Escolar Documentos
Profissional Documentos
Cultura Documentos
Thermodynamics 3 (Work and Heat)
Enviado por
SweetMainaTítulo original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Thermodynamics 3 (Work and Heat)
Enviado por
SweetMainaDireitos autorais:
Formatos disponíveis
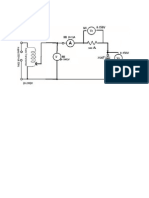
Battery motor system with a fan
Figure-1
.
Baked Potato
Surrounding T1 System (baked potato) T2 T2 > T1
Figure-2
Observation: In both figures 1 & 2, there is an energy transfer between the system and surrounding. In figure 1, the system comprising of battery and motor is raising the weight. This is ,in effect , a force acting through a distance. Therefore work is done on the surrounding by the system. In figure 2, the system comprising of a baked potato will eventually get cooled to the surrounding temperature due to heat energy from the baked potato to the surrounding.
Energy transfer across the boundary is taking place in two distinct forms- heat and work.
Heat is defined as the form of energy that is transferred between two systems ( or a system and its surroundings) by virtue of a temperature difference. Work is said to be done by a system if the sole effect on things external to the system can be reduced to the raising of a weight. The weight may not be actually be raised but the net effect external to the system would be the raising of weight.
Weight is replaced by a fan
Figure-3
The weight is raised with the pulley driven by a motor.
Sign convention
Heat
Surrounding System Work
Surrounding
Heat System Work
Work is positive Heat is negative
Work is Negative Heat is positive
Boundary work/P dV work
Cylinder
Stops
Sand grains
P, V
P1=P-dP, V1=V+dV Piston
P2=P1-dP, V2=V1+dV
Pn,Vn
Gas
W = Force X distance = (Pressure x area) x distance = P ( A Z ) = P dV For a process 1-2 , W1-2 = PdV
It is assumed the system moves through equilibrium states ( 1,2,..n) and there is no friction between the piston and cylinder.
Representation of expansion work in PV plot.
The area under the process curve on a PV diagram is equal, in magnitude, to the work done during a quasi-equilibrium process of a closed system.
Vn
Work is a path function
Choose the correct answer: a) b) c) d)
Pdv work in various quasi static processes a) Process in which pressure is constant ( isobaric process) W1-2= PdV = P(V2-V1)
b) Process in which volume is constant( isochoric process) W1-2= PdV =0
c) Process in which PV = C W1-2 = PV l n ( V2/V1) d) Process in which PV n = C (poly tropic constant) Work done W1-2 = (P2V2-P1V1)/(1-n) n 1
When the gas is considered as ideal gas, the above equation become
Work done W1-2 = mR(T2-T1)/(1-n)
10
Boundary work or P dV at constant pressure (isobaric process ) A frictionless pistoncylinder device contains 5 kg of steam at 400 kPa and 200 deg C. Heat is now transferred to the steam until the temperature reaches 250 deg C. If the piston is not attached to a shaft and its mass is constant, determine the work done by the steam during this process. Represent the process in the P-V diagram and discuss
Steam M= 5 kg P1= 400 kPa, T1= 200 Deg C T2= 250 Deg C
Heat Sp. Vol v1= 0.5343 m3/kg Sp. Vol v2 = 0.5952 m3/kg
11
Boundary work for a constant volume process (isochoric process)
Air P1= 500 kPa T1= 150 Deg C
Heat
P2= 400 KPa T2= 65 Deg C
A rigid tank contains air at 500 kPa and 150C. As a result of heat transfer to the surroundings, the temperature and pressure inside the tank drop to 65C and 400 kPa, respectively. Determine the boundary work done during this process.
A rigid tank Class activity: To represent the process in the P-V diagram and discuss
12
Isothermal compression of an ideal gas
A pistoncylinder device initially contains 0.4 m3 of air at 100 k Pa and 80C. The air is now compressed to 0.1 m3 in such a way that the temperature inside the cylinder remains constant. Determine the work done during this process.
Air is compressed at T0=80 deg C V1=0.4 m3 P1= 100 kPa V2= 0.1 m3
For ideal gas at constant temperature, PV=mRT0
Workdone = PV ln ( V /V ) 2 1
13
Você também pode gostar
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNo EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceNota: 4 de 5 estrelas4/5 (895)
- Indicating InstrumentsDocumento26 páginasIndicating InstrumentsSweetMaina100% (1)
- Never Split the Difference: Negotiating As If Your Life Depended On ItNo EverandNever Split the Difference: Negotiating As If Your Life Depended On ItNota: 4.5 de 5 estrelas4.5/5 (838)
- Hall Effect NewDocumento5 páginasHall Effect NewSweetMainaAinda não há avaliações
- The Yellow House: A Memoir (2019 National Book Award Winner)No EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Nota: 4 de 5 estrelas4/5 (98)
- RC Series CircuitDocumento4 páginasRC Series CircuitSweetMainaAinda não há avaliações
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNo EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeNota: 4 de 5 estrelas4/5 (5794)
- Capillary Rise MethodDocumento2 páginasCapillary Rise MethodSweetMaina67% (3)
- Reel Face: Real Face:: Titanic (1997)Documento2 páginasReel Face: Real Face:: Titanic (1997)SweetMainaAinda não há avaliações
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNo EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaNota: 4.5 de 5 estrelas4.5/5 (266)
- Macroscopic Microscopic: Magnetism, Electricity, and Surface Tension. The Magnetic, Electric, and SystemDocumento5 páginasMacroscopic Microscopic: Magnetism, Electricity, and Surface Tension. The Magnetic, Electric, and SystemSweetMainaAinda não há avaliações
- The Little Book of Hygge: Danish Secrets to Happy LivingNo EverandThe Little Book of Hygge: Danish Secrets to Happy LivingNota: 3.5 de 5 estrelas3.5/5 (400)
- Data Structure Lecture 7 TreeDocumento49 páginasData Structure Lecture 7 TreeSweetMainaAinda não há avaliações
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNo EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureNota: 4.5 de 5 estrelas4.5/5 (474)
- Importance of Business CommunicationDocumento2 páginasImportance of Business CommunicationSweetMainaAinda não há avaliações
- Resolve Cross Cultural MisunderstandingDocumento4 páginasResolve Cross Cultural MisunderstandingSweetMainaAinda não há avaliações
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNo EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryNota: 3.5 de 5 estrelas3.5/5 (231)
- Oral Presentation SkillsDocumento8 páginasOral Presentation SkillsSweetMaina100% (1)
- G.D Topic 2Documento4 páginasG.D Topic 2SweetMainaAinda não há avaliações
- The Emperor of All Maladies: A Biography of CancerNo EverandThe Emperor of All Maladies: A Biography of CancerNota: 4.5 de 5 estrelas4.5/5 (271)
- Breathing: Joshua, Youssef, and ApolloDocumento28 páginasBreathing: Joshua, Youssef, and ApolloArvinth Guna SegaranAinda não há avaliações
- The Unwinding: An Inner History of the New AmericaNo EverandThe Unwinding: An Inner History of the New AmericaNota: 4 de 5 estrelas4/5 (45)
- Lesson 4 Present Simple and Present Continuous Part 2Documento2 páginasLesson 4 Present Simple and Present Continuous Part 2DeniseVuoto100% (1)
- 4.1 Genetic Counselling 222Documento12 páginas4.1 Genetic Counselling 222Sahar JoshAinda não há avaliações
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNo EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersNota: 4.5 de 5 estrelas4.5/5 (345)
- MidtermDocumento8 páginasMidtermBrian FrenchAinda não há avaliações
- Team of Rivals: The Political Genius of Abraham LincolnNo EverandTeam of Rivals: The Political Genius of Abraham LincolnNota: 4.5 de 5 estrelas4.5/5 (234)
- 0606 - s03 - 2 - 0 - QP PENTING KE 2Documento8 páginas0606 - s03 - 2 - 0 - QP PENTING KE 2Titin ChayankAinda não há avaliações
- Learning Competency: Explain The Postulates of The Cell TheoryDocumento4 páginasLearning Competency: Explain The Postulates of The Cell TheoryPamela Isabelle TabiraraAinda não há avaliações
- Use of ICT in School AdministartionDocumento32 páginasUse of ICT in School AdministartionSyed Ali Haider100% (1)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNo EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreNota: 4 de 5 estrelas4/5 (1090)
- Waterfront Development Goals and ObjectivesDocumento2 páginasWaterfront Development Goals and ObjectivesShruthi Thakkar100% (1)
- Isolasi Dan Karakterisasi Runutan Senyawa Metabolit Sekunder Fraksi Etil Asetat Dari Umbi Binahong Cord F L A Steen S)Documento12 páginasIsolasi Dan Karakterisasi Runutan Senyawa Metabolit Sekunder Fraksi Etil Asetat Dari Umbi Binahong Cord F L A Steen S)Fajar ManikAinda não há avaliações
- Jain Gayatri MantraDocumento3 páginasJain Gayatri MantraShil9Ainda não há avaliações
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyNo EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyNota: 3.5 de 5 estrelas3.5/5 (2259)
- Grenade FINAL (Esl Song Activities)Documento4 páginasGrenade FINAL (Esl Song Activities)Ti LeeAinda não há avaliações
- Reflection (The We Entrepreneur)Documento2 páginasReflection (The We Entrepreneur)Marklein DumangengAinda não há avaliações
- 7A Detailed Lesson Plan in Health 7 I. Content Standard: Teacher's Activity Students' ActivityDocumento10 páginas7A Detailed Lesson Plan in Health 7 I. Content Standard: Teacher's Activity Students' ActivityLeizel C. LeonidoAinda não há avaliações
- Sandra Lippert Law Definitions and CodificationDocumento14 páginasSandra Lippert Law Definitions and CodificationЛукас МаноянAinda não há avaliações
- Servicenow Rest Cheat SheetDocumento3 páginasServicenow Rest Cheat SheetHugh SmithAinda não há avaliações
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)No EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Nota: 4.5 de 5 estrelas4.5/5 (121)
- G12 PR1 AsDocumento34 páginasG12 PR1 Asjaina rose yambao-panerAinda não há avaliações
- Asher - Bacteria, Inc.Documento48 páginasAsher - Bacteria, Inc.Iyemhetep100% (1)
- CH 2 & CH 3 John R. Schermerhorn - Management-Wiley (2020)Documento9 páginasCH 2 & CH 3 John R. Schermerhorn - Management-Wiley (2020)Muhammad Fariz IbrahimAinda não há avaliações
- ART 6 LEARNING PACKET Week2-3Documento10 páginasART 6 LEARNING PACKET Week2-3Eljohn CabantacAinda não há avaliações
- Rules and IBA Suggestions On Disciplinary ProceedingsDocumento16 páginasRules and IBA Suggestions On Disciplinary Proceedingshimadri_bhattacharje100% (1)
- Teaching Philosophy StatementDocumento25 páginasTeaching Philosophy Statementtchrdale27Ainda não há avaliações
- VlsiDocumento216 páginasVlsisenthil_5Ainda não há avaliações
- Kepimpinan BerwawasanDocumento18 páginasKepimpinan BerwawasanandrewanumAinda não há avaliações
- NB-CPD IR 4r1 - Guidance For SGs On Their Role and Working MethodsDocumento19 páginasNB-CPD IR 4r1 - Guidance For SGs On Their Role and Working MethodsmingulAinda não há avaliações
- SKZ TrackDocumento6 páginasSKZ TrackOliviaAinda não há avaliações
- Appendix I Leadership Questionnaire: Ior Description Questionnaire (LBDQ - Form XII 1962) - The Division IntoDocumento24 páginasAppendix I Leadership Questionnaire: Ior Description Questionnaire (LBDQ - Form XII 1962) - The Division IntoJoan GonzalesAinda não há avaliações
- Watchitv Portable: Iptv Expert Analysis Application: Key ApplicationsDocumento5 páginasWatchitv Portable: Iptv Expert Analysis Application: Key ApplicationsBen PoovinAinda não há avaliações
- Mil CompiledDocumento62 páginasMil CompiledDan VegaAinda não há avaliações
- PANIC Origin Story (Part 1)Documento6 páginasPANIC Origin Story (Part 1)EpicReads100% (3)
- Adult Consensual SpankingDocumento21 páginasAdult Consensual Spankingswl156% (9)