Escolar Documentos
Profissional Documentos
Cultura Documentos
Resolution in Chromatography
Enviado por
Sean CollinsDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Resolution in Chromatography
Enviado por
Sean CollinsDireitos autorais:
Formatos disponíveis
Column Resolution
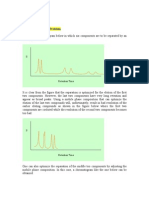
The resolution RS of a column tells us how far apart two bands are relative to their widths. The resolution provides a quantitative measure of the ability of the column to separate two analytes, Rs= 2(t R2 t R1) W A+ W B
It is evident that a resolution of 1.5 gives an essentially complete separation of A and B, whereas a resolution of 0.75 does not. At a resolution of 1.0, zone A contains 4%B and zone B contains 4% of zone A. The resolution of a given stationary phase can be improved by increasing the length of the column and thus increasing the number of theoretical plates. An adverse consequence of the added plates, however, is an increase in the time required for separating the components. Optimization techniques Variation in plate height The resolution of a column improves as the square root of the number of plates it contains increases. The increasing of the number of plates is expensive in terms of time unless the increase is achieved by reducing the plate height and not by increasing column length. Methods of reducing plate height, including the particle size of the packing material, the diameter of the column, and the thickness of the liquid film. Variation in the retention factor Often, a separation can be improved significantly by manipulation of the retention factor k B . Increases in k B generally enhance resolution (but at the expense of elution time). Usually, the easiest way to improve resolution is by optimizing k. For gaseous mobile phases, k can often be improved by temperature changes. For liquid mobile phases, changes in the solvent composition often permit manipulation of k to yield better separations. Variations in the Selectivity Factor Optimizing k and increasing N are not sufficient to give a satisfactory separation of two solutes in a reasonable time when approaches unity. A means must be sought to increase while maintaining k in the range of 1 to 10. Several options are available; in decreasing order of desirability, as determined by promise and convenience, the options are 1. changing the composition of the mobile phase 2. changing the column temperature 3. changing the composition of the stationary phase Skoog, West et al, Fundamentals of analytical chemistry, 8th ed, ISBN-13: 978-0-03-035523-3
4. using special chemical effects Increases in temperature usually cause increases in k but have little effect on values in liquidliquid and liquid-solid chromatography. In contrast, with ion-exchange chromatography, temperature effects can be large enough to make exploration of this option worthwhile before resorting to a change in column packing material. A final method of enhancing resolution is to incorporate into the stationary phase a species that complexes or otherwise interacts with one or more components of the sample.
Skoog, West et al, Fundamentals of analytical chemistry, 8th ed, ISBN-13: 978-0-03-035523-3
Você também pode gostar
- Lecture 2, Fundamentals HPLCDocumento36 páginasLecture 2, Fundamentals HPLCChevuru ManushaAinda não há avaliações
- Practice Problem Set 12 HPLCDocumento12 páginasPractice Problem Set 12 HPLCYocobSamandrewsAinda não há avaliações
- Gas Chromatography (GC) Lecture NotesDocumento23 páginasGas Chromatography (GC) Lecture NotesXin Nix100% (4)
- Chromatography PDFDocumento27 páginasChromatography PDFconstantinAinda não há avaliações
- 3b. Packec Column DesignDocumento48 páginas3b. Packec Column DesignFahim RahmanAinda não há avaliações
- Basic Chromatography Notes 1Documento27 páginasBasic Chromatography Notes 1Aufa InsyirahAinda não há avaliações
- Some Important Points To Remember!: 1.) Typical Response Obtained by Chromatography (I.e., A Chromatogram)Documento51 páginasSome Important Points To Remember!: 1.) Typical Response Obtained by Chromatography (I.e., A Chromatogram)Sabariah OthmanAinda não há avaliações
- Phase (Which May Be A Gas, A Liquid or A Supercritical Fluid) - The Mobile Phase IsDocumento5 páginasPhase (Which May Be A Gas, A Liquid or A Supercritical Fluid) - The Mobile Phase IsasjawolverineAinda não há avaliações
- Principles of Gas ChromatographyDocumento17 páginasPrinciples of Gas ChromatographyAtef LasheenAinda não há avaliações
- On The Line Q Degradation in Hydrogen Masers: L. Busca, H. SchwedaDocumento9 páginasOn The Line Q Degradation in Hydrogen Masers: L. Busca, H. SchwedaschwedaAinda não há avaliações
- Chromatography: Definition and OverviewDocumento9 páginasChromatography: Definition and OverviewPradipkumar PanchalAinda não há avaliações
- HPLC A Practical GuideDocumento139 páginasHPLC A Practical Guidevazram100% (1)
- Application of MachineDocumento21 páginasApplication of MachineSengatan LebahAinda não há avaliações
- Basic Gas ChromatographyDocumento42 páginasBasic Gas ChromatographyFaizAinda não há avaliações
- Experiment of Gas ChromatographyDocumento11 páginasExperiment of Gas ChromatographyMohd Sukri DaudAinda não há avaliações
- Problem Set 9 Chromatographic SeparationsDocumento10 páginasProblem Set 9 Chromatographic SeparationsMenaka MadusankaAinda não há avaliações
- A Technical Guide For Static Headspace Analysis Using GCDocumento20 páginasA Technical Guide For Static Headspace Analysis Using GCjacko9393100% (1)
- Chapter 26 - Gas Chromatography1Documento19 páginasChapter 26 - Gas Chromatography1PARIKAinda não há avaliações
- Tech Data 8APPENDIXAPolymerQuenchingDocumento13 páginasTech Data 8APPENDIXAPolymerQuenchingBalram JiAinda não há avaliações
- Models - Chem.microreactor OptimizationDocumento16 páginasModels - Chem.microreactor OptimizationSaif EvonyAinda não há avaliações
- Characteristics of Liquid: Stationary Phases and Column Evaluation For Gas ChromatographyDocumento16 páginasCharacteristics of Liquid: Stationary Phases and Column Evaluation For Gas ChromatographyHelena OregónAinda não há avaliações
- Gas ChromatographyDocumento152 páginasGas Chromatographypatilamardip007100% (1)
- HPLC 08Documento13 páginasHPLC 08Vikas SharmaAinda não há avaliações
- Experiment 10Documento59 páginasExperiment 10Karina NarcisoAinda não há avaliações
- Basic Gas ChromatographyDocumento42 páginasBasic Gas ChromatographyNofrizalAinda não há avaliações
- Basic ChromatographyDocumento41 páginasBasic ChromatographyLindelwa MthembuAinda não há avaliações
- GC Terms and Techniques GlossaryDocumento5 páginasGC Terms and Techniques GlossaryClaudia GarcíaAinda não há avaliações
- Atom Base Titration NotesDocumento4 páginasAtom Base Titration NotesVooy RajAinda não há avaliações
- Chapter 26 Chromatographic SeparationDocumento32 páginasChapter 26 Chromatographic SeparationBhavesh NayakAinda não há avaliações
- Unit Number 1: GAS ABSORPTIONDocumento22 páginasUnit Number 1: GAS ABSORPTIONISAAC STANLYAinda não há avaliações
- Chromatography MethodDocumento19 páginasChromatography MethodHafiz AzizAinda não há avaliações
- Chmb16 Final NotesDocumento15 páginasChmb16 Final NotesCleveland BrownAinda não há avaliações
- CM2192 CheAnalytical Chemistry Cheat Analytical Chemistry Cheat Sheet 2Documento1 páginaCM2192 CheAnalytical Chemistry Cheat Analytical Chemistry Cheat Sheet 2dorothyhuangAinda não há avaliações
- 422 Sol 26Documento5 páginas422 Sol 26Merna El SayeghAinda não há avaliações
- CHM510-e - LaboratoryManual Sem October 2020-Februari2021Documento10 páginasCHM510-e - LaboratoryManual Sem October 2020-Februari2021Amirah AzlanAinda não há avaliações
- Chromatographic Techniques of AnaylsisDocumento39 páginasChromatographic Techniques of Anaylsispratik satiAinda não há avaliações
- Multiphase ReactorDocumento37 páginasMultiphase ReactorMaria Charlene Caraos TapiaAinda não há avaliações
- Scale Up of BioreactorDocumento8 páginasScale Up of BioreactoraathiraAinda não há avaliações
- Study Notes: The GC ColumnDocumento16 páginasStudy Notes: The GC ColumnLaxmi Kant PrasadAinda não há avaliações
- Exp 06 07 GC FA22 - Protocol - Updated20221024Documento6 páginasExp 06 07 GC FA22 - Protocol - Updated20221024Nathan MeierAinda não há avaliações
- F 39250232446Documento4 páginasF 39250232446Maulana YusufAinda não há avaliações
- Measurement of Damköhler Number Using X-Ray CT in Heavy Oil Carbonate Reservoir AcidizingDocumento6 páginasMeasurement of Damköhler Number Using X-Ray CT in Heavy Oil Carbonate Reservoir Acidizingmostafa abdulkareemAinda não há avaliações
- The General Elution ProblemDocumento3 páginasThe General Elution ProblemgoodigoodiAinda não há avaliações
- Practice Problem Set 11 Gas ChromatographyDocumento11 páginasPractice Problem Set 11 Gas ChromatographyJeslynOngAinda não há avaliações
- 10 Text & ExamplsDocumento46 páginas10 Text & ExamplstarhuniAinda não há avaliações
- Color I MeterDocumento8 páginasColor I MeterVishal GoswamiAinda não há avaliações
- The Chromatographic Process ExplainedDocumento40 páginasThe Chromatographic Process ExplainedFarida NurAinda não há avaliações
- Kromatografi Agam Pemisahan KimiaDocumento4 páginasKromatografi Agam Pemisahan KimiaAgam PriamAinda não há avaliações
- GC Column Efficiency and Band BroadeningDocumento25 páginasGC Column Efficiency and Band BroadeningPrabneeshAinda não há avaliações
- Gas Chromatography Principles and ApplicationsDocumento51 páginasGas Chromatography Principles and ApplicationsDeepak SharmaAinda não há avaliações
- Warwick Thesis WrapDocumento8 páginasWarwick Thesis Wrapafknoaabc100% (2)
- GC - Method DevelopmentDocumento38 páginasGC - Method DevelopmentNur 'AinAinda não há avaliações
- Informe 1 AnalisisDocumento23 páginasInforme 1 AnalisisCarla ParraAinda não há avaliações
- Chap-3-1 GC Slides-1Documento72 páginasChap-3-1 GC Slides-1lishan asefaAinda não há avaliações
- Thermometric Titrimetry: International Series of Monographs in Analytical ChemistryNo EverandThermometric Titrimetry: International Series of Monographs in Analytical ChemistryAinda não há avaliações
- Swelling Concrete in Dams and Hydraulic Structures: DSC 2017No EverandSwelling Concrete in Dams and Hydraulic Structures: DSC 2017Ainda não há avaliações
- Carbon Dioxide Thermodynamic Properties Handbook: Covering Temperatures from -20° to 250°C and Pressures up to 1000 BarNo EverandCarbon Dioxide Thermodynamic Properties Handbook: Covering Temperatures from -20° to 250°C and Pressures up to 1000 BarAinda não há avaliações
- IGCSE Chemistry Test 1Documento1 páginaIGCSE Chemistry Test 1Sean CollinsAinda não há avaliações
- IGCSE Physics Lens Test 1Documento1 páginaIGCSE Physics Lens Test 1Sean CollinsAinda não há avaliações
- Chromatographic SeparationsDocumento2 páginasChromatographic SeparationsSean CollinsAinda não há avaliações
- Plasma SourcesDocumento2 páginasPlasma SourcesSean CollinsAinda não há avaliações
- Eg. 30-2 of TextbookDocumento1 páginaEg. 30-2 of TextbookSean CollinsAinda não há avaliações
- Origins of Atomic SpectraDocumento2 páginasOrigins of Atomic SpectraSean CollinsAinda não há avaliações
- Band Broadening and Column Efficiency in ChromatographyDocumento1 páginaBand Broadening and Column Efficiency in ChromatographySean CollinsAinda não há avaliações
- IGCSE Chemistry Sheet 1Documento1 páginaIGCSE Chemistry Sheet 1Sean Collins100% (1)
- Migration Rates of Solutes in ChromatographyDocumento2 páginasMigration Rates of Solutes in ChromatographySean Collins100% (3)
- Background Correction in AASDocumento1 páginaBackground Correction in AASSean CollinsAinda não há avaliações
- Atomic SpectrosDocumento1 páginaAtomic SpectrosSean CollinsAinda não há avaliações
- Atomic Absorption SpectrometryDocumento3 páginasAtomic Absorption SpectrometrySean CollinsAinda não há avaliações
- Wavelength Selectors For IR SpectrophotometryDocumento2 páginasWavelength Selectors For IR SpectrophotometrySean CollinsAinda não há avaliações
- Instruments For Infrared AbsorptionDocumento2 páginasInstruments For Infrared AbsorptionSean CollinsAinda não há avaliações
- Ultraviolet and Visible SpectrosDocumento2 páginasUltraviolet and Visible SpectrosSean CollinsAinda não há avaliações
- Infrared Absorption SpectrosDocumento2 páginasInfrared Absorption SpectrosSean CollinsAinda não há avaliações
- Instruments For Optical SpectrometryDocumento2 páginasInstruments For Optical SpectrometrySean CollinsAinda não há avaliações
- Infrared Absorption SpectraDocumento1 páginaInfrared Absorption SpectraSean CollinsAinda não há avaliações
- Emission SpectraDocumento2 páginasEmission SpectraSean CollinsAinda não há avaliações
- Absorption SpectraDocumento2 páginasAbsorption SpectraSean CollinsAinda não há avaliações
- PolarographyDocumento2 páginasPolarographySean Collins67% (3)
- Spectroscopic MeasurementsDocumento1 páginaSpectroscopic MeasurementsSean CollinsAinda não há avaliações
- Voltammetry (General)Documento2 páginasVoltammetry (General)Sean CollinsAinda não há avaliações
- Introduction To Spectrochemical MethodsDocumento2 páginasIntroduction To Spectrochemical MethodsSean CollinsAinda não há avaliações
- Hydrodynamic VoltammetryDocumento2 páginasHydrodynamic VoltammetrySean Collins67% (3)
- A. 1986. Fryer, H. Lowry Protein Assay Using An Automatic Microtiter Plate SpectrophotometerDocumento6 páginasA. 1986. Fryer, H. Lowry Protein Assay Using An Automatic Microtiter Plate SpectrophotometerJorge Luis RomeroAinda não há avaliações
- Ouchterlony Double Diffusion (For Antibody Titeration Ration)Documento4 páginasOuchterlony Double Diffusion (For Antibody Titeration Ration)kiedd_04100% (7)
- Enzyme Isolation and Purification TechniquesDocumento15 páginasEnzyme Isolation and Purification TechniquesAnnadurai PillaiAinda não há avaliações
- Lesson Plan Che 515 Instrumental Chemistry For Engineers Faculty of Chemical Engineering Universiti Teknologi MaraDocumento5 páginasLesson Plan Che 515 Instrumental Chemistry For Engineers Faculty of Chemical Engineering Universiti Teknologi MaraMuhamad Baihakhi ShamsudinAinda não há avaliações
- Blaetterkatalog Sim0422Documento36 páginasBlaetterkatalog Sim0422PY WangAinda não há avaliações
- PCR Technologies: A Technical GuideDocumento164 páginasPCR Technologies: A Technical Guidebotond77Ainda não há avaliações
- Quiz 389 DayupDocumento2 páginasQuiz 389 DayupDennis Gutierrez RiojaAinda não há avaliações
- Assay of BZK-Cl Alkyl Components Method VerificationDocumento5 páginasAssay of BZK-Cl Alkyl Components Method VerificationmohsenAinda não há avaliações
- TLC Analysis of Pigments from Siling LabuyoDocumento3 páginasTLC Analysis of Pigments from Siling LabuyoMagat AlexAinda não há avaliações
- Ecm555 ProtocolDocumento13 páginasEcm555 ProtocolaidanaAinda não há avaliações
- Experiment 4Documento5 páginasExperiment 4imenmezhoud1122Ainda não há avaliações
- Jurnal AbcDocumento8 páginasJurnal AbcuswatunAinda não há avaliações
- Dna Hybridization PDFDocumento3 páginasDna Hybridization PDFMaria100Ainda não há avaliações
- 5 Minute Analysis of Vitamin D in Serum by LC/MS/MSDocumento3 páginas5 Minute Analysis of Vitamin D in Serum by LC/MS/MSSuyog patil100% (1)
- Distillation Column Design 2Documento26 páginasDistillation Column Design 2ferns_120% (1)
- KT67 GeNei Genomic DNA Extrcation Teaching KitDocumento11 páginasKT67 GeNei Genomic DNA Extrcation Teaching KitHemant Kawalkar100% (1)
- LAB 085 Laboratory Analytical Determinations SampleDocumento2 páginasLAB 085 Laboratory Analytical Determinations SamplePrashansa ShresthaAinda não há avaliações
- Taqpath Proamp Master Mixes: Quick ReferenceDocumento2 páginasTaqpath Proamp Master Mixes: Quick ReferenceFabio RossiAinda não há avaliações
- Sneha Saha Pharm Microbiology CA1Documento6 páginasSneha Saha Pharm Microbiology CA1Suman bhattacharjeeAinda não há avaliações
- General QIAcuity PresentationDocumento15 páginasGeneral QIAcuity PresentationHairul SaprudinAinda não há avaliações
- Whole-Genome Sequencing An Effective Strategy For Insertion Analysis of Foreign Genes in Transgenic PlantsDocumento10 páginasWhole-Genome Sequencing An Effective Strategy For Insertion Analysis of Foreign Genes in Transgenic PlantsLuis Chavez SantamarinaAinda não há avaliações
- Phos Tag - GUIDEBOOK 2016Documento32 páginasPhos Tag - GUIDEBOOK 2016Susan HsiaoAinda não há avaliações
- Lab Report IDocumento11 páginasLab Report IElle B.Ainda não há avaliações
- Pulsed Field Gel Electrophoresis (PFGE) Molecular Biology Microbe NotesDocumento8 páginasPulsed Field Gel Electrophoresis (PFGE) Molecular Biology Microbe NotesJocesesAinda não há avaliações
- Unicellular and Multicellular OrganisemsDocumento54 páginasUnicellular and Multicellular Organisemsmenaga ilangkovanAinda não há avaliações
- ChromatographyDocumento2 páginasChromatographyEaEamAinda não há avaliações
- Histopathology & Cytopathology Flowchart-1Documento4 páginasHistopathology & Cytopathology Flowchart-1shivam jaiswalAinda não há avaliações
- HPLC Advantages vs GCDocumento68 páginasHPLC Advantages vs GCRobert CoffinAinda não há avaliações
- Lab Cells Under A MicroscopeDocumento3 páginasLab Cells Under A MicroscopeAndrea ColliaAinda não há avaliações
- SBT1043 Biotechnology Concepts and Techniques Test 1Documento7 páginasSBT1043 Biotechnology Concepts and Techniques Test 1Alia HanisaAinda não há avaliações