Escolar Documentos
Profissional Documentos
Cultura Documentos
Separation Processes - Tutorial 3: DR Colin Hare
Enviado por
Tara EdwardsDescrição original:
Título original
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Separation Processes - Tutorial 3: DR Colin Hare
Enviado por
Tara EdwardsDireitos autorais:
Formatos disponíveis
Separation Processes Tutorial 3
Dr Colin Hare
Chemical Engineering School of Process, Environmental and Materials Engineering University of Leeds
Tel. 32407 E-Mail c.l.hare@leeds.ac.uk
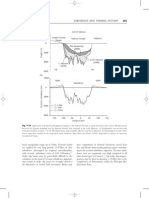
Example 1: Raoults Law, Flash and Batch Distillation
A binary mixture has the equilibrium vapour pressure data shown in the table, measured at a total pressure of 101 kPa. (a) Use Raoults law to calculate the vapour and liquid compositions in equilibrium (in mole fractions, y and x) for this system. (b) A mixture of 35 mole% A and 65 mole% B is subjected to flash distillation at a feed rate of 750 kmol/hr. Determine the fraction of A in the distillate and bottom product for the following fractions of feed vapourised: (i) 0% (ii) 25% (iii) 50% (iv) 75% (v) 100%. (c) If 100 kmol of a binary mixture of 60 mole% A is distilled in a batch operation until the still only contains 20 mole% A, calculate the total distillate collected and the average composition of the distillate.
T (oC) 125 118 110 103 98 92 87 84
P0A (kPa) 0 273 227 192 169 152 138 126
P0B (kPa) 101 82 69 62 56 51 45 44
82
81 80
116
108 101
40
40 0
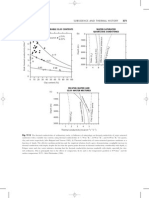
Example 2: McCabe-Thiele Method
A distillation column operating at 1 atm is to be designed for separating a mixture of compounds A and B. The feed is 35 mole% A and the feed flow rate is 750 kmole/hr with q = 0.5. A distillate composition of 95 mole% A and a bottoms composition of not more than 5 mole% A are desired. The reflux ratio is 2. Determine: (a) total number of equilibrium stages (b) optimum feed plate location (c) distillate and bottoms flow rates (Equilibrium data for the binary system at 1 atm pressure are given in the table).
T (oC) 100 95 91 87 83 79 76 74
xA 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7
yA 0.0 0.4 0.55 0.63 0.7 0.76 0.82 0.88
72
70 69
0.8
0.9 1.0
0.92
0.96 1.0
Example 3: Ponchon-Savarit Method
A distillation column operating at 1 atm is to be designed for separating a binary mixture of A and B. The feed is 40 mass% A and the feed flow rate is 250 kg/hr of saturated liquid. A distillate composition of 98 mass% A and a bottoms composition of not more than 10 mass% A are desired. The heat duty of the condenser is 280 MJ/hr and the value of HB is -1050 kJ/kg. Using the Ponchon Savarit method, determine the number of ideal stages required to carry out the separation under these conditions. (Equilibrium and enthalpy data for the mixture at 1 atm pressure are given in the table). Note, HL refers to the enthalpy of the mixture at boiling point, HV refers to the enthalpy of the mixture at dew point.
T (oC) 110 105 101 98 95 92 88 84 82 81
xA 0
yA 0
HL (kJ/kg) 1500 1100 850 700 600 500 400 350 300 250
HV (kJ/kg) 3000 2800 2600 2510 2475 2400 2300 2200 2100 1950
0.09 0.26 0.18 0.55 0.26 0.73 0.3 0.4 0.67 0.78 0.82 0.9
0.53 0.86 0.78 0.94 0.89 0.98
80.5 80
0.98 0.99 1 1
210 200
1800 1500
Example 4: Lewis-Matheson Method
A distillation column operating at 1 atm is to be designed for separating a mixture of component A, B and C. The feed has mole fractions of 0.5, 0.4 and 0.1 of A, B and C, respectively. The feed flow rate is 500 kmole/hr. A distillate composition of 92 mole% A and 7 mole% B and a bottom product with not more than 12 mole% A are desired. Assuming a reflux ratio of 2, determine: The top and bottom product flow rates and compositions. The number of ideal stages required to carry out the separation, using the Lewis-Matheson method. Where AC, BC and CC are 2.6, 1.2 and 1, respectively. q=1
Example 5: Underwood & Fenske equations
A distillation column operating at 1 atm is to be designed for separating a mixture of components A, B, C and D. Feed and distillation fractions are given in the table below, determine: The top and bottom product flow rates and bottom product composition. The light and heavy key components. The minimum reflux ratio for the separation to be achieved when (i) q = 0, (ii) q = 0.25, (iii) q = 0.5, (iv) q = 0.75 and (v) q = 1. The minimum number of plates required at total reflux by using the Fenske equation. (Note AB refers to the relative volatility of the light key with respect to the heavy key). Component xF xD xB 125 118 2.3 1.5 0.50 0.25 0.80 0.15 0 ?
110
103
1.2
1
0.20
0.05
0.05
0
?
?
Example 5 (continued): Gillilands empirical relationship
Using the values of Nm and Rm determined previously when q = 1, determine the number of stages required for reflux ratios of 1.5, 2, 3, 5, 10 and 20.
Você também pode gostar
- Mass Transfer Tutorial: Distillation Example Problem 2: Mccabe-Thiele MethodDocumento11 páginasMass Transfer Tutorial: Distillation Example Problem 2: Mccabe-Thiele MethodTapiwa KapondaAinda não há avaliações
- Assignment Problems Batch I (R.No. 102117001 To 102117011)Documento7 páginasAssignment Problems Batch I (R.No. 102117001 To 102117011)Nishanth ChandranAinda não há avaliações
- Tutorial DistillationDocumento3 páginasTutorial DistillationManu Indivare Nundoolall100% (1)
- Physical Chemistry of Polyelectrolyte SolutionsNo EverandPhysical Chemistry of Polyelectrolyte SolutionsMitsuru NagasawaAinda não há avaliações
- Tutorial-Chapter 2 (June - Oct 2013)Documento5 páginasTutorial-Chapter 2 (June - Oct 2013)paulineanakmawatAinda não há avaliações
- Set 4Documento3 páginasSet 4Ibtisam FarhaniAinda não há avaliações
- TutrealDocumento2 páginasTutrealsaint deanAinda não há avaliações
- New Problems Chapter 26Documento3 páginasNew Problems Chapter 26KaakmmAinda não há avaliações
- Ponchon-Savarit Method Draft 01Documento7 páginasPonchon-Savarit Method Draft 01Joanna remAinda não há avaliações
- Distillation - Self Study QuestionsDocumento8 páginasDistillation - Self Study QuestionsEsther MaidenAinda não há avaliações
- Distillation TutorialDocumento17 páginasDistillation TutorialXin-YiWoon100% (1)
- Distillation Aspen HysysDocumento66 páginasDistillation Aspen HysysCzarina MasicatAinda não há avaliações
- Assignment 2Documento3 páginasAssignment 2deepika snehi100% (1)
- Continuous Distillation Practice 1Documento35 páginasContinuous Distillation Practice 1Najmul Puda PappadamAinda não há avaliações
- 1165r05320801 Mass Transfer Operations IIDocumento9 páginas1165r05320801 Mass Transfer Operations IIsobichemAinda não há avaliações
- Che 246 - Mass Transfer and Unit Operations Tutorial-Chapter 2 (Distillation)Documento5 páginasChe 246 - Mass Transfer and Unit Operations Tutorial-Chapter 2 (Distillation)fatien zakariaAinda não há avaliações
- Rr320801masstransferoperationsiiDocumento8 páginasRr320801masstransferoperationsiikorangaprakashAinda não há avaliações
- 1174rr320801 Mass Transfer Operations IIDocumento8 páginas1174rr320801 Mass Transfer Operations IIsobichemAinda não há avaliações
- Individual Assignment 200412Documento2 páginasIndividual Assignment 200412Zaidi ZakariaAinda não há avaliações
- DistillationDocumento6 páginasDistillationanita_shar29Ainda não há avaliações
- Problems in Mass TransferDocumento3 páginasProblems in Mass TransferAngelica Joyce BenitoAinda não há avaliações
- Distillation Exercises - Set 1Documento3 páginasDistillation Exercises - Set 1Fred VoAinda não há avaliações
- Diploma Examination, May 2015: (Petroleum Refining Engineering) 110. DistillationDocumento2 páginasDiploma Examination, May 2015: (Petroleum Refining Engineering) 110. DistillationgebremichaelAinda não há avaliações
- Liquid-Liquid Extraction OTKDocumento38 páginasLiquid-Liquid Extraction OTKJaffarudin Janu WahyudiAinda não há avaliações
- MTO AssignmentDocumento4 páginasMTO AssignmentBishal LamichhaneAinda não há avaliações
- 2023 SPU260S Tutorial 3 QuestionsDocumento6 páginas2023 SPU260S Tutorial 3 QuestionsMABUKE NDINAINWI INNOCENTIAAinda não há avaliações
- Single Stage PDFDocumento52 páginasSingle Stage PDFThelunatic ModAinda não há avaliações
- Liquid Liquid ExtractionDocumento40 páginasLiquid Liquid ExtractionMohsin Ehsan100% (1)
- MSOCHA3 - Tutorial 4 - LU 3 - Liquid-Liquid ExtractionDocumento7 páginasMSOCHA3 - Tutorial 4 - LU 3 - Liquid-Liquid ExtractionTshwarelo MahlakoaneAinda não há avaliações
- MSOCHA3 Tutorial 1 Multicomponent AbsorptionDocumento5 páginasMSOCHA3 Tutorial 1 Multicomponent AbsorptionTshwarelo MahlakoaneAinda não há avaliações
- CHE311 Practice Problems 2012Documento9 páginasCHE311 Practice Problems 2012Albert HuynhAinda não há avaliações
- Assignment 3Documento4 páginasAssignment 3Yi Hong LowAinda não há avaliações
- Mass Transfer Design Question PaperDocumento11 páginasMass Transfer Design Question PaperAbdul Majid IaAinda não há avaliações
- Nptel Mto.2 DistillationDocumento3 páginasNptel Mto.2 Distillationmaddukuri jagadeesh babuAinda não há avaliações
- DistillationDocumento40 páginasDistillationEbook Download100% (2)
- Chemical Reaction Set4ansDocumento4 páginasChemical Reaction Set4ansffffffAinda não há avaliações
- Set 7 AnsDocumento4 páginasSet 7 AnsArturo Hernández MoralesAinda não há avaliações
- #Separation Tut2Documento1 página#Separation Tut2ibtihal esamAinda não há avaliações
- Mass Transfer Operations II Rr320801Documento8 páginasMass Transfer Operations II Rr320801Nagwa MansyAinda não há avaliações
- 7HC - Mt-Ii Oct-98,99 Apr-00Documento7 páginas7HC - Mt-Ii Oct-98,99 Apr-00Ahmed AliAinda não há avaliações
- MT Answer KeyDocumento41 páginasMT Answer Keykaviyas.21chemAinda não há avaliações
- Master Complex Columns and Four Assumptions Problems 2020 Set - 5Documento7 páginasMaster Complex Columns and Four Assumptions Problems 2020 Set - 5vikyappleAinda não há avaliações
- ChE212 Topic 04 A NotesDocumento4 páginasChE212 Topic 04 A NotesGino Paul MarasiganAinda não há avaliações
- SHMT Midterm Examination Paper-2018Documento1 páginaSHMT Midterm Examination Paper-2018MuhammadTanzeeLUsmanAinda não há avaliações
- HomeworkDocumento3 páginasHomeworkJudluzAinda não há avaliações
- Tute 3Documento4 páginasTute 3ArunAinda não há avaliações
- CHE 502 Tutorial 5Documento3 páginasCHE 502 Tutorial 5Ibnu HamidAinda não há avaliações
- LLE TutoDocumento2 páginasLLE TutoizzAinda não há avaliações
- Lecture 11-ADocumento19 páginasLecture 11-AChirag GargAinda não há avaliações
- Tutorial 2-1Documento3 páginasTutorial 2-1pritiksha.mathapersadAinda não há avaliações
- Tutorials QuestionsDocumento3 páginasTutorials Questionskarri100% (1)
- Distillation HWDocumento1 páginaDistillation HWKhin OoAinda não há avaliações
- Separation Processes IDocumento3 páginasSeparation Processes IAmesh Chiyogami100% (1)
- Tutorial 5drtuhDocumento2 páginasTutorial 5drtuhFikrie MuhdAinda não há avaliações
- Tutorial Absorption 2022Documento27 páginasTutorial Absorption 2022Mars Studio0% (1)
- Chapter 11Documento12 páginasChapter 11kumar_chemicalAinda não há avaliações
- The Oil Depletion ProtocolDocumento53 páginasThe Oil Depletion ProtocolTara EdwardsAinda não há avaliações
- Permeabilities Key MDDocumento8 páginasPermeabilities Key MDTara EdwardsAinda não há avaliações
- Executive Summary For GulaimDocumento2 páginasExecutive Summary For GulaimTara EdwardsAinda não há avaliações
- WattenbergDocumento38 páginasWattenbergTara EdwardsAinda não há avaliações
- Toluene DisproportionDocumento4 páginasToluene DisproportionTara EdwardsAinda não há avaliações
- Exercises For Lecture x2Documento8 páginasExercises For Lecture x2Tara EdwardsAinda não há avaliações
- Example 1: Underwood & Fenske equations: Component α x x xDocumento8 páginasExample 1: Underwood & Fenske equations: Component α x x xTara EdwardsAinda não há avaliações
- PEME2210 Engineering Science 2 Heat Transfer: Tutorial 1 (Solution)Documento2 páginasPEME2210 Engineering Science 2 Heat Transfer: Tutorial 1 (Solution)Tara EdwardsAinda não há avaliações
- Depth Section: Nondeposition Basin FloorDocumento0 páginaDepth Section: Nondeposition Basin FloorTara EdwardsAinda não há avaliações
- Application To Petroleum Play AssessmentDocumento0 páginaApplication To Petroleum Play AssessmentTara EdwardsAinda não há avaliações
- 8.4.1 Carbonate StratigraphyDocumento0 página8.4.1 Carbonate StratigraphyTara EdwardsAinda não há avaliações
- Fig. 9.10 Application of Flexural Backstripping Technique To The Valencia Trough, A Young Rift Basin in The Western MediterraneanDocumento0 páginaFig. 9.10 Application of Flexural Backstripping Technique To The Valencia Trough, A Young Rift Basin in The Western MediterraneanTara EdwardsAinda não há avaliações
- Esogenesis and Telogenesis of Sandstones: Cements Such As Chlorite, Which May Inhibit QuartzDocumento0 páginaEsogenesis and Telogenesis of Sandstones: Cements Such As Chlorite, Which May Inhibit QuartzTara EdwardsAinda não há avaliações
- Mesogenetic (Beyond The Influence of Surficial Processes) Karst Systems That Enhance Vertical Conductivity of FluidsDocumento0 páginaMesogenetic (Beyond The Influence of Surficial Processes) Karst Systems That Enhance Vertical Conductivity of FluidsTara EdwardsAinda não há avaliações
- Water-Saturated Quartzose Sandstones: Subsidence and Thermal HistoryDocumento0 páginaWater-Saturated Quartzose Sandstones: Subsidence and Thermal HistoryTara EdwardsAinda não há avaliações
- Sedimentology Lecture Notes (Week 5 Test)Documento4 páginasSedimentology Lecture Notes (Week 5 Test)Tara EdwardsAinda não há avaliações
- Limak 2017 Annual ReportDocumento122 páginasLimak 2017 Annual Reportorcun_ertAinda não há avaliações
- Practice Exam 1Documento7 páginasPractice Exam 1425Ainda não há avaliações
- Civ-Su-6001-C - Design of BuildingsDocumento37 páginasCiv-Su-6001-C - Design of BuildingsBolarinwaAinda não há avaliações
- Course Material Fees: Terms 1190 - 1193Documento8 páginasCourse Material Fees: Terms 1190 - 1193Frances Ijeoma ObiakorAinda não há avaliações
- Data Structure Algorithm Using C PresentationDocumento245 páginasData Structure Algorithm Using C PresentationdhruvwAinda não há avaliações
- University of Tennessee - ChattanoogaDocumento34 páginasUniversity of Tennessee - ChattanoogaMALIK ZARYABBABARAinda não há avaliações
- DualityDocumento27 páginasDualitySuprabhat TiwariAinda não há avaliações
- GL 314Documento2 páginasGL 314Vinayak SinghAinda não há avaliações
- Activation and Deactivation of CatalystsDocumento16 páginasActivation and Deactivation of Catalystsshan0214Ainda não há avaliações
- UT TransducersDocumento20 páginasUT TransducersSamanyarak AnanAinda não há avaliações
- CPS 800 12 900 10 Parts List 2012 02 ENG 2205 6006 51 PDFDocumento51 páginasCPS 800 12 900 10 Parts List 2012 02 ENG 2205 6006 51 PDFMar SolAinda não há avaliações
- Transfer Action in Sap HRDocumento3 páginasTransfer Action in Sap HRKarthi MrvkAinda não há avaliações
- SunstarDocumento189 páginasSunstarSarvesh Chandra SaxenaAinda não há avaliações
- Chapter 2 Magnetic Effects of Current XDocumento25 páginasChapter 2 Magnetic Effects of Current XPawan Kumar GoyalAinda não há avaliações
- Green Line TransformerDocumento4 páginasGreen Line TransformerwakasAinda não há avaliações
- CE 411 Lecture 03 - Moment AreaDocumento27 páginasCE 411 Lecture 03 - Moment AreaNophiAinda não há avaliações
- Forklift Operator Evaluation Form: Operator Behaviors Rating Comments Pre-Use InspectionDocumento2 páginasForklift Operator Evaluation Form: Operator Behaviors Rating Comments Pre-Use InspectionXionAinda não há avaliações
- Uses of The Components of Crude Oil As FuelsDocumento6 páginasUses of The Components of Crude Oil As FuelsPearl LawrenceAinda não há avaliações
- PDK Repair Aftersales TrainingDocumento22 páginasPDK Repair Aftersales TrainingEderson BJJAinda não há avaliações
- Particle Swarm Optimization - WikipediaDocumento9 páginasParticle Swarm Optimization - WikipediaRicardo VillalongaAinda não há avaliações
- .Preliminary PagesDocumento12 páginas.Preliminary PagesKimBabAinda não há avaliações
- RT 67Documento11 páginasRT 67dinesh kumarAinda não há avaliações
- DRM Transmitter PresentationDocumento22 páginasDRM Transmitter PresentationJuan Jose PerezAinda não há avaliações
- ISO - 3601-2 O-Rings HousingDocumento56 páginasISO - 3601-2 O-Rings HousingAlexey FlidliderAinda não há avaliações
- Omron ManualDocumento44 páginasOmron ManualHaroDavidAinda não há avaliações
- Acids and Bases Part 3 (Weak Acids) EdexcelDocumento2 páginasAcids and Bases Part 3 (Weak Acids) EdexcelKevin The Chemistry TutorAinda não há avaliações
- Ex Delta Ex Delta - Dia: OVAL CorporationDocumento8 páginasEx Delta Ex Delta - Dia: OVAL CorporationDaniela GuajardoAinda não há avaliações
- AK Carbon Steel PB 201307Documento70 páginasAK Carbon Steel PB 201307SilveradoAinda não há avaliações
- Class 7 Science Electric Current and Its EffectsDocumento7 páginasClass 7 Science Electric Current and Its Effectsshanna_heenaAinda não há avaliações
- SAP Hybris Thinking Outside The Box. PART 1Documento18 páginasSAP Hybris Thinking Outside The Box. PART 1Rauf AlievAinda não há avaliações