Escolar Documentos
Profissional Documentos
Cultura Documentos
Matter and Change
Enviado por
Yadana1Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Matter and Change
Enviado por
Yadana1Direitos autorais:
Formatos disponíveis
BELLWORK
What is matter?
What is mass and how does it
compare to weight?
What word is used to describe
the amount of space an object
takes up?
1.
2.
3.
Matter and Change
Unit III Properties of Matter
Matter and Change
1.
2.
3.
Anything that takes up space
and has mass.
Weight is a measure of the pull
of gravity on an object; mass is
the amount of matter the object
contains.
Volume.
Matter and Change
What states of matter are
represented in the photograph?
What must you do to a
substance to change its
physical state?
Matter and Change
Matter and Change
The mass of an object is the
amount of matter the object
contains. A golf ball has a greater
mass than a table tennis ball. The
golf ball, therefore, contains more
matter.
Matter and Change
Matter and Change
Materials differ in terms of the kind of
matter they contain.
Table sugar is always 100% sucrose.
It always has the same chemical
composition.
Matter that has a uniform and definite
composition is called a substance.
Matter and Change
Matter and Change
Substances contain only one kind
of matter.
Which of these two is a substance?
H20 or Lemonade?
Matter and Change
All samples of a substance have the
same physical properties.
All crystals of sucrose taste sweet
and dissolve completely in
water.
Matter and Change
A physical property is a quality
or condition of a substance that
can be observed or measured
without changing the
substances composition.
Matter and Change
Examples of physical properties
include:
1.- COLOR
2.- SOLUBILITY
3.- ODOR
4.- HARDNESS
Matter and Change
5.- DENSITY
6.- MELTING POINT
7.- BOILING POINT
Matter and Change
Physical properties help chemists
identify a substance based on their
characteristics.
Which substance is this?
Colorless liquid that boils at 100
C and melts at 0 C
???????
Colorless liquid that boils at 78 C
and melts at -117 C ???????
Matter and Change
Important properties of the States of Matter
Property
vapor
Shape
Volume
Expansion
on heating
Compressibility
Solid
Liquid
Gas or
Definite
Definite
Indefinite
Definite
Indefinite
Indefinite
Very slight
Moderate
Great
Almost
incompressible
Almost
incompressible
Readily
compressibl
e
Matter and Change
SOLIDS
Coal, sugar, ice and iron.
Definite shape and volume.
The shape doesnt depend on the
shape of their container.
The particles are packed tightly
together, they are almost
incompressible.
Solids expand only lightly when heated
Matter and Change
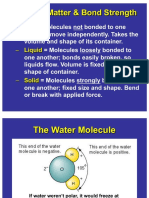
LIQUIDS
Water, milk and blood.
Takes the shape of their container (flows).
Particles are packed closely together, but
not rigidly packed.
The volume that a liquid occupies is
always constant, no matter what shape it
takes.
Almost incompressible, with a tendency
to expand when heated.
Matter and Change
GASES
Takes the shape and form of their
container (flowable).
Particles are spaced far apart.
Gases expand without limit to fill any
space.
Gases are easily compressed.
Matter and Change
VAPOR
The gaseous state of a substance that
is generally a liquid or solid at room
temperature.
Steam (the gaseous state of matter)
is referred to as a vapor because
water is a liquid at room temperature.
Moist air contains water vapor.
Matter and Change
PHYSICAL CHANGE
A type of change that alters a
given material without changing
its chemical composition.
Examples include: cutting,
grinding, bending, the melting of
the metal gallium, the freezing of
water and the condensation of
steam to water.
Matter and Change
PHYSICAL CHANGE
Other verbs or actions related to
physical changes include: boil,
freeze, dissolve, melt, condense,
break, split, crack, grind, cut, crush
and bend.
This powerpoint was kindly donated to
www.worldofteaching.com
http://www.worldofteaching.com
Is home to well over a thousand powerpoints
submitted by teachers. This a free site. Please visit
and I hope it will help in your teaching
Você também pode gostar
- Matter and ChangeDocumento23 páginasMatter and ChangeDionisio BrinosaAinda não há avaliações
- Matter and States of ChangeDocumento23 páginasMatter and States of ChangeDionisio BrinosaAinda não há avaliações
- ParticleDocumento143 páginasParticlespamiplviewAinda não há avaliações
- Matter and Energy PDFDocumento2 páginasMatter and Energy PDFOlatzPérezFernándezAinda não há avaliações
- Classifying Matter: Describing the Three States of Solids, Liquids, and GasesDocumento28 páginasClassifying Matter: Describing the Three States of Solids, Liquids, and GasesJohn Michael DitchonAinda não há avaliações
- Science8 Q3 Week2Documento21 páginasScience8 Q3 Week2Kathrina De SenaAinda não há avaliações
- States of MattersDocumento13 páginasStates of MattersZahraa HerekAinda não há avaliações
- Chemistry 2nd Topic BridgingDocumento24 páginasChemistry 2nd Topic BridgingRhean ParbaAinda não há avaliações
- Matter Propertiesand Changes Complete UNITth GradeDocumento22 páginasMatter Propertiesand Changes Complete UNITth GradeSheilaAinda não há avaliações
- MatterDocumento52 páginasMatterapi-217729044Ainda não há avaliações
- GR 9 Chapter 2 (2.1,2.2) The Nature of MatterDocumento48 páginasGR 9 Chapter 2 (2.1,2.2) The Nature of MatterPRUTHIVI BEHRAAinda não há avaliações
- States of Matter: and Its Physical ChangesDocumento18 páginasStates of Matter: and Its Physical ChangescassierottoAinda não há avaliações
- 2.1 Solids Liquids and GasesDocumento9 páginas2.1 Solids Liquids and GasesnidhiAinda não há avaliações
- Chem F1 U2 NoteDocumento3 páginasChem F1 U2 NoteumaymaupdirahmanmohamedAinda não há avaliações
- Matter in Our SurroundingsDocumento37 páginasMatter in Our SurroundingsMaghil shreeAinda não há avaliações
- Matter IntroductionDocumento33 páginasMatter Introductionapi-313400286Ainda não há avaliações
- Materials & Their Structure: 6Th GradeDocumento59 páginasMaterials & Their Structure: 6Th GradeMoosa Mohammed PatelAinda não há avaliações
- Properties of MatterDocumento56 páginasProperties of MatterD. Rakesh KumarAinda não há avaliações
- Properties of Matter and Changes in MatterDocumento55 páginasProperties of Matter and Changes in MatterPrinces April ArrezaAinda não há avaliações
- Interactive Textbook2 1 States of MatterDocumento5 páginasInteractive Textbook2 1 States of Matterapi-240094705Ainda não há avaliações
- Properties of MatterDocumento25 páginasProperties of MatterAl-Afiq AlbukharyAinda não há avaliações
- 0708 MatterDocumento20 páginas0708 MatterLuisa RagueroAinda não há avaliações
- Matter:: It's What The World Is Made ofDocumento20 páginasMatter:: It's What The World Is Made ofSUDHIR NAIRAinda não há avaliações
- Matter PPT 1Documento48 páginasMatter PPT 1Vlad KaneAinda não há avaliações
- Science Matter TeacherDocumento77 páginasScience Matter Teacherapi-661751964Ainda não há avaliações
- Lesson 1 - States of MatterDocumento26 páginasLesson 1 - States of Matterapi-268442191Ainda não há avaliações
- 3 States of MatterDocumento19 páginas3 States of Matterwahyuno100% (1)
- Matter and Its PropertiesDocumento17 páginasMatter and Its PropertiesMely CarinoAinda não há avaliações
- MatterDocumento22 páginasMatterRon GruellaAinda não há avaliações
- Science - MatterDocumento32 páginasScience - MatterBbia Channelle BautistaAinda não há avaliações
- F I C H A #4-2021-Resuelto INGLESDocumento10 páginasF I C H A #4-2021-Resuelto INGLESGiane BonziAinda não há avaliações
- Why Can The Smell of Durians Be Detected Even Very Far Away ?Documento38 páginasWhy Can The Smell of Durians Be Detected Even Very Far Away ?Kavitha ThayagarajanAinda não há avaliações
- MATTER AND ITS PROPERTIESDocumento57 páginasMATTER AND ITS PROPERTIESArvin Corpuz DiamanteAinda não há avaliações
- 2 Classification of Matter PowerPointDocumento58 páginas2 Classification of Matter PowerPointPrasatha PattarasirinAinda não há avaliações
- CBSE Class 9 Science Chapter 1 Matter in Our Surroundings Revision NotesDocumento22 páginasCBSE Class 9 Science Chapter 1 Matter in Our Surroundings Revision NotesBriti DubeyAinda não há avaliações
- Solids, Liquids, and Gases: States of Matter Changes of State Gas BehaviorDocumento16 páginasSolids, Liquids, and Gases: States of Matter Changes of State Gas BehaviorYoAinda não há avaliações
- Separating Mixtures: Chemical ChangesDocumento1 páginaSeparating Mixtures: Chemical ChangesQuinie Rose OngcoyAinda não há avaliações
- SLIDE 1 - Sci 07 (Matter)Documento81 páginasSLIDE 1 - Sci 07 (Matter)Allen Joy LazoAinda não há avaliações
- What Is MatterDocumento20 páginasWhat Is MatterKarena WahimanAinda não há avaliações
- 2.1 StudentDocumento5 páginas2.1 StudentDariana FloresAinda não há avaliações
- States of MatterDocumento23 páginasStates of MatterAngel MulyadiAinda não há avaliações
- How Do You Group Objects?Documento29 páginasHow Do You Group Objects?Joy PaasAinda não há avaliações
- Lecture 1Documento16 páginasLecture 1wmemo42Ainda não há avaliações
- Matter in Oursurroundings-Ix Chemistry Chapter 1Documento9 páginasMatter in Oursurroundings-Ix Chemistry Chapter 1Hyder AliAinda não há avaliações
- 5 States of MatterDocumento21 páginas5 States of MatterUnknown ScoutAinda não há avaliações
- chem notesDocumento59 páginaschem notesKyi ThitsarAinda não há avaliações
- Grade 5 Properties and Structure of Matter Powerpoint AllDocumento60 páginasGrade 5 Properties and Structure of Matter Powerpoint Allapi-25442847475% (4)
- Igcse Chemistry Revision Final!!Documento126 páginasIgcse Chemistry Revision Final!!sohaila ibrahim100% (1)
- States of MatterDocumento40 páginasStates of Matterjocelyn.matigaAinda não há avaliações
- 3 States of Matter and Changes of StateDocumento21 páginas3 States of Matter and Changes of StateAlyssa Mae Dapadap1996Ainda não há avaliações
- Can You Find The Three States of MatterDocumento19 páginasCan You Find The Three States of Matterapi-205655271Ainda não há avaliações
- Matter PowerpointDocumento10 páginasMatter PowerpointTallia LewisAinda não há avaliações
- MatterDocumento48 páginasMatterReneAinda não há avaliações
- Matter 1220239046970201 8Documento83 páginasMatter 1220239046970201 8ManuelPauloAcogidoAinda não há avaliações
- BL Skill CH.4 Notes Page and True FalseDocumento12 páginasBL Skill CH.4 Notes Page and True Falselamouna.lamittaAinda não há avaliações
- States of Matter and Changes of StateDocumento19 páginasStates of Matter and Changes of StateShaniya RomeAinda não há avaliações
- 12 WaterDocumento18 páginas12 WaterROGELIO TORRESAinda não há avaliações
- Phases of MatterDocumento18 páginasPhases of MatterAivy YlananAinda não há avaliações
- Chapter 5 Loading BobDocumento2 páginasChapter 5 Loading BobYadana1Ainda não há avaliações
- Strength of Material PDFDocumento272 páginasStrength of Material PDFYadana1Ainda não há avaliações
- GPS - Global Positioning SystemDocumento12 páginasGPS - Global Positioning SystemYadana1Ainda não há avaliações
- Work and Energy TutorialDocumento5 páginasWork and Energy TutorialYadana1Ainda não há avaliações
- Bjectives: Chapter 1: Introduction To Control Systems ODocumento41 páginasBjectives: Chapter 1: Introduction To Control Systems OYadana1Ainda não há avaliações
- Aerospace Legislation 1Documento45 páginasAerospace Legislation 1Yadana1Ainda não há avaliações
- Impact Load - Strain EnergyDocumento8 páginasImpact Load - Strain EnergyYadana1Ainda não há avaliações
- Basic ChemistryDocumento11 páginasBasic ChemistryYadana1Ainda não há avaliações
- Wave and SoundDocumento25 páginasWave and SoundYadana1Ainda não há avaliações
- ColumnsDocumento5 páginasColumnsYadanaAinda não há avaliações
- Dynamics (Linear Impulse and Momentum)Documento4 páginasDynamics (Linear Impulse and Momentum)YadanaAinda não há avaliações
- Dynamics (Linear Kinematic)Documento4 páginasDynamics (Linear Kinematic)Yadana1Ainda não há avaliações
- Kinematics of Rigid Body - Absolute MotionDocumento5 páginasKinematics of Rigid Body - Absolute MotionYadana1Ainda não há avaliações
- What Makes Good SupervisorDocumento16 páginasWhat Makes Good SupervisorIndra bayuAinda não há avaliações
- PhysicsDocumento5 páginasPhysicsYadana1100% (1)
- Forces PDFDocumento35 páginasForces PDFYadana1Ainda não há avaliações
- Frequency Response and Control StabilityDocumento61 páginasFrequency Response and Control StabilityYadana1Ainda não há avaliações
- MixturesDocumento22 páginasMixturesYadana1Ainda não há avaliações
- ColumnsDocumento5 páginasColumnsYadanaAinda não há avaliações
- Atomic TheoryDocumento186 páginasAtomic TheoryYadana1Ainda não há avaliações
- EQM Rectangular Cordinetes PRB SolutionDocumento16 páginasEQM Rectangular Cordinetes PRB SolutionYadana1Ainda não há avaliações
- Aircraft Structures IIDocumento106 páginasAircraft Structures IIvodmox75% (4)
- Major Repair of Composite MaterialsDocumento9 páginasMajor Repair of Composite MaterialsYadana1Ainda não há avaliações
- 27 AnswersDocumento25 páginas27 AnswersYadana1Ainda não há avaliações
- Aircraft Structures 1Documento100 páginasAircraft Structures 1vodmox100% (2)
- AC Electrical System1Documento26 páginasAC Electrical System1Yadana1Ainda não há avaliações
- AMS 5 - 1 Landing Gear Components and MaintenanceDocumento63 páginasAMS 5 - 1 Landing Gear Components and MaintenanceYadana1100% (1)
- 12 Auto Pilot IntroDocumento13 páginas12 Auto Pilot IntroYadana1Ainda não há avaliações
- Iron RecyclingDocumento10 páginasIron RecyclingYousef SailiniAinda não há avaliações
- Practice Problem Sets on Voltaic CellsDocumento3 páginasPractice Problem Sets on Voltaic CellsChristian Angelo NECESITOAinda não há avaliações
- HUGPS209Documento13 páginasHUGPS209Aditya KavuluriAinda não há avaliações
- Proposal ChlorobenzeneDocumento12 páginasProposal ChlorobenzeneDavid Akomolafe100% (1)
- Laboratory Report Sheet 02 Properties of SolidsDocumento10 páginasLaboratory Report Sheet 02 Properties of SolidsCzarina RelleveAinda não há avaliações
- 6 1 UpDocumento102 páginas6 1 Upsoraandreea20Ainda não há avaliações
- Pour Point TestingDocumento10 páginasPour Point TestingAAKASHAinda não há avaliações
- Chemical GroutingDocumento27 páginasChemical GroutingDhananjay ShahAinda não há avaliações
- 3.1 Sample Collection, Preservation and Storage: 3.1.1 Collecting Water SamplesDocumento16 páginas3.1 Sample Collection, Preservation and Storage: 3.1.1 Collecting Water Sampleshandoyo_eko20017573Ainda não há avaliações
- MSE Admission and Degree RequirementsDocumento6 páginasMSE Admission and Degree Requirementsdeathbuddy_87Ainda não há avaliações
- Synthesis of Fluorescein Dye UsingDocumento6 páginasSynthesis of Fluorescein Dye UsingMoises BarraganAinda não há avaliações
- Applied Thermodynamics For Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, KharagpurDocumento19 páginasApplied Thermodynamics For Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, KharagpurTommyVercettiAinda não há avaliações
- Explosion Welding: A Solid-State Process for Joining Dissimilar MetalsDocumento23 páginasExplosion Welding: A Solid-State Process for Joining Dissimilar MetalsAnurag SinghAinda não há avaliações
- Proses ManufakturDocumento5 páginasProses ManufakturAchmad HabibieAinda não há avaliações
- The Group 1a and Group 2a Elements PDFDocumento36 páginasThe Group 1a and Group 2a Elements PDFEZ RioAinda não há avaliações
- Scale-Up Problems MoldDocumento3 páginasScale-Up Problems MoldKeehong KimAinda não há avaliações
- Hempel - Hempadur Multi-Strength 45753 (Cold Climate)Documento3 páginasHempel - Hempadur Multi-Strength 45753 (Cold Climate)CallumWoodwardAinda não há avaliações
- Environmental EngineeringDocumento5 páginasEnvironmental EngineeringOktrian SinathryaAinda não há avaliações
- Mop Strip 1217 MSDSDocumento2 páginasMop Strip 1217 MSDSSage Chemical InternationalAinda não há avaliações
- TCCDocumento48 páginasTCCMahin ShaAinda não há avaliações
- 10th Chemistry HB-1Documento4 páginas10th Chemistry HB-1Ahm MalikAinda não há avaliações
- Maneurop NTZ Low Temp - Refrigeration CompressorsDocumento32 páginasManeurop NTZ Low Temp - Refrigeration CompressorsMacSparesAinda não há avaliações
- Sand MouldingDocumento33 páginasSand MouldingSuman DeyAinda não há avaliações
- Practical Wet Test Acid Radical-1Documento5 páginasPractical Wet Test Acid Radical-1psyxs4tsv9Ainda não há avaliações
- Topical Tinctures: Submitted byDocumento17 páginasTopical Tinctures: Submitted byAhmed Imran100% (1)
- Carbohydrates NotesDocumento9 páginasCarbohydrates NotesAshley Saron100% (1)
- Price List Travo LasDocumento21 páginasPrice List Travo Laspei sajaAinda não há avaliações
- Restriction Mapping GuideDocumento4 páginasRestriction Mapping GuideWency Joy ObreroAinda não há avaliações
- ThesisDocumento242 páginasThesisali.umrani4538Ainda não há avaliações
- NFPA 10 - Selection of Fire Extinguishers PDFDocumento9 páginasNFPA 10 - Selection of Fire Extinguishers PDFHSE nest100% (1)