Escolar Documentos
Profissional Documentos
Cultura Documentos
Group1 Report Phase Equilibrium
Enviado por
JobertCastillanesDireitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Group1 Report Phase Equilibrium
Enviado por
JobertCastillanesDireitos autorais:
Formatos disponíveis
Phase
Equilibrium
Reporters:
Cruz, Jerico
Caringal, Angelo
Castillanes, Jobert
Dion, Canlas
Candidato, Vince
Taedo, Cassidy
ABSTRACT
The recent interest in the determination of the phase
diagrams for many systems involving water as one
component has been in those systems which are of
greatest importance to clay mineralogy. The importance
of both published and more recent work on synthesis of
the clay is demonstrated in the ability to prepare
chemically pure, mineralogically homogeneous kaolinites,
montmorillonites, micas, and chlorites. The stability and
identification of the clay minerals are discussed in relation
to some of the major problems of clay mineralogy.
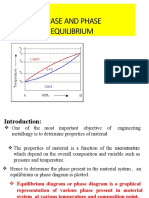
INTRODUCTION
Studies of phase equilibria are highly relevant to many areas of geosciences
because in most cases, mineral systems have the time to reach thermodynamic
equilibrium and lack the energy input needed to sustain disequlibrium. With
increasing pressure, most crystal structures that are stable at low pressure (e.g. 1
bar) are replaced by denser structures. This has important implications for Earth's
interior as well as for the synthesis of novel materials for industrial applications. A
significant area of mineral physics research is therefore dedicated towards
discovering new phases that may be present either in the deep earth or within
other planets and characterizing their physical and chemical properties.
Equilibrium relations between phases at high pressure are governed by the same
thermodynamic principles that operate at low pressure.
A given mineral crystallizes as a stable phase only within the
restricted ranges of pressure and temperature. Subjecting the
mineral to conditions that fall outside its stability will cause
another mineral that is table under those conditions to crystallize
in its place.
Testing mineral stability: laboratory experiment looking at the T, P,
water vapour pressure,etc)
Note: Phase diagrams
I. Introduction
Concepts that are particularly important to
understand are: Gibbs Free energy, enthalpy,
entropy, chemical activity, Clausius Clapeyron
equation, endothermic and exothermic reactions
and the phase rule.
II. Description of Terms:
1) Phase part or parts of a system occupying a specific volume and having
a uniform physical and chemical characteristics which distinguish it from all
other parts of the system.
2) System a collection of geological phases
a) Open system one that is free to exchange both matter and energy with its
surroundings
b) Closed system - one that is sealed with respect to the transfer of matter, but that
can still exchange energy with the surroundings.
c) Isolated system - one that is incapable of exchanging both mass and energy with its
surroundings.
II. Description of Terms:
3) Component basic chemical constituents of a system, of which the various
phases are composed; comprises the minimum number of chemical (atomic
and molecular) species required to specify completely the compositions of all
the phases present.
III. Equilibirum
A. Thermal equilibirum
All parts of the system have the same temperature; there is no net transfer of
heat.
B. Chemical equilibrium
Distribution of components among the phases of a system has become
constant, showing no net change with time; the flux of atoms across the
crystal boundary is the same in both directions.
Note: disequilibrium
IV. The Gibbs Phase Rule
Q: How many phases can be in equilibrium with each other at any one time?
(William Gibbs-1870- pioneer of modern thermodynamics)
Phase Rule- expresses the number of phases that can coexist in mutual
equilibrium () in terms of the number of components (C) in the system
and variance (F).
Variance = number of degrees of freedom
+ F = C + 2
Degrees of freedom of a system is the number of variables (i.e., temperature,
pressure and concentration of the components, which must be arbitrarily fixed in
order that the condition of the system may be completely defined.
Phase Characteristic
A phase does not have to be BOTH physically and chemically distinct
Example:
1.Water and ice
2.Oil and water
Equilibrium Assemblages
At equilibrium, the mineralogy (and the composition of each mineral)
is determined by T, P, and X Mineral paragenesis refers to such an
equilibrium mineral assemblage Relict minerals or later alteration
products are thereby excluded from consideration unless specifically
stated
Driving Forces and Gibbs Free Energy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It
includes theinternal energy, which is the energy required to create a system,
and the amount of energy required to make room for it by displacing
itsenvironment and establishing its volume and pressure.
Enthalpy is athermodynamic potential. It is a state function and
anextensive quantity. The unit of measurement in theInternational System of
Units (SI) for enthalpy is the joule, but other historical, conventional units are still
in use, such as the small and the large calorie.
ENTROPY
a measure of the unavailable energy in a closed
thermodynamic system that is also usually
considered to be a measure of the system's
disorder, that is a property of the system's state,
and that varies directly with any reversible change
in heat in the system and inversely with the
temperature of the system;
CHEMICAL ACTIVITY
The active mass or chemical activity of any chemical system is
simply the chemical activity of its components, which are the
reactants or the products of a chemical reaction. The rate of the
chemical reaction CaCO3 --> CaO + CO2 is then proportional to the
activity of CaCO3, while the rate of the reverse reaction, CaO + CO2
--> CaCO3 is proportional both to the activity of CaO and to the
activity of CO2. Likewise, the driving force of the reaction of CaCO3
is directly proportional to the activity of CaCO3 while the driving
force of the reverse reaction is directly proportional both to the
activity of CaO and to the activity of CO2.
Clausius Clapeyron equation
The relationship between the temperature of a liquid and its vapor pressure is
not a straight line. The vapor pressure of water, for example, increases
significantly more rapidly than the temperature of the system. This behavior
can be explained with the Clausius-Clapeyron equation.
The Clausius-Clapeyron Relation
Along the phase boundary:
P
T
P
T
phase
boundary
Since S1 - S2 = L/T (L is the latent heat), we
arrive at the Clausius-Clapeyron Relation :
For the slope of the
boundary we have:
ENDOTHERMIC AND EXOTHERMIC
REACTIONS
exothermic:
1. release heat, therefore the room temperature will increase
2. H = negative (kJ mol^-1)
3. bond will be formed, and energy will be release in form of heat, the
molecules that are formed had a lower kinetic energy but very stabil
endothermic:
1. absorb heat, therefore the room temperature will decrease
2. H = positive (kJ mol^-1)
3. bonds will broke down, because the energy of reactants are less than the
energy of products in the chemical reaction
PHASE RULE
The Phase Rule describes the possible
number of degrees of freedom in a (closed)
system at equilibrium, in terms of the
number of separate phases and the
number of chemical constituents in the
system. It was deduced from
thermodynamic principles by J. W. Gibbs in
the 1870s.
PHASE RULE
Phase diagrams in P-T space (Reaction or equilibrium between
compounds)
NaAlSi2O6 + SiO2 = NaAlSi3O8
Jadeite (px)
silica pyroxene
Note: reaction or equilibrium boundary
Gibbs Phase Rule
The Phase Rule- expresses the number of phases that can coexist in mutual
equilibrium
F = C - P+ 2
F is the degree of freedom
P - number of phases
C minimum number of components.
Note: the 2 represent 2 intensive parameters particularly temperature and
pressure.
High Pressure Phase Equilibrium Studies
There are a number of different types of high pressure studies
related to phase equilibria. There are true phase equilibrium
studies that seek to characterize the range of pressure,
temperature or chemical activity (e.g. for O2) over which a given
phase (or phase assemblage) is stable.
Synthesis studies seek to discover and produce new phases.
However, they are distinct from phase equilibrium studies. There
are a number of circumstances that can lead to minerals forming
outside their thermodynamic stability field. Another major area of
interest is phase transformation mechanisms and kinetics.
Reversals: the difference between synthesis
and phase equilibrium studies
One of the most important
features of a good phase
boundary determination is the
reversal. A reversal is a pair of
experiments where the reactants
and products in one experiment
become the products and
reactants in another experiment.
Reversals: the difference between synthesis
and phase equilibrium studies
The position of the phase boundary can be said to be somewhere between
these bracketing experiments. In-situ experiments (e.g. synchrotron x-ray
diffraction) are particularly efficient for conducting phase equilibrium studies
because the transformation from one phase assemblage to the other can be
run first one way and then back the other at a number of pressure and
temperatures during a single experiment.
Since phase boundaries are lines (or curves if volatiles are present) at minimum two
reversal are required to fully describe a phase boundary. Reversals are necessary for two
reasons:
1)Due to kinetics, phases can persist in some cases indefinitely outside of their stability
field. Merely witnessing a phase at a given P and T, especially at lower temperatures,
does not guarantee that it is the stable phase.
2) As mentioned above, phases can be synthesized outside of their stability field. Some
of the reasons that this can occur are related to a phenomena referred to as Ostwald's
step rule which says that the first phase to from in a reaction is not always the one with
the lowest free energy but the phase with a free energy closest to the free energy of the
reactants. In order to start with a sample that is highly homogeneous, experimentalists
may choose a gel or glass; the free energy of these reactants can be quite large.
Reactants ground in a mortar and pestle can be highly strained (e.g. Kingma et al., 1993)
which can also substantially raise their free energy. So for example, coesite can be
produced from highly strained quartz at P and T conditions in the quartz stability field
(Green, 1972). For these reasons if one wants to measure the position of a phase boundary
or confirm that a given phase in fact has a stability field, reversals are required.
Discovery and Synthesis of New Phases
The simplest way to discover a new
phase is to put reactants of interest
together and bring them to some
new pressure temperature condition
and either observe them with x-rays
at temperature and pressure or
quench them and use x-ray
diffraction to identify the phases
present and search for new phases.
However, there are more
sophisticated strategies for searching
P,T chemistry spaces for new phases.
Discovery and Synthesis of New Phases
However, there are more sophisticated strategies for searching P,T chemistry
spaces for new phases. One strategy that has been around for a long time is
to use one's knowledge of phase relations in one chemical system to make
intelligent guesses about phase relations in another similar chemical system.
For example, the observation that Mg2GeO4 formed in both an olivine and a
denser spinel structured polymorph, led researchers in the 1930's to suggest
that (Mg, Fe)2SiO4 olivine might also have a spinel structured polymorph which
could account for the increase in seismic velocity at ~400 km depth. Many
compounds that share the same stoichiometry have phase diagrams that
share the same topology.
Phase Equilibria Relevant to the Deep Earth
Phase diagrams illustrate the stability of phases in Pressure,Temperature,
chemical activity space. Many igneous and metamorphic systems contain
up to 11 significant chemical components. This means that a full depiction of
phase equilibria in these systems requires 13 dimension. Petrologists have
devised a host of sections, pseudo sections and projections to create
illustrative 2D diagrams from these multi-dimensional spaces. Mineral physics
has mostly confined itself to working in relatively simple chemical systems
(e.g. MgO-SiO2) that serve as a proxy for the chemically more complex Earth.
Another strategy is to look at changes with pressure and temperature in a
single bulk composition (e.g. MORB or pyrolite). A selection of relevant phase
diagrams is given below.
Phases: Kyanite, Sillimanite and Andalusite
Component: Al2SiO5
Pressure and Temperature Phase
Diagram (One component)
Analyze at point X, Y and Z.
Analyze point F
Binary Diagrams
Composition is now variable
Condensed phase rule
P + F = C + 1
Note: pressure is no longer a variable: only Temperature and composition
matter.
Crystallization in systems with no solid
solution
no compounds/solid
solutions in solid state
only single phase liquid
at high temperatures
partial melting at
intermediate
temperatures
Liquidus Curve- specifies
the maximum
temperature at which
crystals can co-exist
with the melt in
thermodynamic
equilibrium
Tie Line- links together
the compositions of two
phases which can
coexist stably
Analyze point x1 and E
Liquidus
Curve
System with a complete Solid Solution
Example would be plagioclase
m, cools down,
encounters liquidus at
a where plag b will
begin to crystallize
Because b is more
calcic, it will deplete
the melt with
anorthite and thereby
enrich it with albite
The changing melt
composition causes a
corresponding
evolution in the
equilibrium
composition of the
plagioclase crystals.
LEVER RULE
The lever rule is a tool used to determine weight percentages of each
phase of a binary equilibrium phase diagram. It is used to determine the
percent weight of liquid and solid phases for a given binary composition
and temperature that is between the liquidus and solidus.
Consider a cooling alloy at the composition and temperature
marked on the diagram. As shown on the phase diagram, the
alloy is, at the given temperature, a mixture of alpha and liquid
phases - but what are their exact compositions at this
temperature?
An isothermal (constant temperature) line through the alloy's position on the phase
diagram when it is in a two phase field, intersecting the two adjacent solubility curves, is
called a tie line (yes, that's the horizontal yellow line on the diagram).The ends of the tie
lines show the compositions of the two phases that exist in equilibrium with each other at
this temperature. From the diagram we know that alpha and liquid phases will exist. The tie
line shows that the alpha phase is 5.2%B and the liquid phase is 34.5%B at this temperature.
Remember, though, that the overall composition of the sample is unchanged - we are only
discovering the compositions of the constituent phases within the sample.
For a cooling alloy at composition C
o
and temperature T
x
, tie lines may
be used to answer questions such as:
what phases are present ?
what are their compositions ?
if the temperature is reduced to T
y
, how do the compositions of the two
phases vary ?
The answer to "what phases are present ?" is easy. Composition C
o
and
temperature T
x
meet in the beta + liquid phase field, so these are the two
phases present.
To answer "what are their compositions ?" we must draw a horizontal tie
line from the point to the nearest phase diagram boundaries. The tie line
shows us that the compositions are:
Liquid: X wt% B
Beta: Y wt% B
To answer the last question "if the temperature is reduced to T
y
, how do the compositions of the
two phases vary?"consider the new tie-line, shown in yellow on the diagram.The compositions of
liquid and beta phase have both decreased in wt%B to:
Liquid: X' wt% B
Beta: Y' wt% B
Thus, both the liquid and the beta phases are getting richer in A as the sample is cooled.
Now that we know the compositions of the two phases, we need to
find how much of each phase exists at the given temperature.
The ratio of the two phases present can be found by using the lever
rule.At first sight the lever rule can appear confusing. It is really invoking
the conservation of mass, and can be proved mathematically, as shown
below the diagram.
Essentially, we start off with an overall composition of our alloy - C
o
. From
the tie-line we know that the two phases at a given temperature have
two different compositions, but overall the amounts of these two
compositions must add up to the alloy's overall composition, C
o
.
This is the basis for the lever rule. Using the lever rule itself is very simple,
we'll show you with a diagram.....
Basically, the proportions of the phases present are given by the relative
lengths of the tie line. So, the proportions of alpha and liquid present on
the diagram (showing a portion of the whole phase diagram) are:
X Y
X+Y and X+Y
Simple, isn't it ?
But... which equation corresponds to which phase ?
Now, consider the same alloy as it crosses the liquidus line. It seems
reasonable to assume that, at this point, the alloy will be nearly all liquid.
Looking at the diagram it can be seen that Y1 is very small here and so
must be the proportion of alpha present. Similarly X1 is relatively large and
so it corresponds to the amount of liquid.
So, the left side of the tie line gives the proportion of the liquid phase (the
phase to the right), and the right side of the tie line gives the proportion of
the alpha phase (the phase on the left).
Remember: you use the length of the line which is furthest from the phase in
which you are interested.
Distances along the tie line can be found very simply by using a ruler on
an accurate phase diagram or, more correctly, by using data from the
composition axis (the x-axis).For example, on the diagram shown, the
percentage of alpha present can be calculated from the three pieces of
composition data given:
Fraction of alpha = (34.5 - 23.7) / (34.5 - 5.2) = 0.3686
Thus, percentage of alpha = 0.3686 x 100 = 36.86%
and, as the alpha and the liquid make up 100% of the alloy's
composition:
Percentage of liquid = 100 - 36.86 = 63.14%
Magmatic Processes
Some useful terms:
Primary magma - magma originating in (mantle) source
directly from melting.
Primitive magma - magma that undergo minimal
differentiation.
Parental magma - least differentiated magma in a series
leading to evolved rocks.
Magmatic Differentiation:
the process whereby, magma originally homogeneous
splits up into contrasted parts, which may form separate
bodies of rocks or may remain within the boundaries of
single unitary mass.
It is usually favored by two factors:
a.Rate of cooling.
b.Settling of early crystallized heavy minerals.
Fractional Crystallization
Separation of crystals from liquid
Gravitative settling or flotation play a significant role
ASSIMILATION
It is the method of creating different daughter magmas from a parent is by
having the latter react with its wall rocks
assimilation is accompanied by crystallization, it is likely that both fractional
crystallization and assimilation will take place simultaneously.
Combined Process (AFC = Assimilation +
Fractional Crystallization)
HOT (1200C)
basaltic magma
Blocks of continental
crust fall into basaltic
magma and dissolve.
basaltic magma +
assimilated blocks =
andesitic magma
Heat transfer from
hot, basaltic magma
melts wall rock
magma composition progressively
changes as crystal are physically
removed from the magma
Incongruent Melting
Incongruent melting occurs when a substance does not melt
uniformly and decomposes into another substance. For example,
potassium feldspar (KAlSi3O8) decomposes to leucite (KAlSi2O6)
when it melts. The decomposition is not complete, however. Most of
the feldspar does melt, a portion of it decomposes to leucite and
some quartz (SiO2) is left over, since the chemical formulas of
potassium feldspar and leucite differ by SiO2. Another mineral that
melts incongruently is enstatite (MgSiO3), which decomposes to
forsterite (Mg2SiO4). Enstatite does melt congruently between
pressures of 2.5 and 5.5 kilobars.
Congruent Melting
Congruent melting occurs during melting of a
compound when the composition of the liquid that
forms is the same as the composition of the solid. It
can be contrasted with incongruent melting. This
generally happens in two- component systems.
Congruent melting VS Incongruent melting
In congruent melting of rocks, the
minerals simply melt and join the liquid. In
incongruent melting, a new solid minerals
precipitates from the melt.
For example of a gneiss containing quartz, biotite and
sillimanite is melted, then the result is a liquid plus the
new solid mineral cordierite (magnesium iron aluminum
cyclosilicate)
Você também pode gostar
- Rotating Electrode Methods and Oxygen Reduction ElectrocatalystsNo EverandRotating Electrode Methods and Oxygen Reduction ElectrocatalystsAinda não há avaliações
- Metallurgy of Superconducting Materials: Treatise on Materials Science and Technology, Vol. 14No EverandMetallurgy of Superconducting Materials: Treatise on Materials Science and Technology, Vol. 14Thomas LuhmanAinda não há avaliações
- Electrochemical CellsDocumento63 páginasElectrochemical CellsHoongAinda não há avaliações
- Preparative Methods in Solid State ChemistryNo EverandPreparative Methods in Solid State ChemistryPaul HagenmullerAinda não há avaliações
- Advanced Catalysts and Nanostructured Materials: Modern Synthetic MethodsNo EverandAdvanced Catalysts and Nanostructured Materials: Modern Synthetic MethodsAinda não há avaliações
- Surface Physics of Materials: Materials Science and TechnologyNo EverandSurface Physics of Materials: Materials Science and TechnologyAinda não há avaliações
- Materials Engineering: Proceedings of the First International Symposium, University of the Witwatersrand, Johannesburg, South Africa, November 1985No EverandMaterials Engineering: Proceedings of the First International Symposium, University of the Witwatersrand, Johannesburg, South Africa, November 1985J. V. BeeAinda não há avaliações
- Electronic Structure and Surfaces of Sulfide Minerals: Density Functional Theory and ApplicationsNo EverandElectronic Structure and Surfaces of Sulfide Minerals: Density Functional Theory and ApplicationsAinda não há avaliações
- Fracture of Metals: An Advanced TreatiseNo EverandFracture of Metals: An Advanced TreatiseH. LiebowitzAinda não há avaliações
- Electronic Absorption Spectra and Geometry of Organic Molecules: An Application of Molecular Orbital TheoryNo EverandElectronic Absorption Spectra and Geometry of Organic Molecules: An Application of Molecular Orbital TheoryNota: 5 de 5 estrelas5/5 (1)
- Elements of Structures and Defects of Crystalline MaterialsNo EverandElements of Structures and Defects of Crystalline MaterialsAinda não há avaliações
- Coordination Chemistry: Invited Lectures Presented at the 20th International Conference on Coordination Chemistry, Calcutta, India, 10-14 December 1979No EverandCoordination Chemistry: Invited Lectures Presented at the 20th International Conference on Coordination Chemistry, Calcutta, India, 10-14 December 1979D. BanerjeaAinda não há avaliações
- Conductometric TitrationsDocumento19 páginasConductometric Titrationsusman_uet08100% (5)
- Introduction to the Theory of Atomic Spectra: International Series of Monographs in Natural PhilosophyNo EverandIntroduction to the Theory of Atomic Spectra: International Series of Monographs in Natural PhilosophyNota: 5 de 5 estrelas5/5 (1)
- Advances in Materials: Proceedings of a Symposium Organised by the North Western Branch of the Institution of Chemical Engineers Held at Manchester, 6–9 April, 1964No EverandAdvances in Materials: Proceedings of a Symposium Organised by the North Western Branch of the Institution of Chemical Engineers Held at Manchester, 6–9 April, 1964Ainda não há avaliações
- The Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelNo EverandThe Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelAinda não há avaliações
- Current Topics in Amorphous Materials: Physics & TechnologyNo EverandCurrent Topics in Amorphous Materials: Physics & TechnologyY. SakuraiNota: 5 de 5 estrelas5/5 (1)
- Lab Report Corrosion-1Documento10 páginasLab Report Corrosion-1areniqwardiah_918730100% (1)
- Heat Engines Lab PDFDocumento24 páginasHeat Engines Lab PDFAnonymous ee5dOjAinda não há avaliações
- Catalyst Synthesis TechniquesDocumento19 páginasCatalyst Synthesis Techniquescoolcupidguy100% (1)
- Ce6306 Som PDFDocumento55 páginasCe6306 Som PDFHemakumar ManoharanAinda não há avaliações
- Solidification of Metals (To Be Completed) : Prof. H. K. Khaira Professor, Deptt. of MSME M.A.N.I.T., BhopalDocumento62 páginasSolidification of Metals (To Be Completed) : Prof. H. K. Khaira Professor, Deptt. of MSME M.A.N.I.T., BhopalIndranil Bhattacharyya100% (1)
- Inorganic Chemistry ExpDocumento46 páginasInorganic Chemistry Exppc355chyi100% (3)
- Physical Electronics Slides of Chapter 7 All SlidesDocumento45 páginasPhysical Electronics Slides of Chapter 7 All SlidesLina Al-SalehAinda não há avaliações
- Ions in Solution Lab (Retake)Documento4 páginasIons in Solution Lab (Retake)Evan PfeiferAinda não há avaliações
- Chapter 6 Phase DiagramsDocumento73 páginasChapter 6 Phase DiagramsSaiful AzrieAinda não há avaliações
- Phase Equilibria in Ceramic SystemsDocumento43 páginasPhase Equilibria in Ceramic SystemsAlexAinda não há avaliações
- Phase Equilibria and Phase TransformationDocumento57 páginasPhase Equilibria and Phase TransformationAzhan Haqimi100% (1)
- Coordination ChemistryDocumento25 páginasCoordination Chemistrypatel_346879839Ainda não há avaliações
- Heat ProblemsDocumento17 páginasHeat ProblemsAhmed AliAinda não há avaliações
- V Naoh (ML) PH: Otentiometric ItrationDocumento9 páginasV Naoh (ML) PH: Otentiometric ItrationradyjrAinda não há avaliações
- Catalysis & Catalysts: Facts and Figures About CatalystsDocumento88 páginasCatalysis & Catalysts: Facts and Figures About CatalystskeatyAinda não há avaliações
- V. Gravimetric AnalysisDocumento14 páginasV. Gravimetric AnalysisMark Christian GuintoAinda não há avaliações
- Section 3 EnergeticsDocumento47 páginasSection 3 Energeticsapi-3734333Ainda não há avaliações
- D and F BlockDocumento30 páginasD and F BlockTS SPORTZAinda não há avaliações
- AdsorptionDocumento22 páginasAdsorptionaleena'Ainda não há avaliações
- Midterm Review SolutionsDocumento16 páginasMidterm Review SolutionsKate SongAinda não há avaliações
- Temperature & Density For CastingsDocumento46 páginasTemperature & Density For CastingsRobinson GnanaduraiAinda não há avaliações
- Experiment 1 - DistillationDocumento22 páginasExperiment 1 - DistillationJhef ebuengaAinda não há avaliações
- Iron-Carbon Phase DiagramsDocumento25 páginasIron-Carbon Phase DiagramsTisza_MAinda não há avaliações
- Phase Diagrams PDFDocumento56 páginasPhase Diagrams PDFAbir Roy100% (1)
- Reduction Oxidation Cycling of Metal OxidesDocumento281 páginasReduction Oxidation Cycling of Metal OxidesAngel RumboAinda não há avaliações
- UNIT 4 Metal FinishingDocumento17 páginasUNIT 4 Metal FinishingVasudev GuptaAinda não há avaliações
- 5 CH241 Stereochemistry 8th EdDocumento84 páginas5 CH241 Stereochemistry 8th EdEfrain AnayaAinda não há avaliações
- Magnetism Notes CompleteDocumento11 páginasMagnetism Notes CompleteSathya Sai Kumar Yeluri100% (1)
- E302 Title PageDocumento1 páginaE302 Title PageJobertCastillanesAinda não há avaliações
- Rhodonite Tremolite: AnorthiteDocumento2 páginasRhodonite Tremolite: AnorthiteJobertCastillanesAinda não há avaliações
- Jobert O. CastillanesDocumento3 páginasJobert O. CastillanesJobertCastillanesAinda não há avaliações
- Structural Map of The PhilippinesDocumento4 páginasStructural Map of The PhilippinesJobertCastillanesAinda não há avaliações
- Math16l Project Castillanes 2013-2014 4qDocumento5 páginasMath16l Project Castillanes 2013-2014 4qJobertCastillanesAinda não há avaliações
- Structural Map of The PhilippinesDocumento3 páginasStructural Map of The PhilippinesJobertCastillanesAinda não há avaliações
- EOS Residuales Thermodynamics & Chemicals Kinetics (ChBC-34)Documento292 páginasEOS Residuales Thermodynamics & Chemicals Kinetics (ChBC-34)swerty619Ainda não há avaliações
- Thermodynamics Lecture NotesDocumento99 páginasThermodynamics Lecture NotesprashantcuriostudyAinda não há avaliações
- Thermodynamics of Material AssignmentDocumento7 páginasThermodynamics of Material AssignmentRanjit Kumar Dehury 1631002550% (2)
- 16a.triangular Plots in Metamorphic PetrologyDocumento14 páginas16a.triangular Plots in Metamorphic PetrologyAsmita BhattacharyaAinda não há avaliações
- Syllabus Chemical EngineeringDocumento83 páginasSyllabus Chemical EngineeringSHARAD SHARMAAinda não há avaliações
- Process Dynamics and Control Notes PDFDocumento81 páginasProcess Dynamics and Control Notes PDFBongibethu Msekeli Hlabano100% (2)
- Lead-Silver System and Its Explanation: Phase Diagram: FreezingDocumento3 páginasLead-Silver System and Its Explanation: Phase Diagram: FreezingVora AyushAinda não há avaliações
- Pet 326: Reservoir Phase Behaviour: Lecture NotesDocumento79 páginasPet 326: Reservoir Phase Behaviour: Lecture NotesAkande Ayodeji100% (2)
- Supplementary Information: Revisiting The Classic Activity Coefficient ModelsDocumento35 páginasSupplementary Information: Revisiting The Classic Activity Coefficient ModelsLeonardo ReyesAinda não há avaliações
- Fluid Phase EquilibriaDocumento19 páginasFluid Phase EquilibriaYli S'cAinda não há avaliações
- Phase Equilibrium Diagram': Phase, Phase Transformation, Phase Diagram, Phase Rule & Degress of FreedomDocumento20 páginasPhase Equilibrium Diagram': Phase, Phase Transformation, Phase Diagram, Phase Rule & Degress of FreedomGlesie Devara CabacangAinda não há avaliações
- L8 Binary Phase Diagrams PDFDocumento78 páginasL8 Binary Phase Diagrams PDFSudeepta MondalAinda não há avaliações
- Multiparametric Equation State Span2000Documento22 páginasMultiparametric Equation State Span2000Fátima ReyesAinda não há avaliações
- Phase and Phase EquilibriumDocumento40 páginasPhase and Phase EquilibriumNyanda MadiliAinda não há avaliações
- Gas Extraction An Introduction To Fundamentals of Supercritical Fluids and The Application To Separation Processes by Prof. Dr.-Ing. Gerd Brunner (Auth.) PDFDocumento396 páginasGas Extraction An Introduction To Fundamentals of Supercritical Fluids and The Application To Separation Processes by Prof. Dr.-Ing. Gerd Brunner (Auth.) PDFMUHAMMAD KHOLIDAinda não há avaliações
- Phase Rule: Ternary Liquid System: Physical Chemistry Laboratory Experiment II-2Documento9 páginasPhase Rule: Ternary Liquid System: Physical Chemistry Laboratory Experiment II-2Mohini BajajAinda não há avaliações
- Chapter 7 PDFDocumento76 páginasChapter 7 PDFNurfatini CheAinda não há avaliações
- Lec 10Documento13 páginasLec 10Rohan TiwariAinda não há avaliações
- B.SC - Chemistry.2022-23 Naan Mudhalvan Scheme PDFDocumento75 páginasB.SC - Chemistry.2022-23 Naan Mudhalvan Scheme PDFapstvlAinda não há avaliações
- EntropyDocumento11 páginasEntropyGerman ChiappeAinda não há avaliações
- Smith ch08Documento55 páginasSmith ch08張子恆Ainda não há avaliações
- Fe-Pb-S (Iron-Lead-Sulfur)Documento2 páginasFe-Pb-S (Iron-Lead-Sulfur)Regina H ChAinda não há avaliações
- Degrees of Freedom in Dynamic and Static SystemsDocumento8 páginasDegrees of Freedom in Dynamic and Static Systemsbarbara_ropeAinda não há avaliações
- User Guide Gpec 2012 1Documento27 páginasUser Guide Gpec 2012 1João GuilhermeAinda não há avaliações
- Solubility of DrugsDocumento147 páginasSolubility of Drugsharshagadia234Ainda não há avaliações
- Chemistry PPT Cse BDocumento12 páginasChemistry PPT Cse BSamiksha ChichwareAinda não há avaliações
- Engineering Thermodynamics, 7Th Ed., Mcgraw-Hill, 2005Documento2 páginasEngineering Thermodynamics, 7Th Ed., Mcgraw-Hill, 2005ne gerek var anonimAinda não há avaliações
- VLLE and VLE of The System Water Ethanol HeptaneDocumento5 páginasVLLE and VLE of The System Water Ethanol HeptaneArun EbenezerAinda não há avaliações
- Chemistry For Engineers (PDFDrive)Documento422 páginasChemistry For Engineers (PDFDrive)Heinrich Galamgam100% (6)