Escolar Documentos
Profissional Documentos
Cultura Documentos
Inorganic Chemistry II
Enviado por
Alvin Garcia PalancaDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Inorganic Chemistry II
Enviado por
Alvin Garcia PalancaDireitos autorais:
Formatos disponíveis
INORGANIC CHEMISTRY II
SMEP METALLURGICAL ENGINEERING
LECTURE SERIES
EQUILIBRIUM PRINCIPLES AND ITS
APPLICATIONS TO IONIC EQUILIBRIA IN
AQUEOUS SOLUTIONS
REACTION RATES
Reaction Rate- describes how fast the
concentration of a reactant or product changes
with time
Rate of change [A] = delta [A]/ delta time
Rate of reaction [A] = - rate of change [A]
Instantaneous Rate of Reaction- determined
from the slope of a tangent lines to a
concentration-time graph
Initial Rate of Reaction- reaction rate when
the reactants are first brought together.
RATE LAW
The rate of reaction depends on the concentrations of
reactants.
aA + bB + gG + hH
The exponents, m,n.. are not generally related to the
stoichiometric coefficients. They are the order of
reactions. The overall order of reaction is the sum of
all the exponents.
Rate constant, k- relates the rate of reaction to
reactant concentrations, the larger the value, the
faster the reaction goes.
ZERO ORDER REACTIONS
The zero-order reaction as a rate equation in which
the sum of the exponents m+n+.. is equal to 0.
A products
The concentration-time graph is a straight line with a
negative slope
The rate of reaction, which is equal to k and remains
constant throughout the reaction, is the negative of
the slope of this line.
The units of k are the same as the units of the rate of
a concentration (mol/L-s)
Integrated rate equation:
y= mx + b [A]t = -kt + [A]
0
FIRST ORDER REACTIONS
The first order reaction has a rate equation in
which the sum of the exponents is equal to 1. A
common type is a single reactant decomposes
into products.
A products
e.g. 2 H
2
O
2
2 H
2
O + O
2
Integrated rate equation:
Half life of a reaction is the time required for
one-half of a reactant to be consumed.
| | A k reaction of rate =
| |
2 2
O H k reaction of rate =
| |
| |
kt
A
A
t
=
0
ln
| | | |
0
ln ln A kt A
t
+ =
| |
k
t
2 ln
2 / 1
=
SECOND ORDER REACTIONS
The second order reaction has a rate
equation in which the sum of the
exponents is equal to 2.
A products
Integrated rate equation:
| |
2
A k reaction of rate =
| | | |
0
1 1
A
kt
A
t
+ =
THE EFFECT OF TEMPERATURE ON
REACTION RATES
RT E
a
Ae k
/
=
|
|
.
|
\
|
=
2 1 1
2
1 1
ln
T T R
E
k
k
a
ACIDS AND BASES
EQUILIBRIUM CONSTANT, K
c
- allows us
to calculate equilibrium concentrations of
reactants and products
aA + bB = gG +hH
When we reverse an equation, we invert the value of Kc
When we multiply the coefficients in a balanced equation
by a corresponding factor, we raise the equilibrium
constant to the corresponding power.
When we divide the coefficients in a balanced equation by
a common factor, we take the corresponding root of the
equilibrium constant.
| | | |
| | | |
b a
h g
c
B A
H G
K =
When the individual equations are combined their
equilibrium constants are multiplied to obtain the
equilibrium constant for the net reaction.
Equilibrium constant expressions do not contain
concentration terms for solid or liquid phase of a
single component (pure solids or liquids)
A reaction is most likely to reach a state of
equilibrium in which significant quantities of both
reactants and products are present if the numerical
value of Kc is neither very large nor very small,
roughly from about 10
-10
to 10
+10
.
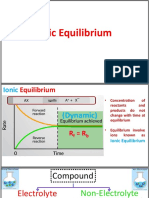
When an equilibrium system is subjected to a change
in temperature, pressure, or concentration of reacting
species, the system responds by attaining a new
equilibrium that partially offsets the impact of the
change
Reaction quotient, Qc ratio of
initial concentrations in a reaction
mixture that has the same form as
the equilibrium constant expression
If Q
c
=K
c
a reaction is at equilibrium
If Q
c
<K
c
a net reaction proceeds from
left to right (forward reaction)
If Q
c
>K
c
a net reaction proceeds from
right to left (reverse direction)
ARRHENIUS THEORY
In aqueous solution a strong electrolyte exists only in
the form of ions, whereas a weak electrolyte exists
partly as ions and partly as molecules.
A neutralization reaction involves the combination of
hydrogen ions and hydroxide ions to form water.
HCl H
+
+ Cl
-
NaOH Na
+
+OH
-
Neutralization reaction:
H
+
+ Cl
-
+ Na
+
+OH
-
Na
+
+ Cl
-
+ H
2
O
Acid base salt
Net ionic equation: H
+
+ OH
-
H
2
O
BRONSTED-LOWRY THEORY
Acid is proton donor, and a base is a proton
acceptor
NH3 + H2O NH4+ + OH-
base acid acid base
amphiprotic substances- can act either as an
acid or a base (H
+
)
amphoteric substances- can act either as an
acidic or basic oxide (Al
2
O
3
), associated with
elements having electronegativities in an
intermediate range.
LEWIS ACID AND BASE THEORY
Lewis acid-base theory is not limited to
reactions involving H
+
and OH
-
, it
extends to reactions in gases and in
solids.
Lewis acid is a species (atom, ion or
molecule) that is an electron pair
acceptor and a Lewis base is a species
that is an electron pair donor.
SELF IONIZATION OF WATER AND THE pH SCALE
Self Ionization of water- for each H2O molecule that acts as
an acid another acts as base, and hydronium (H3O+) and
hydroxide (OH-) ions are formed.
H
2
O + H
2
O H3O
+
+ OH
-
K
c
=[ H
3
O
+
][OH
-
]
At 25
o
C: [ H3O+]=[OH-]= 1.0 x 10
-7
M
Kw= [ H3O+][OH-]=1.0 x 10
-14
pH- potential of hydrogen ion
pH= - log [H3O
+
]
pOH= - log [OH
-
]
pKw = pH + pOH= 14
Percent ionization- gives the proportion
of ionized molecules on a percentage basis.
Percent ionization of a weak acid or a weak
base increases as the solution becomes
more dilute.
Polyprotic or polybasic acids- acids with
more than one ionizable H atom per
molecule.
% 100
3
x
HA of molarity initial
HA from derived O H molarity
ionization percent
+
=
Hydrolysis- a reaction between an ion and
water
Salts of strong bases and strong acids do not
hydrolyze, pH=7
Salts of strong bases and weak acids hydrolyze,
pH>7 (anion acts as base)
Salts of weak bases and strong acids hydrolyze,
pH<7 (cation acts as acid)
Salts of weak bases and weak acids hydrolyze,
pH depends on the relative values of Ka and Kb
for the ions (cations are acids, anions are bases)
Solutions of Weak Acids/Bases and Strong
Acids/Bases
The common ion effect is the suppression of the
ionization of a weak electrolyte caused by the
addition of an ion that is also a product of the
ionization equilibrium of weak electrolyte.
When a strong electrolyte supplies the common ion
(H3O
+
for acids, and OH
-
for bases) the equilibrium
shifts.
Solutions of Weak Acids/Bases and Their Salts
The salt of a weak acid/base is a strong electrolyte-
its ions become completely dissociated from one
another in aqueous solution. The presence of the
common ion suppresses the ionization of the weak
acid/base.
Buffer solutions
The pH values of buffer solutions change only
very slightly on the addition of small amounts of
either an acid or a base.
Buffer solutions require two components, one of
which is able to neutralize acids and the other
able to neutralize bases, but the two
components must not neutralize each other.
Common buffer solutions are a mixture of a
weak acid and its conjugate base or a weak
base and its conjugate acid.
Buffer capacity- the amount of acid or base that a
buffer can neutralize before its pH change
appreciably. The maximum buffer capacity exists
when the concentration of a weak acid and its
conjugate base are kept large and approximately
equal to each other.
Buffer range- pH range in which a buffer effectively
neutralizes added acids and bases and maintains a
fairly constant pH.
A range of 2 pH units is the maximum range to which a
buffer solution should be exposed.
| |
| | acid
base conjugate
pK pH
a
log + =
Complex ion- is a polyatomic cation or anion
composed of a central metal ion to which other
groups (molecules or ions) are bonded. Substances
containing complex ions belong to a category of
compounds called coordination compounds.
Kf- formation constant is the equilibrium constant
that is used to deal with a complex ion equilibrium, it
describes the formation of a complex ion from a
central ion and its ligands.
K
f
are usually large numbers which distinguish K
f
from
other equilibrium constants.
POSITIVE IONS BEHAVIOR AND
DETERMINATION
Qualitative Analysis of Cations- aims at identifying the cations present in
a mixture but not their quantities
Cations are divided into five groups depending on differing solubilities of their
compounds.
Group I: Ag
+
, Hg
2
2+
, Pb
2+
Precipitated in 1 M HCl
Group II: Bi
3+
, Cd
2+
, Cu
2+
, Hg
2+
, (Pb
2+
), Sb
3+
and Sb
5+
, Sn
2+
and Sn
4+
Precipitated in 0.1 M H
2
S solution at pH 0.5
Group III: Al
3+
, (Cd
2+
), Co
2+
, Cr
3+
, Fe
2+
and Fe
3+
, Mn
2+
, Ni
2+
, Zn
2+
Precipitated in 0.1 M H
2
S solution at pH 9
Group IV: Ba
2+
, Ca
2+
, K
+
, Mg
2+
, Na
+
, NH
4+
Ba
2+
, Ca
2+
, and Mg+ are precipitated in 0.2 M (NH
4
)
2
CO
3
solution at pH 10;
the other ions are soluble
Group V: The resulting solution consists of the soluble ions in water, Na
+
,
K
+
, NH
4+
Dissolving Metal sulfides
Increase the solubility of any sulfide by allowing
it to react with acid.
Use an oxidizing acid such as HNO
3
3CuS
(s)
+ 8 H
+
(aq)
+ 2NO
3
(aq)
2 Cu
2+
(aq)
+ 3 S
(s)
+2 NO
(g)
+ 4 H
2
O
A few metal sulfides dissolve in basic solution
with a high concentration of HS-. The subgroup
consisting of HgS , PbS, CuS , Bi
2
S
3
and CdS
remains undissolved after treatment with an
alkaline solution with an excess of HS- but
As
2
S
3
. Sb
2
S
3
and SnS
2
dissolve.
VOLUMETRIC AND
GRAVIMETRIC ANALYSIS
Volumetric analysis- a technique that employs the
measurement of volumes to determine quantitatively
the amount of a substance in solution. In any reaction
between two or more species, the reaction equation
will show the stoichiometric ratio of reacting species.
Gravimetric analysis- based upon the measurement
of mass. The precipitation method of gravimetric
analysis involves isolation of an ion in solution by a
precipitation reaction, filtering, washing the
precipitate free of contaminants, conversion of the
precipitate to a product of known composition, and
finally weighing the precipitate and determining its
mass by difference. From the mass and known
composition of the precipitate, the amount of the
original ion can be determined.
Você também pode gostar
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNo EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersAinda não há avaliações
- Practice Makes Perfect in Chemistry: Acids, Bases, and SaltsNo EverandPractice Makes Perfect in Chemistry: Acids, Bases, and SaltsAinda não há avaliações
- Structure and Reactivity: Acids and Bases, Polar and Nonpolar MoleculesDocumento53 páginasStructure and Reactivity: Acids and Bases, Polar and Nonpolar MoleculesAdzimahAinda não há avaliações
- Chapter 7 - EquilibriumDocumento8 páginasChapter 7 - EquilibriumstudyforiittomeetbtsAinda não há avaliações
- Power Pointpresentation On Ionic Equilibrium and Concept of PHDocumento31 páginasPower Pointpresentation On Ionic Equilibrium and Concept of PHritik12041998Ainda não há avaliações
- Chem 30 Course Summary 4Documento10 páginasChem 30 Course Summary 4dutritinh0806Ainda não há avaliações
- Class 11 Chemistry Notes 2023-24 8. Redox ReactionsDocumento40 páginasClass 11 Chemistry Notes 2023-24 8. Redox ReactionsAyushi Shah100% (1)
- In Sustainable Design and Development Si Edition 1st Edition by Striebig Ogundipe Papadakis Isbn 1133629784 9781133629788Documento36 páginasIn Sustainable Design and Development Si Edition 1st Edition by Striebig Ogundipe Papadakis Isbn 1133629784 9781133629788lee.caldwell560100% (12)
- Chem 142 - N Lab Acid Base Equilibria and Buffer Solutions 2022Documento64 páginasChem 142 - N Lab Acid Base Equilibria and Buffer Solutions 2022Jahred CantornaAinda não há avaliações
- Solution Manual For Engineering Applications in Sustainable Design and Development 1St Edition by Striebig Ogundipe and Papadakis Isbn 1133629776 978113362977 Full Chapter PDFDocumento36 páginasSolution Manual For Engineering Applications in Sustainable Design and Development 1St Edition by Striebig Ogundipe and Papadakis Isbn 1133629776 978113362977 Full Chapter PDFlee.caldwell560100% (11)
- Engineering Applications in Sustainable Design and Development 1st Edition by Striebig Ogundipe and Papadakis ISBN Solution ManualDocumento51 páginasEngineering Applications in Sustainable Design and Development 1st Edition by Striebig Ogundipe and Papadakis ISBN Solution Manualann100% (21)
- L4. Acids. Bases. PH Water Ionization: H O H + Ho +Documento4 páginasL4. Acids. Bases. PH Water Ionization: H O H + Ho +anaAinda não há avaliações
- Aqueous Solutions and Chemical EquilibriaDocumento54 páginasAqueous Solutions and Chemical EquilibriaJulius FrondaAinda não há avaliações
- Unit 18 - Acids and Bases HL NotesDocumento38 páginasUnit 18 - Acids and Bases HL NotesAdham SalmanAinda não há avaliações
- Chapter 4 Part-1 Sawyer's BookDocumento11 páginasChapter 4 Part-1 Sawyer's BookRegina MardatillahAinda não há avaliações
- Precipitation Reactions: 3 (Aq) (S) (S) 3 (Aq)Documento25 páginasPrecipitation Reactions: 3 (Aq) (S) (S) 3 (Aq)RonaldAinda não há avaliações
- Module 2 (B)Documento35 páginasModule 2 (B)SoniAinda não há avaliações
- Equilibrium - RevisionDocumento4 páginasEquilibrium - RevisionsatishAinda não há avaliações
- Ch.9 Aqueous Solutions & Chemical EquilibriaDocumento18 páginasCh.9 Aqueous Solutions & Chemical EquilibriaHazel TampilicAinda não há avaliações
- Uzair ChemistryDocumento6 páginasUzair ChemistryTalbia SyedAinda não há avaliações
- Equilibrium 3Documento8 páginasEquilibrium 3francis JASAinda não há avaliações
- 11 Chemistry Notes Ch07 EquilibriumDocumento8 páginas11 Chemistry Notes Ch07 EquilibriumShishirRanjanAinda não há avaliações
- Analytical ChemistryDocumento120 páginasAnalytical ChemistryJomed BarallasAinda não há avaliações
- L4 Acids Bases PH 2020Documento4 páginasL4 Acids Bases PH 2020anaAinda não há avaliações
- Chapter 2 Structure and Reactivity Alkanes: Chemistry 140A Winter 2014 (K. Albizati)Documento27 páginasChapter 2 Structure and Reactivity Alkanes: Chemistry 140A Winter 2014 (K. Albizati)Michael SeoAinda não há avaliações
- Ionic Equilibrium-Study MaterialDocumento32 páginasIonic Equilibrium-Study MaterialAhmed ShaalanAinda não há avaliações
- Critical Review of Rate Constant For Reaction of Hydrated ElectronsDocumento21 páginasCritical Review of Rate Constant For Reaction of Hydrated ElectronsumairyaqubqaziAinda não há avaliações
- Chapter-7: EquilibriumDocumento8 páginasChapter-7: EquilibriumAbhayAinda não há avaliações
- Equilibrium Equilibrium in Physical ProcessesDocumento6 páginasEquilibrium Equilibrium in Physical ProcessesSteveMathewKuruvillaAinda não há avaliações
- General Chemistry 2 Final Exam ReviewerDocumento6 páginasGeneral Chemistry 2 Final Exam ReviewerZyriel SaavedraAinda não há avaliações
- Equillibrium RevisionDocumento6 páginasEquillibrium RevisionMahesh BabuAinda não há avaliações
- Chemistry UNIT 4 NotesDocumento10 páginasChemistry UNIT 4 NotesSammit NadkarniAinda não há avaliações
- Ionic EquilibriumDocumento14 páginasIonic Equilibrium8842 AnuragAinda não há avaliações
- Types of ElectrolytesDocumento24 páginasTypes of ElectrolytesPranoy Baishya100% (1)
- Chapter 7 EquilibriumDocumento20 páginasChapter 7 EquilibriumNitish MehraAinda não há avaliações
- Introduction 1Documento13 páginasIntroduction 1yousernameAinda não há avaliações
- Câu Hỏi Thi FinalDocumento12 páginasCâu Hỏi Thi FinalDuy Do MinhAinda não há avaliações
- 1st Acid-Base LectureDocumento33 páginas1st Acid-Base Lecturesoma_92Ainda não há avaliações
- Chemical Equilibrium 1Documento49 páginasChemical Equilibrium 1samarthasai2006Ainda não há avaliações
- Chemistry Content Palm CardsDocumento52 páginasChemistry Content Palm Cardsk.gardnerAinda não há avaliações
- Chapter 4Documento4 páginasChapter 4Joshua DubluisAinda não há avaliações
- Wa0025.Documento7 páginasWa0025.Uday BhaskarAinda não há avaliações
- Equilibrium Cheat Sheet InhouseDocumento2 páginasEquilibrium Cheat Sheet InhouseShirleyLinAinda não há avaliações
- Ionic EquilibriumDocumento31 páginasIonic EquilibriumharshitAinda não há avaliações
- QC-1 Assn TanvirDocumento11 páginasQC-1 Assn TanvirRA TanvirAinda não há avaliações
- Chapter 2b-Water and The Aqueous EnvironmentDocumento25 páginasChapter 2b-Water and The Aqueous EnvironmentAra Jean AgapitoAinda não há avaliações
- Chem Note 4Documento5 páginasChem Note 4Gee BandongAinda não há avaliações
- 101 CHM EqualDocumento24 páginas101 CHM EqualDave LarryAinda não há avaliações
- Redox Reactions NotesDocumento17 páginasRedox Reactions NotesTejas SinghAinda não há avaliações
- Ionic EquilibriumDocumento35 páginasIonic EquilibriumYak Raj PandeyAinda não há avaliações
- Che 91166 4pagesDocumento0 páginaChe 91166 4pagesapi-218511741Ainda não há avaliações
- The Chemistry of Water: 2.1. Water As A SolventDocumento6 páginasThe Chemistry of Water: 2.1. Water As A SolventEva MoonAinda não há avaliações
- Focus Coaching Centre Cet - Neet-Crash Course: Subject: Chemistry Unit 7-Eqilibrium Resource Person: Priyanka RajaramDocumento13 páginasFocus Coaching Centre Cet - Neet-Crash Course: Subject: Chemistry Unit 7-Eqilibrium Resource Person: Priyanka RajaramPriyankaAinda não há avaliações
- Balancing Redox ReactionsDocumento3 páginasBalancing Redox ReactionsMargaretAinda não há avaliações
- L1 - Intro Biochem & Water PropertiesDocumento31 páginasL1 - Intro Biochem & Water Propertieshaiqalfariq07Ainda não há avaliações
- SOURCE: General Chemistry: Principles and Modern Applications 10Documento3 páginasSOURCE: General Chemistry: Principles and Modern Applications 10Jerremiah YuAinda não há avaliações
- Critical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsNo EverandCritical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsAinda não há avaliações
- Organic Chemistry Study Guide: Key Concepts, Problems, and SolutionsNo EverandOrganic Chemistry Study Guide: Key Concepts, Problems, and SolutionsNota: 3.5 de 5 estrelas3.5/5 (10)
- Energy Design GuidelineDocumento120 páginasEnergy Design GuidelineAlvin Garcia PalancaAinda não há avaliações
- Palawan MainteDocumento91 páginasPalawan MainteAlvin Garcia PalancaAinda não há avaliações
- Inorganic Chemistry IIDocumento23 páginasInorganic Chemistry IIAlvin Garcia PalancaAinda não há avaliações
- Energy MGT PalawanDocumento65 páginasEnergy MGT PalawanAlvin Garcia PalancaAinda não há avaliações
- Fire Involving Radioactive Source: Judix/Grieza/RudolfDocumento10 páginasFire Involving Radioactive Source: Judix/Grieza/RudolfAlvin Garcia PalancaAinda não há avaliações
- Transport Accident Involving A Radioactive Source: Hanna Saco Philip CaluyaDocumento13 páginasTransport Accident Involving A Radioactive Source: Hanna Saco Philip CaluyaAlvin Garcia PalancaAinda não há avaliações
- Radioactive Source Is Leaking: Michael Alvarez Christian EraDocumento8 páginasRadioactive Source Is Leaking: Michael Alvarez Christian EraAlvin Garcia PalancaAinda não há avaliações
- Engine Oil Replacement: An Operator Is About To Get Off A Hydraulic Excavator, While The Engine Oil Is Being ReplacedDocumento1 páginaEngine Oil Replacement: An Operator Is About To Get Off A Hydraulic Excavator, While The Engine Oil Is Being ReplacedAlvin Garcia PalancaAinda não há avaliações
- EHPT22Documento1 páginaEHPT22Alvin Garcia PalancaAinda não há avaliações
- Deburring Work: A Worker Is Deburring A Steel Plate With An Air SunderDocumento1 páginaDeburring Work: A Worker Is Deburring A Steel Plate With An Air SunderAlvin Garcia PalancaAinda não há avaliações
- A Worker Is Drilling A Hole On Work With A Bench Drilling MachineDocumento1 páginaA Worker Is Drilling A Hole On Work With A Bench Drilling MachineAlvin Garcia PalancaAinda não há avaliações
- Grinding Work: A Worker Is Grinding Small Parts, Holding It With A PlierDocumento1 páginaGrinding Work: A Worker Is Grinding Small Parts, Holding It With A PlierAlvin Garcia PalancaAinda não há avaliações
- Emergency Response On The Incident of Missing Radioactive SourceDocumento9 páginasEmergency Response On The Incident of Missing Radioactive SourceAlvin Garcia PalancaAinda não há avaliações
- Heat Treatment of Parts For CorrectionDocumento1 páginaHeat Treatment of Parts For CorrectionAlvin Garcia PalancaAinda não há avaliações
- On-Site Gas Welding Work: A Service Mechanic Is Flame-Cutting A Steel Plate at An In-Field Repair SiteDocumento1 páginaOn-Site Gas Welding Work: A Service Mechanic Is Flame-Cutting A Steel Plate at An In-Field Repair SiteAlvin Garcia PalancaAinda não há avaliações
- EHPT27Documento1 páginaEHPT27Alvin Garcia PalancaAinda não há avaliações
- Bolt Tightening: A Worker Is Retightening A Cylinder Pin Stopper BoltDocumento1 páginaBolt Tightening: A Worker Is Retightening A Cylinder Pin Stopper BoltAlvin Garcia PalancaAinda não há avaliações
- Transportation: Dismantled From A Hydraulic Excavator, A Boom Is Going To Be Moved With An Overhead CraneDocumento1 páginaTransportation: Dismantled From A Hydraulic Excavator, A Boom Is Going To Be Moved With An Overhead CraneAlvin Garcia PalancaAinda não há avaliações
- EHPT26Documento1 páginaEHPT26Alvin Garcia PalancaAinda não há avaliações
- EHPT20Documento1 páginaEHPT20Alvin Garcia PalancaAinda não há avaliações
- EHPT23Documento1 páginaEHPT23Alvin Garcia PalancaAinda não há avaliações
- Replacement of Cylinder: A Mechanic Is Driving A Connecting Pin in The Arm Cylinder Replacement WorkDocumento1 páginaReplacement of Cylinder: A Mechanic Is Driving A Connecting Pin in The Arm Cylinder Replacement WorkAlvin Garcia PalancaAinda não há avaliações
- EHPT21Documento1 páginaEHPT21Alvin Garcia PalancaAinda não há avaliações
- Sanity Check PresentationDocumento28 páginasSanity Check PresentationAlvin Garcia PalancaAinda não há avaliações
- Bailey Net 90Documento4 páginasBailey Net 90Alvin Garcia PalancaAinda não há avaliações
- EHPT18Documento1 páginaEHPT18Alvin Garcia PalancaAinda não há avaliações
- EHPT19Documento1 páginaEHPT19Alvin Garcia PalancaAinda não há avaliações
- EHPT1Documento1 páginaEHPT1Alvin Garcia PalancaAinda não há avaliações
- Rio Tuba FR Vol - I Main Report 090618Documento114 páginasRio Tuba FR Vol - I Main Report 090618Alvin Garcia PalancaAinda não há avaliações
- Product Catalog: Drilling & CompletionsDocumento33 páginasProduct Catalog: Drilling & CompletionsSergioBernardesAinda não há avaliações
- Gastric AnalysisDocumento23 páginasGastric AnalysisAvi VermaAinda não há avaliações
- Ionic EquilibriumDocumento38 páginasIonic EquilibriumSwara BhideAinda não há avaliações
- Syllabus IOC I Pharm.D (C.L Baid)Documento4 páginasSyllabus IOC I Pharm.D (C.L Baid)giridharan rajendranAinda não há avaliações
- Application of Neutralization TitrationsDocumento21 páginasApplication of Neutralization TitrationsAdrian NavarraAinda não há avaliações
- Physical Chemistry I (Solid State) : DR Fatah EltaboniDocumento20 páginasPhysical Chemistry I (Solid State) : DR Fatah EltaboniDina Garan100% (1)
- Yab1 033 - Analytical ChemistryDocumento12 páginasYab1 033 - Analytical Chemistrymaster guardianAinda não há avaliações
- European Journal of Biomedical AND Pharmaceutical SciencesDocumento14 páginasEuropean Journal of Biomedical AND Pharmaceutical SciencesSACHIN BHASKAR NARKHEDEAinda não há avaliações
- International Journal of Innovative Pharmaceutical ResearchDocumento8 páginasInternational Journal of Innovative Pharmaceutical ResearchDavid GuzmanAinda não há avaliações
- A Robust Liquid Chromatographic Method For Confirmation of Drug Stability of Azithromycin in Bulk Samples, Tablets and SuspensionsDocumento11 páginasA Robust Liquid Chromatographic Method For Confirmation of Drug Stability of Azithromycin in Bulk Samples, Tablets and SuspensionsCesar RodriguezAinda não há avaliações
- Kimia Anugrah Ricky Wijaya PDFDocumento9 páginasKimia Anugrah Ricky Wijaya PDFdian pingkiAinda não há avaliações
- 4.7 Titration Curves Indicators and Buffers Questions OnlyDocumento13 páginas4.7 Titration Curves Indicators and Buffers Questions Onlyilias1973100% (1)
- Experiment 9 (UV-Vis) - Lab ManualDocumento2 páginasExperiment 9 (UV-Vis) - Lab ManualJoseph JoeAinda não há avaliações
- Aspirin Determination Colorimetric MethodDocumento10 páginasAspirin Determination Colorimetric MethodSamantha Alexandra Vélez SalinasAinda não há avaliações
- IMA Questions PaperDocumento17 páginasIMA Questions PaperAj ShindeAinda não há avaliações
- Lab Report-13: Environmental Chemistry (ENE-213) Course Instructor: Dr. Sofia BaigDocumento7 páginasLab Report-13: Environmental Chemistry (ENE-213) Course Instructor: Dr. Sofia BaigHaniya SiddiqueAinda não há avaliações
- Amino Acids by Paper ChromatographyDocumento3 páginasAmino Acids by Paper ChromatographynaomiAinda não há avaliações
- Acids and BasesDocumento26 páginasAcids and BasesGaayathiriAinda não há avaliações
- Molarity of Concentrated Reagents - (WWW - RhodiumDocumento2 páginasMolarity of Concentrated Reagents - (WWW - RhodiumŠĭlệncěIšmyPŕIdệAinda não há avaliações
- Experiment No. 9 Introduction To Chromatography: I. DataDocumento3 páginasExperiment No. 9 Introduction To Chromatography: I. DataKleya ParreñoAinda não há avaliações
- Expt.1 BiochemDocumento4 páginasExpt.1 BiochemMc de RamosAinda não há avaliações
- CHEM 301 LEC - Analytical Chemistry I LectureDocumento5 páginasCHEM 301 LEC - Analytical Chemistry I LectureDaniel TanAinda não há avaliações
- Jurnal Praktikum Dasar-Dasar Pemisahan Kimia Pembuatan Membran Polysulfon (PSF)Documento9 páginasJurnal Praktikum Dasar-Dasar Pemisahan Kimia Pembuatan Membran Polysulfon (PSF)Rizki AuAinda não há avaliações
- Acid Base ActivityDocumento2 páginasAcid Base ActivityNICOLE CA�ARESAinda não há avaliações
- Acids and BasesDocumento26 páginasAcids and BasesBharat SinghAinda não há avaliações
- RecrystallizationDocumento5 páginasRecrystallizationHannako NgohayonAinda não há avaliações
- 12 Chemistry Chapter 3 Assignment 5Documento2 páginas12 Chemistry Chapter 3 Assignment 5sansharmajsAinda não há avaliações
- Comparative Study of RP-HPLC and UV Spectrophotometric Techniques For The Simultaneous Determination of Amoxicillin and Cloxacillin in CapsulesDocumento6 páginasComparative Study of RP-HPLC and UV Spectrophotometric Techniques For The Simultaneous Determination of Amoxicillin and Cloxacillin in Capsulesiabureid7460Ainda não há avaliações
- Optical Min5Documento19 páginasOptical Min5DesyAAlvridaAinda não há avaliações
- YMC Solutions For BioseparationsDocumento4 páginasYMC Solutions For BioseparationsM.AhmedAinda não há avaliações
- CHEM1001 Acid Base Part 2Documento36 páginasCHEM1001 Acid Base Part 2Dlcm Born To WinAinda não há avaliações