Escolar Documentos
Profissional Documentos
Cultura Documentos
CPE1002 Chap13 2011
Enviado por
Shazriel Yushamiel YusoffDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
CPE1002 Chap13 2011
Enviado por
Shazriel Yushamiel YusoffDireitos autorais:
Formatos disponíveis
Law Jeng Yih Ext: 2107
Multiphase
What is multiphase????
A system consists more than one phase such boiling water: liquid / steam (gas) Melting ice: solid / liquid Brewing a cup of coffee: Hot liquid of water and solid ground coffee bean
LAW JENG YIH
Volatility = degree of species tends to transfer to vapor from liquid or solid Antoine Equation (most common)
B log 10 P A T C
*
Example of process: Evaporation, Drying, Humidification (involve transfer of liquid into the gas phase) Condensation, Dehumidification (involve transfer of the condensable species from gas to liquid phase)
Superheated vapor: A vapor present in a gas in less than its saturation amount!
In the definitions to be given, the term saturation refers to any gas-vapor combination, while humidity refers to specifically to an airwater system.
EXAMPLE 1a :
EXAMPLE 1a :
EXAMPLE 1b :
EXAMPLE 1b :
Raoultss Law :
p A y A P x A p A (T )
*
Where :
xA mole fraction in liquid yA mole fraction in gas
Henrys Law :
p A y A P x A H A (T )
*
Where :
xA mole fraction in liquid yA mole fraction in gas HA(T) is Henrys Law constant
When a liquid is a mixture of several component: - when it is heated slowly at const P, the temp at which the first vapor bubble forms is the bubble-point temperature at the given P.
- when a gas (vapor) is cooled slowly at const P, the temp at which the first liq droplet forms is the dew-point temperature at the given P.
Assuming an ideal solution and Raoults law applies, the partial pressures of vapor components:
pi xi pi (Tbp ), i A, B,...
*
Since we have A, B, present in the system, the sum of partial pressures must be the total pressure (Daltons law)
P xA p (Tbp ) xB pB (Tbp ) ...
* A *
The bubble-point temperature may be calculated by trial & error as the Tbp satisfies this eqn.
Again, a gas phase that contains condesable components A, B, C, at fix P. At Tdp it will be equilibrium with the 1st liq that forms. Assuming Raoults law applies, the liq phase mole fraction:
The mole fractions of liq components must sum to 1: yAP yB P
yi P xi * , i A, B,... pi (Tdp ) ..., 1
p (Tdp )
* A
pB (Tdp )
The dew-point temperature may be calculated by trial & error.
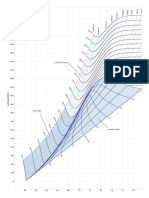
Vapor-liq equilibrium calc for binary (twocomponent) systems can be simplified using a Txy diagram at a fix pressure.
Liq phase: T vs xA Vapor phase: T vs yA A is usually the more volatile component. To determine a Tbp for a given liq composition, go to the liq curve and read the desired temperature.
Move horizontally to the vapor curve to determine the vapor composition in equilibrium with the liq. To determine a Tdp for a given vapor mixture, look at specified mole frac of A in vapor phase, go to the vapor curve and read the desired temperature. Move horizontally to the liq curve to determine the liq composition in equilibrium with the vapor.
If a noncondensable species is present in the gas phase, must use eqn to calc dew point.
If water and methyl isobutyl ketone (MIBK) are mixed at 25C, A single phase formed if the mixture contains > 98% water or 97.7% MIBK.
otherwise, it separates into two liq phases:
98% water, 2% MIBK 97.7% MIBK, 2.3% water Termed partially miscible Termed immiscible if 2% MIBK or 2.3% water is negligible.
If a third substance is added to a two-phase liquid mixture, it distributes itself according to its relative solubility in each phase.
Water-MIBK-acetone at 25C. Here the solute acetone is completely miscible with the two solvents H2O and MIBK, although the two solvents are only partially miscible with each other. Each apex of the triangle represents a single component. Edges represent binary solutions. Exp. edge b represents solutions of H2O and acetone. K mixture of 20.0 wt% MIBK, 65.0% acetone, 15.0% water.
Any mixture whose composition falls in region A (exp. K), is single-phase liquid. Region B represents a two-phase region which will split up into two layers in equilibrium with each other. The lines within region B is known as a tie line. Such lines, connect the compositions of two phases in equilibrium with each other.
Exp. overall composition at M (55.0 wt% water, 15% acetone, 30.0% MIBK) results the mixture separates into two phases: L (85 wt% water, 12% acetone, 3% MIBK) N (4 wt% water, 20% acetone, 76% MIBK) When a mixture does not fall on a tie line, interpolation between the lines is necessary to determine composition of each phase.
THANK YOU
Você também pode gostar
- SAT Practice Book 2009 2010Documento204 páginasSAT Practice Book 2009 2010Fedrick Tharun T100% (1)
- A B C D: Choose Only One Answer For Each QuestionDocumento10 páginasA B C D: Choose Only One Answer For Each QuestionAchitt AchitAinda não há avaliações
- Student Projects For Distillation PDFDocumento186 páginasStudent Projects For Distillation PDFHugh Manta100% (2)
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsNo EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsNota: 5 de 5 estrelas5/5 (1)
- Vapor Liquid EquilibriumDocumento39 páginasVapor Liquid EquilibriumTouhid IslamAinda não há avaliações
- Chapter - 2 DistillationDocumento75 páginasChapter - 2 DistillationJACOB DAVEAinda não há avaliações
- Chapter 1 DistillationDocumento110 páginasChapter 1 DistillationSiti Nurshahira80% (5)
- 7 1. Vapor Liquid EquilibriumDocumento9 páginas7 1. Vapor Liquid Equilibriumwaseemkhan49Ainda não há avaliações
- Chapter Two: Properties of Pure SubstanceDocumento53 páginasChapter Two: Properties of Pure SubstanceHabtamu Tkubet EbuyAinda não há avaliações
- Bodybuilder Guidelines: Update: 2011-22Documento438 páginasBodybuilder Guidelines: Update: 2011-22thkimzone73100% (12)
- Once Through: Steam GeneratorsDocumento21 páginasOnce Through: Steam GeneratorsrajrampallyAinda não há avaliações
- Distillation Lecture Note-2Documento20 páginasDistillation Lecture Note-2BasseyAinda não há avaliações
- Chapter 1Documento111 páginasChapter 1Radhi Abdullah100% (1)
- High-Pressure Fluid Phase Equilibria: Phenomenology and ComputationNo EverandHigh-Pressure Fluid Phase Equilibria: Phenomenology and ComputationAinda não há avaliações
- Design of Helical Pier Foundations in Frozen GroundDocumento6 páginasDesign of Helical Pier Foundations in Frozen GroundCortesar ManuAinda não há avaliações
- Chapter 8 Phase DiagramsDocumento18 páginasChapter 8 Phase DiagramsWynlor AbarcaAinda não há avaliações
- CHE325 Note1 From DR AyoolaDocumento30 páginasCHE325 Note1 From DR AyoolaPreciousAinda não há avaliações
- Lecture1 PDFDocumento10 páginasLecture1 PDFEjaz AnwarAinda não há avaliações
- CMT 405 - Distillation PDFDocumento72 páginasCMT 405 - Distillation PDFMuhammad Azri HaziqAinda não há avaliações
- Chapter 6 - Multiphase Systems: CBE2124, LevickyDocumento27 páginasChapter 6 - Multiphase Systems: CBE2124, LevickyRimmonAinda não há avaliações
- Bubble - Dew PointDocumento5 páginasBubble - Dew PointKhaing Myint MyatAinda não há avaliações
- DistillatnDocumento149 páginasDistillatnVinayak ThalangeAinda não há avaliações
- Vapor-Liquid Equilibrium (Vle) - PortalDocumento39 páginasVapor-Liquid Equilibrium (Vle) - PortalKaizerAinda não há avaliações
- PT Chapter 6Documento64 páginasPT Chapter 6shubhamAinda não há avaliações
- Lecture #2-Physical Chemistry - 8 (L2-P-2)Documento10 páginasLecture #2-Physical Chemistry - 8 (L2-P-2)احمد الدلالAinda não há avaliações
- Capitulo 1 Termoquimica 2Documento41 páginasCapitulo 1 Termoquimica 2adrianaAinda não há avaliações
- Properties of Pure SubstanceDocumento26 páginasProperties of Pure SubstanceMahadi HasanAinda não há avaliações
- Goal 1: Design A Flash DrumDocumento16 páginasGoal 1: Design A Flash DrumGago_88Ainda não há avaliações
- Norkus Paper Rev0 FinalDocumento18 páginasNorkus Paper Rev0 Finaljlcheefei9258Ainda não há avaliações
- Chapter 19 (Humidification Operations)Documento25 páginasChapter 19 (Humidification Operations)adekAinda não há avaliações
- Phase BehaviorDocumento10 páginasPhase Behaviorfri_13thAinda não há avaliações
- Vapor Pressure: Pº Pressure of A Substance in Equilibrium With Its Pure Condensed (Liquid or Solid) PhaseDocumento32 páginasVapor Pressure: Pº Pressure of A Substance in Equilibrium With Its Pure Condensed (Liquid or Solid) Phaseashoku2Ainda não há avaliações
- Phase DiagramsDocumento19 páginasPhase Diagramsget2cs100% (1)
- Distillation & BPDocumento12 páginasDistillation & BPAmirahKamaruddinAinda não há avaliações
- Transcript - Multicomponent Flash Calculations VideoDocumento3 páginasTranscript - Multicomponent Flash Calculations VideoChristopher RileyAinda não há avaliações
- CL-201 Chapter 6 Multiphase Systems (Compatibility Mode) PDFDocumento42 páginasCL-201 Chapter 6 Multiphase Systems (Compatibility Mode) PDFSuman MandalAinda não há avaliações
- Chapter 3. Pure SubstanceDocumento49 páginasChapter 3. Pure SubstanceMuhammad Awais MalikAinda não há avaliações
- Distillation - Lectures 1 To 6 PDFDocumento45 páginasDistillation - Lectures 1 To 6 PDFMayank PrasadAinda não há avaliações
- Phase BehaviorDocumento30 páginasPhase Behaviorebrahim ftiesAinda não há avaliações
- Chp10 Notes-PHASE EQUI PrintDocumento24 páginasChp10 Notes-PHASE EQUI PrintNurul FarhanaAinda não há avaliações
- Phase Diagrams of MixturesDocumento24 páginasPhase Diagrams of MixturesmohammedAinda não há avaliações
- Properties of Pure SubstancesDocumento11 páginasProperties of Pure SubstancesTirth SolankiAinda não há avaliações
- Properties of Pure SubstancesDocumento53 páginasProperties of Pure Substancesفضائح لا تصدقAinda não há avaliações
- Pure QuestionsDocumento4 páginasPure QuestionssreejishmAinda não há avaliações
- Humidification OperationsDocumento79 páginasHumidification OperationsmirzaAinda não há avaliações
- 3chapter IIIDocumento30 páginas3chapter IIILuAinda não há avaliações
- 3chapter IIIDocumento30 páginas3chapter IIIMuhammad Randy AkbarAinda não há avaliações
- Chemistry General Sem IV Part 1Documento10 páginasChemistry General Sem IV Part 1Cristiano RonaldoAinda não há avaliações
- The Fascinating World Of: ThermodynamicsDocumento36 páginasThe Fascinating World Of: ThermodynamicsSai Swaroop MandalAinda não há avaliações
- Properties of Pure Substances: Thermodynamics: An Engineering ApproachDocumento35 páginasProperties of Pure Substances: Thermodynamics: An Engineering ApproachAsmawi Mohd KhailaniAinda não há avaliações
- Phase EquilibriaDocumento21 páginasPhase EquilibriasuperchellyAinda não há avaliações
- Experiment D1 - Distillation ColumnDocumento16 páginasExperiment D1 - Distillation ColumnchaitanyaAinda não há avaliações
- UntitledDocumento11 páginasUntitledTural EmirliAinda não há avaliações
- Properties of Pure Substances: Thermodynamics: An Engineering ApproachDocumento35 páginasProperties of Pure Substances: Thermodynamics: An Engineering ApproachmohamadfaizalrosliAinda não há avaliações
- Chapter 3Documento28 páginasChapter 3ambatarAinda não há avaliações
- Clitical PointDocumento23 páginasClitical PointWawanW36Ainda não há avaliações
- Thermodynamics I Properties of Pure Substances: Mohsin Mohd SiesDocumento39 páginasThermodynamics I Properties of Pure Substances: Mohsin Mohd SiesDhruv SinghAinda não há avaliações
- Midterm CaeDocumento17 páginasMidterm CaeDianne AlarconAinda não há avaliações
- Phase RuleDocumento21 páginasPhase RuleRajat KaliaAinda não há avaliações
- Chapter Three Volumetric Properties of Pure FluidsDocumento10 páginasChapter Three Volumetric Properties of Pure FluidsWendell Kim LlanetaAinda não há avaliações
- Phase Equilibria Two-Component System: I. Liquid-Liquid System Ideal SolutionDocumento16 páginasPhase Equilibria Two-Component System: I. Liquid-Liquid System Ideal SolutionChelsea MartinezAinda não há avaliações
- Physical Chemistry II (TKK-1237) : Review ReviewDocumento7 páginasPhysical Chemistry II (TKK-1237) : Review ReviewUlvatus Sa' DiyahAinda não há avaliações
- JSF + JPA + JasperReports (Ireport) Part 2 - Ramki Java BlogDocumento7 páginasJSF + JPA + JasperReports (Ireport) Part 2 - Ramki Java BlogMartin MurciegoAinda não há avaliações
- FP 3000 PDFDocumento1 páginaFP 3000 PDFClaudio Godoy ZepedaAinda não há avaliações
- 417 Model E Alarm Check ValvesDocumento4 páginas417 Model E Alarm Check ValvesM Kumar MarimuthuAinda não há avaliações
- XG5000 Manual (2009.10.26) (Eng)Documento645 páginasXG5000 Manual (2009.10.26) (Eng)wanderly_40100% (1)
- Tlsiw - Class X - Project Details - 2023-24Documento2 páginasTlsiw - Class X - Project Details - 2023-24how toAinda não há avaliações
- Python Unit 1Documento18 páginasPython Unit 1Rtr. Venkata chetan Joint secretaryAinda não há avaliações
- Change LogDocumento3 páginasChange Logyoga hendriyantoAinda não há avaliações
- Fahad H. Ahmad (+92 323 509 4443) : Kinetic Particle Theory (5070 Multiple Choice Questions)Documento51 páginasFahad H. Ahmad (+92 323 509 4443) : Kinetic Particle Theory (5070 Multiple Choice Questions)Ali AshrafAinda não há avaliações
- EC205 Mathematics For Economics and Business: The Straight Line and Applications IIDocumento3 páginasEC205 Mathematics For Economics and Business: The Straight Line and Applications IIpereAinda não há avaliações
- Physics Lab - Detailed - Answer KeyDocumento6 páginasPhysics Lab - Detailed - Answer KeyJasdeepSinghAinda não há avaliações
- RCC-DI-AC PipeDocumento10 páginasRCC-DI-AC PipeNaveen NagisettiAinda não há avaliações
- Low Temperature Plastics - EnsingerDocumento4 páginasLow Temperature Plastics - EnsingerAnonymous r3MoX2ZMTAinda não há avaliações
- ECE ExperimentDocumento13 páginasECE Experimentasm98090% (1)
- All Graphs and Charts Available in Show MeDocumento16 páginasAll Graphs and Charts Available in Show MeGANGA TAGRAAinda não há avaliações
- Mollier Enthalpy Entropy Chart For Steam - US UnitsDocumento1 páginaMollier Enthalpy Entropy Chart For Steam - US Unitslin tongAinda não há avaliações
- Data Download CMM366A-4G V1.0 enDocumento16 páginasData Download CMM366A-4G V1.0 enSuramanAinda não há avaliações
- W.R. Klemm (Auth.) - Atoms of Mind - The - Ghost in The Machine - Materializes-Springer Netherlands (2011)Documento371 páginasW.R. Klemm (Auth.) - Atoms of Mind - The - Ghost in The Machine - Materializes-Springer Netherlands (2011)El equipo de Genesis ProjectAinda não há avaliações
- Gas Turbine Compressor WashingDocumento8 páginasGas Turbine Compressor Washingwolf_ns100% (1)
- Asm 10Documento4 páginasAsm 10Tukaram ParabAinda não há avaliações
- Presentation5 EV ArchitectureDocumento26 páginasPresentation5 EV ArchitectureJAYKUMAR MUKESHBHAI THAKORAinda não há avaliações
- Ruby On Rails 3 Cheat SheetDocumento7 páginasRuby On Rails 3 Cheat SheetJarosław MedwidAinda não há avaliações
- Gregory, Robert Wayne Et Al. - 'The Role of Artificial Intelligence and Data Network Effects For Creating User Value'Documento18 páginasGregory, Robert Wayne Et Al. - 'The Role of Artificial Intelligence and Data Network Effects For Creating User Value'DylanOSullivanAinda não há avaliações
- 100 TOP Real Time Objective C Multiple Choice Questions and Answers PDF DownloadDocumento22 páginas100 TOP Real Time Objective C Multiple Choice Questions and Answers PDF DownloadNayan BariAinda não há avaliações
- Mit BBM (Ib), Ipm-Session 2.4Documento32 páginasMit BBM (Ib), Ipm-Session 2.4Yogesh AdhateAinda não há avaliações