defining different EEG states. Our study on listening to happy' music /'sad' music compared to that of lecture speech bears it out (Feng and Chen, submitted for publication). Apparently, many variables used for processing the EEG (electrode density, recoding, reference, time-episode, epoch, FFT, frequency bands, effective-energy threshold) require standardization to facilitate useful comparison across conditions and among laboratories. Additionally, the subject variables include the choice of the resting basal state, EC or EO, in the study. The observed 10 folds of alpha variability in EC over EO shall be taken into account. The reason for this in neurophysiology remains totally unknown. Given the high magnitude of spectral field powers in the EC state, it provides an advantage in invoking states with effective EEG reduction. Given the low magnitude of field powers in the EO state, it is an advantage to invoke states which may enhance field powers. The surprising markedly inter-individual variability, as much as 90 folds in alpha-1 and 62 folds in alpha-2 field powers (see Table 1) shall not deter scientific investigation in EEG research. By considering the individual normalization, relative EEG transformation and use of non-parametric statistics with adequate sample size, sensible and viable EEG/brain functions are deemed fully worthy for future investigation. In the field of EEG research, effort should be directed toward the innovative method for standardized automatic quantification of spectral EEG powers and differentiation of EEG states (Chen et al., 2005). To this end, the current study strategy is ready and opens up a new avenue for the examination of the EEG DMN in gender differences, on the development of age-span in normal brain function as well as their changes upon experimental stimulation or task demand. Likewise, the EEG DMN is valuable in the examination of alterations in patients with diseases and the dysfunctional responses to their environment (cf. Laufs et al., 2007; Babiloni et al., 2006). Conclusion In a group of 15 healthy females, the EEG default mode network (DFN) at rest with eyes-closed of the dominant spectral field powers entails a constellation of large low-frequency delta activity at the prefrontal area, much smaller theta activity at fronto-central area, alpha-1 activity at the anterior-posterior area, alpha-2 and beta-1 at the posterior area, and high-frequency beta-2 and gamma activities at the prefrontal area. Compared to the eyes-open resting state, the delta field power is enhanced at the prefrontal area while the theta, alpha-1, alpha-2 and beta-1 powers are reduced in the respective areas. Greater inter-individual variability in field power can be seen in the eyes-closed than the eyes-open state. In the eyes-closed state, the prefrontal delta field power is correlated with both beta-1 and gamma field powers, while these two high-frequency field powers are mutually correlated at the same area. The EEG DMN of the spectral field power in the eyesclosed or in the eyes-open state at rest, devoid of task demands, is readily served as basal brain condition for the comparison of activation challenge or dysfunctional alteration in diseases of the brain. Acknowledgments This study was supported by several grants respectively from the Chinese National Science Foundation for a project on Brain and Anesthesia NSF-30770691, Beijing Municipal Government for Advancement of Sciences, and Capital Medical University for Innovation Awards.
572
A.C.N. Chen et al. / NeuroImage 41 (2008) 561574 Damoiseaux, J.S., Rombouts, S.A., Barkhof, F., Scheltens, P., Stam, C.J., Smith, S.M., Beckmann, C.F., 2006. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A. 103, 1384813853. Damoiseaux, J.S., Beckmann, C.F., Arigita, E.J., Barkhof, F., Scheltens, P., Stam, C.J., Smith, S.M., Rombouts, S.A. in press. Reduced restingstate brain activity in the default network in normal aging. Cereb. Cortex 2. de Munck, J.C., Goncalves, S.I., Huijboom, L., Kuijer, J.P., Pouwels, P.J., Heethaar, R.M., Lopes da Silva, F.H., 2007. The hemodynamic response of the alpha rhythm: an EEG/fMRI study. NeuroImage 35, 11421151. Doppelmayr, M.M., Klimesch, W., Pachinger, T., Ripper, B., 1998. The functional significance of absolute power with respect to event-related desynchronization. Brain Topogr. 11, 133140. Duffy, F.H., Hughes, J.R., Miranda, F., Bernad, P., Cook, P., 1994. Status of quantitative EEG (QEEG) in clinical practice. Clin. Electroencephalogr. 25, 622. Esposito, F., Bertolino, A., Scarabino, T., Latorre, V., Blasi, G., Popolizio, T., Tedeschi, G., Cirillo, S., Goebel, R., Di Salle, F., 2006. Independent component model of the default-mode brain function: Assessing the impact of active thinking. Brain Res. Bull. 70, 263269. Feng, W., Chen, A.C., submitted for publication. Spectral EEG mapping of human emotion in processing of fast (Superman) vs. slow (Schindler's List) music of movie episodes compared to a lecture speech. Fernandez, A., Rodriguez-Palancas, A., Lopez-Ibor, M., Zuluaga, P., Turrero, A., Maestu, F., Amo, C., Lopez-Ibor Jr., J.J., Ortiz, T., 2005. Increased occipital delta dipole density in major depressive disorder determined by magnetoencephalography. J. Psychiatry Neurosci. 30, 1723. Fransson, P., 2005. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum. Brain Mapp. 26, 1529. Fransson, P., 2006. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44, 28362845. Garrity, A.G., Pearlson, G.D., McKiernan, K., Lloyd, D., Kiehl, K.A., Calhoun, V.D., 2007. Aberrant default mode functional connectivity in schizophrenia. Am. J. Psychiatry 164, 450457. Geday, J., Kupers, R., Gjedde, A., 2007. As time goes by: temporal constraints on emotional activation of inferior medial prefrontal cortex. Cereb. Cortex. 17, 27532759. Goncalves, S.I., de Munck, J.C., Pouwels, P.J., Schoonhoven, R., Kuijer, J.P., Maurits, N.M., Hoogduin, J.M., Van Someren, E.J., Heethaar, R.M., Lopes da Silva, F.H., 2006. Correlating the alpha rhythm to BOLD using simultaneous EEG/fMRI: inter-subject variability. NeuroImage 30, 203213. Grady, C.L., Springer, M.V., Hongwanishkul, D., McIntosh, A.R., Winocur, G., 2006. Age-related changes in brain activity across the adult lifespan. J. Cogn. Neurosci. 18, 227241. Greicius, M.D., Flores, B.H., Menon, V., Glover, G.H., Solvason, H.B., Kenna, H., Reiss, A.L., Schatzberg, A.F., 2007. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 62, 429437. Greicius, M.D., Krasnow, B., Reiss, A.L., Menon, V., 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 100, 253258. Greicius, M.D., Menon, V., 2004. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J. Cogn. Neurosci. 16, 14841492. Guerit, J.M., 2005. Neurophysiological patterns of vegetative and minimally conscious states. Neuropsychol. Rehabil. 15, 357371. Guntekin, B., Basar, E., 2007. Emotional face expressions are differentiated with brain oscillations. Int. J. Psychophysiol. 64, 91100. Grigg-Damberger, M., Gozal, D., Marcus, C.L., Quan, S.F., Rosen, C.L., Chervin, R.D., Wise, M., Picchietti, D.L., Sheldon, S.H., Iber, C., 2007. The visual scoring of sleep and arousal in infants and children. J. Clin. Sleep Med. 3, 201240.
References
Alper, K.R., John, E.R., Brodie, J., Gnther, W., Daruwala, R., Prichep, L.S., 2006. Correlation of PET and qEEG in normal subjects. Psychiatry Res. 146, 271282. Andrade, P.E., Bhattacharya, J., 2003. Brain tuned to music. J. R. Soc. Med. 96, 284287. Arns, M., Peters, S., Breteler, R., Verhoeven, L., 2007. Different brain activation patterns in dyslexic children: evidence from EEG power and coherence patterns for the double-deficit theory of dyslexia. J. Integr. Neurosci. 6, 175190. Babiloni, C., Binetti, G., Cassetta, E., Dal Forno, G., Del Percio, C., Ferreri, F., Ferri, R., Frisoni, G., Hirata, K., Lanuzza, B., Miniussi, C., Moretti, D.V., Nobili, F., Rodriguez, G., Romani, G.L., Salinari, S., Rossini, P.M., 2006. Sources of cortical rhythms change as a function of cognitive impairment in pathological aging: a multicenter study. Clin. Neurophysiol. 117, 252268. Barry, R.J., Clarke, A.R., McCarthy, R., Selikowitz, M., 2007. EEG coherence in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Clin. Neurophysiol. 118, 356362. Basar, E., Guntekin, B., Oniz, A., 2006. Principles of oscillatory brain dynamics and a treatise of recognition of faces and facial expressions. Prog. Brain Res. 159, 4362. Begleiter, H., Porjesz, B., 2006. Genetics of human brain oscillations. Int. J. Psychophysiol. 60, 162171. Bellec, P., Perlbarg, V., Jbabdi, S., Plgrini-Issac, M., Anton, J.L., Doyon, J., Benali, H., 2006. Identification of large-scale networks in the brain using fMRI. NeuroImage 29, 12311243. Benbadis, S.R., 2006. The EEG in nonepileptic seizures. J. Clin. Neurophysiol. 23, 340352. Bertrand, O., Tallon-Baudry, C., 2000. Oscillatory gamma activity in humans: a possible role for object representation. Int. J. Psychophysiol. 38, 211223. Birn, R.M., Diamond, J.B., Smith, M.A., Bandettini, P.A., 2006. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage 31, 15361548. Boord, P.R., Rennie, C.J., Williams, L.M., 2007. Integrating brain and body measures: correlations between EEG and metabolic changes over the human lifespan. J. Integr. Neurosci 6, 205218. Brickman, A.M., Paul, R.H., Cohen, R.A., Williams, L.M., MacGregor, K.L., Jefferson, A.L., Tate, D.F., Gunstad, J., Gordon, E., 2005. Category and letter verbal fluency across the adult lifespan: relationship to EEG theta power. Arch. Clin. Neuropsychol. 20, 561573. Buchem, M.A., van Dijk, J.G., Middelkoop, H.A., 2007. EEG correlates in the spectrum of cognitive decline. Clin. Neurophysiol. 118, 19311939. Cacioppo, J.T., 2004. Feelings and emotions: roles for electrophysiological markers. Biol. Psychol. 67, 235243. Cahn, B.R., Polich, J., 2006. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol. Bull. 132, 180211. Calhoun, V.D., Maciejewski, P.K., Pearlson, G.D., Kiehl, K.A., in press. Temporal lobe and default hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum. Brain Mapp. Castellanos, F.X., Margulies, D.S., Kelly, C., Uddin, L.Q., Ghaffari, M., Kirsch, A., Shaw, D., Shehzad, Z., Di Martino, A., Biswal, B., SonugaBarke, E.J., Rotrosen, J., Adler, L.A., Milham, M.P., 2008. CingulatePrecuneus Interactions: A New Locus of Dysfunction in Adult Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 63, 332337. Chen, A.C., Wang, L., Arendt-Nielsen, L., Krupimski, R., 2005. Standardised automatic quantification method in EEG-ERP mapping. Organ. Hum. Brain Mapp. 271. Chen, A.C., Liu, F.J., Wang, L., Arendt-Nielsen, L., 2006. Mode and site of acupuncture modulation in the human brain: 3D (124-ch) EEG power spectrum mapping and source imaging. NeuroImage 29, 10801091. Chornet-Lurbe, A., Oteo, J.A., Ros, J., 2002. Characteristic times in sleepwaking electroencephalograms. Rev. Neurol. 35, 415419. Cummins, T.D., Finnigan, S., Ros, J., 2007. Theta power is reduced in healthy cognitive aging. Int. J. Psychophysiol. 66, 1017.
A.C.N. Chen et al. / NeuroImage 41 (2008) 561574 Gusnard, D.A., Akbudak, E., Shulman, G.L., Raichle, M.E., 2001. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 98 , 42594264. Harrison, B.J., Ycel, M., Pujol, J., Pantelis, C., 2007. Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophr. Res. 91, 8286. Helps, S., James, C., Debener, S., Karl, A., Sonuga-Barke, E.J. in press. Very low frequency EEG oscillations and the resting brain in young adults: a preliminary study of localisation, stability and association with symptoms of inattention. J. Neural. Transm. Hobbs, M.J., Clarke, A.R., Barry, R.J., McCarthy, R., Selikowitz, M., 2007. EEG abnormalities in adolescent males with AD/HD. Clin. Neurophysiol. 118, 363371. Hoogenboom, N., Schoffelen, J.M., Oostenveld, R., Parkes, L.M., Fries, P., 2006. Localizing human visual gamma-band activity in frequency, time and space. NeuroImage 29, 764773. Horovitz, S.G., Fukunaga, M., de Zwart, J.A., van Gelderen, P., Fulton, S.C., Balkin, T.J., Duyn, J.H., 2007. Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG-fMRI study. Hum. Brain Mapp. 38, 306320. Hunter, A.M., Cook, I.A., Leuchter, A.F., 2007. The promise of the quantitative electroencephalogram as a predictor of antidepressant treatment outcomes in major depressive disorder. Psychiatr. Clin. North Am. 30, 105124. Hunter, M.D., Eickhoff, S.B., Miller, T.W., Farrow, T.F., Wilkinson, I.D., Woodruff, P.W., 2006. Neural activity in speech-sensitive auditory cortex during silence. Proc. Natl. Acad. Sci. U. S. A. 103, 189194. Husain, A.M., 2006. Electroencephalographic assessment of coma. J. Clin. Neurophysiol. 23, 208220. Inanaga, K., 1998. Frontal midline theta rhythm and mental activity. Psychiatry Clin. Neurosci. 52, 555566. Kagan-Kushnir, T., Roberts, S.W., Snead III, O.C., 2005. Screening electroencephalograms in autism spectrum disorders: evidence-based guideline. J. Child Neurol. 20 (3), 197206. Klimesch, W., 1997. EEG-alpha rhythms and memory processes. Int. J. Psychophysiol 26, 319340. Klimesch, W., 1999. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 29, 169195. Klimesch, W., Doppelmayr, M., Hanslmayr, S., 2006a. Upper alpha ERD and absolute power: their meaning for memory performance. Prog. Brain Res. 159, 151165. Klimesch, W., Sauseng, P., Hanslmayr, S., 2006b. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev. 53, 6388. Klimesch, W., Sauseng, P., Hanslmayr, S., Gruber, W., Freunberger, R., 2007. Event-related phase reorganization may explain evoked neural dynamics. Neurosci. Biobehav. Rev. 31 (7), 10031016. Laufs, H., Hamandi, K., Salek-Haddadi, A., Kleinschmidt, A.K., Duncan, J.S., Lemieux, L., 2007. Temporal lobe interictal epileptic discharges affect cerebral activity in default mode brain regions. Hum. Brain Mapp 25, 894901. Mantini, D., Perrucci, M.G., Del Gratta, C., Romani, G.L., Corbetta, M., 2007. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. U. S. A. 104, 1317013175. Marsh, R., Zhu, H., Wang, Z., Skudlarski, P., Peterson, B.S., 2007. A developmental fMRI study of self-regulatory control in Tourette's syndrome. Am. J. Psychiatry 164, 955966. Menon, V., Leroux, J., White, C.D., Reiss, A.L., 2004. Frontostriatal deficits in fragile X syndrome: relation to fMR1 gene expression. Proc. Natl. Acad. Sci. U. S. A 101, 36153620. Nagai, Y., Critchley, H.D., Featherstone, E., Trimble, M.R., Dolan, R.J., 2004. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a default mode of brain function. NeuroImage 22 (1), 243251 May. Neuper, C., Wortz, M., Pfurtscheller, G., 2006. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog. Brain Res. 159, 211222. Nuwer, M.R., Comi, G., Emerson, R., Fuglsang-Frederiksen, A., Guerit, J.M., Hinrichs, H., Ikeda, A., Luccas, F.J., Rappelsberger, P., 1999. IFCN standards for digital recording of clinical EEG. The International Fede-
573
ration of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. 52, 1114. Onton, J., Delorme, A., Makeig, S., 2005. Frontal midline EEG dynamics during working memory. NeuroImage 27, 341356. Oostenveld, R., Praamstra, P., 2001. The five percent electrode system for high-resolution EEG and ERP measurements. Clin. Neurophysiol. 112, 713719. Parkes, L.M., Bastiaansen, M.C., Norris, D.G., 2006. Combining EEG and fMRI to investigate the post-movement beta rebound. NeuroImage 29, 685696. Perrin, F., Pernier, J., Bertrand, O., Giard, M.H., Echallier, J.F., 1987. Mapping of scalp potentials by surface spline interpolation. Electroencephalogr. Clin. Neurophysiol. 66, 7581. Pfurtscheller, G., Stancak, A.J., Neuper, C., 1996. Event-related synchronization (ERS) in the alpha band-an electrophysiological correlate of cortical idling: a review. Int. J. Psychophysiol. 24, 3946. Pollen, D.A., Trachtenberg, M.C., 1972. Some problems of occipital alpha block in man. Brain Res. 41, 303314. Preissl, H., Lowery, C.L., Eswaran, H., 2004. Fetal magnetoencephalography: current progress and trends. Exp. Neurol. 190, 2836. Prichep, L.S., 2007. Quantitative EEG and electromagnetic brain imaging in aging and in the evolution of dementia. Ann. N.Y. Acad. Sci. 1097, 156167. Raichle, M.E., MacLeod, A.M., Snyder, A.Z., Powers, W.J., Gusnard, D.A., Shulman, G.L., 2001. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 98, 676682. Raichle, M.E., Snyder, A.Z., 2007. A default mode of brain function: a brief history of an evolving idea. NeuroImage 237, 10831090. Rihs, T.A., Michel, C.M., Thut, G., 2007. Mechanisms of selective inhibition in visual spatial attention are indexed by alpha-band EEG synchronization. Eur. J. Neurosci. 25, 603610. Rombouts, S.A., Barkhof, F., Goekoop, R., Stam, C.J., Scheltens, P., 2005. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum. Brain Mapp. 26, 231239. Rombouts, S.A., Damoiseaux, J.S., Goekoop, R., Barkhof, F., Scheltens, P., Smith, S.M., Beckmann, C.F., in press. Model-free group analysis shows altered BOLD FMRI networks in dementia. Hum. Brain Mapp. Rossini, P.M., Del Percio, C., Pasqualetti, P., Cassetta, E., Binetti, G., Dal Forno, G., Ferreri, F., Frisoni, G., Chiovenda, P., Miniussi, C., Parisi, L., Tombini, M., Vecchio, F., Babiloni, C., 2006. Conversion from mild cognitive impairment to Alzheimer's disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience 143, 793803. Sauseng, P., Hoppe, J., Klimesch, W., Gerloff, C., Hummel, F.C., 2007. Dissociation of sustained attention from central executive functions: local activity and interregional connectivity in the theta range. Eur. J. Neurosci. 25, 587593. Sediri, H., Bourriez, J.L., Derambure, P., 2007. Role of EEG in the diagnosis of brain death. Rev. Neurol. 163, 248253. Smit, D.J., Posthuma, D., Boomsma, D.I., De Geus, E.J., 2007. The relation between frontal EEG asymmetry and the risk for anxiety and depression. Biol. Psychol. 74, 2633. Sorg, C., Riedl, V., Mhlau, M., Calhoun, V.D., Eichele, T., Ler, L., Drzezga, A., Frstl, H., Kurz, A., Zimmer, C., Wohlschlger, A.M., 2007. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 104, 1876018765. Srinivasan, N., 2007. Cognitive neuroscience of creativity: EEG based approaches. Methods 42, 109116. Tandonnet, C., Burle, B., Hasbroucq, T., Vidal, F., 2005. Spatial enhancement of EEG traces by surface Laplacian estimation: comparison between local and global methods. Clin. Neurophysiol. 116, 1824. Tsujimoto, T., Shimazu, H., Isomura, Y., 2006. Direct recording of theta oscillations in primate prefrontal and anterior cingulate cortices. J. Neurophysiol. 95, 29873000. van Albada, S.J., Robinson, P.A., 2007. Transformation of arbitrary distributions to the normal distribution with application to EEG test-retest reliability. J. Neurosci. Methods 16, 205211. van der Hiele, K., Vein, A.A., Reijntjes, R.H., Westendorp, R.G., Bollen, E.L., van Buchem, M.A., van Dijk, J.G., Middelkoop, H.A., 2007. EEG
574
A.C.N. Chen et al. / NeuroImage 41 (2008) 561574 Alzheimer's disease: evidence from resting state fMRI. NeuroImage 31, 496504. Wilding, E.L., Herron, J.E., 2006. Electrophysiological measures of episodic memory control and memory retrieval. Clin. EEG Neurosci. 37, 315321. Wilson, S.M., Molnar-Szakacs, I., Iacoboni, M., 2007. Beyond Superior Temporal Cortex: Intersubject Correlations in Narrative Speech Comprehension. Cereb. Cortex 15 May. Zhao, X.H., Wang, P.J., Li, C.B., Hu, Z.H., Xi, Q., Wu, W.Y., Tang, X.W., 2007. Altered default mode network activity in patient with anxiety disorders: An fMRI study. Eur. J. Radiol 63, 373378.
correlates in the spectrum of cognitive decline. Clin. Neurophysiol. 118, 19311939. Vanhatalo, S., Kaila, K., 2006. Development of neonatal EEG activity: from phenomenology to physiology. Semin. Fetal Neonatal. Med. 11, 471478. Vuilleumier, P., Driver, J., 2007. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philos. Trans. R. Soc. Lond., B Biol. Sci. 362, 837855. Wang, L., Zang, Y., He, Y., Liang, M., Zhang, X., Tian, L., Wu, T., Jiang, T., Li, K., 2006. Changes in hippocampal connectivity in the early stages of
Você também pode gostar
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (121)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2259)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5795)
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1091)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (345)
- RH InheritanceDocumento10 páginasRH InheritanceShemiza Balmacoon0% (1)
- Conceive A BoyDocumento16 páginasConceive A BoyIbn E Mujib Almaliri100% (1)
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
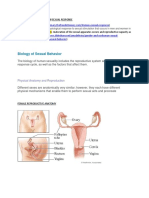
- Understanding The Human Sexual ResponseDocumento12 páginasUnderstanding The Human Sexual ResponseMaan JosueAinda não há avaliações
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- Cavity Preparation in Primary TeethDocumento92 páginasCavity Preparation in Primary TeethDrPratibha Ahirwar100% (7)
- Common Medical AbbreviationsDocumento10 páginasCommon Medical AbbreviationsPrincess MariscotesAinda não há avaliações
- Blood Bank Functioning and Hospital Transfusion CommitteeDocumento13 páginasBlood Bank Functioning and Hospital Transfusion CommitteeTraite Hmar100% (1)
- Odontogenic and Non Odontogenic PainDocumento44 páginasOdontogenic and Non Odontogenic Painjohnjpw 92Ainda não há avaliações
- Dandy Walker SyndromeDocumento9 páginasDandy Walker SyndromeDarshika Vyas MohanAinda não há avaliações
- CatsDocumento14 páginasCatsSuperDAinda não há avaliações
- Physiology of Lactation: Prepared By: Fy MSC NursingDocumento32 páginasPhysiology of Lactation: Prepared By: Fy MSC NursingSùjâl PätídàrAinda não há avaliações
- Yoga of Islamic PrayerDocumento2 páginasYoga of Islamic PrayerMustafa A JariwalaAinda não há avaliações
- Muscle Tissue SummaryDocumento2 páginasMuscle Tissue SummaryLindenScholesAinda não há avaliações
- Physiology of LactationDocumento16 páginasPhysiology of LactationsabaAinda não há avaliações
- Anatomi Organ DR - Siti Rafiah HusainDocumento73 páginasAnatomi Organ DR - Siti Rafiah HusainfitrahfajrianihamingAinda não há avaliações
- Histology of AppendixDocumento1 páginaHistology of Appendixalyson_l100% (1)
- Relacion Molar y Canina 123Documento7 páginasRelacion Molar y Canina 123Soraya Cohen MontesAinda não há avaliações
- Embriology of Digestive System: by Rita RositaDocumento30 páginasEmbriology of Digestive System: by Rita Rositacendy_prastiwiAinda não há avaliações
- Lesson 1.3 Animal Reproduction and Development: Human Reproductive SystemsDocumento15 páginasLesson 1.3 Animal Reproduction and Development: Human Reproductive SystemsCruella MajoAinda não há avaliações
- Oral Histo SiteDocumento2 páginasOral Histo SiteLiau Chu SengAinda não há avaliações
- Bachelor of Physiotherapy - BPT: (4 Year Degree Course)Documento107 páginasBachelor of Physiotherapy - BPT: (4 Year Degree Course)Ritesh TrivediAinda não há avaliações
- The Ovarian Cycle.Documento20 páginasThe Ovarian Cycle.Salman KhanAinda não há avaliações
- I.Objective: Ii - Subject Matter:: Science 5Documento2 páginasI.Objective: Ii - Subject Matter:: Science 5Yvette LeonidaAinda não há avaliações
- Seminar ReportDocumento28 páginasSeminar ReportlaxmikantchhipaAinda não há avaliações
- Female Reproductive Anatomy - KWDocumento25 páginasFemale Reproductive Anatomy - KWabyannaybaAinda não há avaliações
- Muscular System Concept MapDocumento1 páginaMuscular System Concept MapKimsabu Doldam100% (1)
- MakalahDocumento78 páginasMakalahMuhammad HasanAinda não há avaliações
- Assessment Management of Patients With Peripheral Vascular Disorders HypertensionDocumento17 páginasAssessment Management of Patients With Peripheral Vascular Disorders HypertensionJhosita Flora LarocoAinda não há avaliações
- Respiratory System of Frog: External RespirationDocumento3 páginasRespiratory System of Frog: External RespirationShaira CogollodoAinda não há avaliações
- Autopsy: Bocoboc, Castillo, Miguel, Nalupta, RoldanDocumento28 páginasAutopsy: Bocoboc, Castillo, Miguel, Nalupta, RoldanAya CstlAinda não há avaliações
- Woodworth1985 PDFDocumento14 páginasWoodworth1985 PDFacevariel5851Ainda não há avaliações