Escolar Documentos
Profissional Documentos
Cultura Documentos
Lecture 5a Catalysis
Enviado por
Sandeep ChawlaDescrição original:
Direitos autorais
Formatos disponíveis
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoDireitos autorais:
Formatos disponíveis
Lecture 5a Catalysis
Enviado por
Sandeep ChawlaDireitos autorais:
Formatos disponíveis
Enzyme Catalysis
Bill Royer
Office: LRB 921
Phone: x6-6912
I. Transition state theory
II. Mechanisms of catalysis
Acid-base catalysis - Ribonuclease A
Metal ion catalysis - Hammerhead Catalytic RNA
Covalent catalysis - Chymotrypsin
Enzymes have spectacular abilities to accelerate chemical reactions
often by factors of 10
6
-10
14
over non-catalyzed reactions. In this
lecture, we will briefly discuss some of the strategies used by enzymes
to achieve such remarkable rate increases.
I. Transition state theory
Consider the reaction A + B P + Q
where A + B react through transition state, X
, to form products P + Q. K
is the
equilibrium constant between A + B and X
and k' is the rate constant for
conversion of X
to P + Q.
A + B P + Q
X
K
k'
A + B
_
P + Q
AG
reaction
AG
G
Reaction coordinate
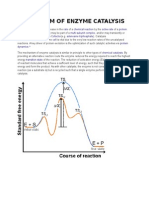
The minimum energy pathway of the reaction
is shown in the reaction coordinate, or
transition state diagram, at left. Chemical
conversion of A + B to P + Q proc eeds
through a transition state X
which is the
least stable (least probable, highest free
energy) spec ies along the pathway.
Molecules that ac hieve the activation energy,
AG
, can go on to react while molecules that
fail to achieve the transition state fall bac k to
the ground state.
The transition state, X
, is metastable. (Unlike a reaction intermediate, the transition
state has only a transient existence, like a pebble balanced on a pin. By definition, a
transition state cannot be isolated.) The transition state can be thought of as sharing
some features of the reactants and some features of the products. That is, some
bonds in the substrate are on their way to being broken and some bonds in the
product are partially formed.
The transition state, X, is in rapid equilibrium with reactants
with equilibrium constant K.
K
=
X
[A] [ B]
-RT lnK
= AG
AG, the activation energy, is the difference in Gibbs free energy between the transition
state, X, and the reactants. Since K is an equilibrium constant, the now familiar
equation applies:
where T is the absolute temperature in degrees Kelvin (C + 273) and R is the gas constant
(1.98 cal / mol / degree). In other words, the frequency with which reactants achieve the
transition state is inversely proportional to the activation energy barrier between the two.
The observed rate of the reaction, k
obs
, will be a
function of the concentration of the reactants, the rate
of conversion of X to P + Q, k', and will decrease
exponentially with an increase in AG.
k = k' e
obs
-AG
/ RT
[A][B]
Thus, the smaller the difference in free energy of the reactants and the transition state, the faster
the reaction proceeds. Enzymatic rate accelerations are achieved by lowering the activation
barrier between reactants and the transition state, thereby increasing the fraction of
reactants able to achieve the transition state. Enzymes reduce the activation barrier by
destabilizing the ground state of enzyme-bound substrates and products, by stabilizing the
transition state, and/or by introducing a new reaction pathway with a different transition state that
has a lower free energy.
A + B
_
P + Q
G
Reaction coordinate
AAG
cat
A+B P+Q
Uncatalyzed
Catalyzed
Enzymes accelerate reactions by lowering the
energy barrier between reactants and products.
AAG = AG - AG
Although less energy is required to form the
transition state in the catalyzed reaction, the
ground states of the free substrates and products
remain the same. The kinetic barrier is lowered
by the same extent for the forward and reverse
reactions. Consequently, a catalyst accelerates
the reaction without affe cting its equilibrium .
uncatalyzed catalyzed
If a catalyst lowers the activation barrier by AAG, the rate of the reaction is enhanced
by the factor e
AAG/RT
. Consequently, a ten-fold rate enhancement requires that AAG =
1.36 kcal/mole, less than the energy of a single hydrogen bond.
(AAG = RTln10 = 1.98 x 10
-3
kcal/mol*K x 298K*ln(10) = 1.36 kcal/mol)
(Nelson & Cox, Lehninger Principles of
Biochemistry, 3rd ed., 2000)
Imaginary enzyme ("stickase") designed to catalyze "cleavage" (breaking) of a metal stick
A
P
G
Reaction coordinate
A
I
k
1
A P k
2
I
k
1
k
2
<
k
1
k
2
>
If the formation of I, an intermediate, from A is
slower than the formation of P from I (k < k )
the activation barrier for the first step must be
higher than the activation barrier for the sec ond
step (thick line). If k is much slower than k ,
conversion of A to I is the rate-determining step
for the reaction. That is, the overall reaction
proceeds at a rate that can be no faster than k .
Conversely, if formation of P from I is much
slower than formation of I from A (k < k ), the
ac tivation barrier for the sec ond step is higher
(thin line) and formation of P from I is
rate-determining.
1 2
2 1
1 2
1
A
k
1
k
2
I P
For a reaction that involves several steps, each step will have a corresponding
transition state.
k
1
< k
2
k
1
> k
2
II. Mechanisms of catalysis
A. Acid-base catalysis
Specific acid or base catalysis - Reaction rate is directly proportional to [H+] or [OH-].
Example: Alkaline hydrolysis of RNA
General acid or base catalysis - Reaction rate is proportional to [Bronsted acid] or
[Bronsted base]
Bronsted acid - species that can donate protons
Bronsted base - species that can combine with a proton
[Imidazole buf fer]
R
a
t
e
[Imidazole buf fer]
R
a
t
e
Specific Base Catalysis General Base Catalysis
pH 7.3
pH 7.0
pH 7.3
pH 7.0
H C
NH
COO
CH C
O
O
-
2
-
+
3
Aspartic acid
Amino Acid pK
a
3.90
|-COOH
H C
NH
COO
CH C
O
O
-
2
-
+
3
Glutamic acid 4.07
-COOH
CH
2
H C
NH
COO
CH
2
-
+
3
Histidine 6.04
imidazole
N
N
H C
NH
COO
CH SH
2
-
+
3
Cysteine 8.33
sulf hydryl
H C
NH
COO
CH
2
-
+
3
Tyrosine 10.13
phenol
OH
H C
NH
COO
CH CH
2
-
+
3
Lysine 10.79
c-amino
CH
2 2
NH
3
+
Amino acids side chains with pKa's in the neutral pH range can
function as Bronsted acids/bases
Biologically important
nucleophilic groups:
Hydroxyl group R-OH R-O: + H
+
-
Sulfhydryl group
R-SH
Adapted from
Voet & Voet,
Biochemistry
R-S:
-
+ H
+
R-NH
3
+
R-NH
2
+ H
+
Amino group
HN NH
+
R
HN N:
R
+ H
+
Imidazole group
Nucleophilic
form
R-NH
2
+ C=O
Biologically important
electrophiles:
C=O
H
+
M
n+
Protons Metal Ions
Carbonyl carbon
O
OHOH
Base
O
O O
O 5'...
P
Pyri mi di ne
O
HO
Base
3'...
O O
-
2',3'-Cyclic phosphate
O
O OH
P O O
O
Pyri mi di ne
O
5'...
H
H O
2
O
O OH
P O O
O
Pyri mi di ne
O
5'...
3' phosphate
O OH
Ribonuclease A
An example of concerted acid-base catalysis - reaction subject to both
general acid and general base catalysis
RNase A (124 residues, mw 13.7 kd) is a digestive enzyme secreted by the pancreas
that catalyzes hydrolysis of phosphodiester backbone of RNA. In first step of the
reaction, cleavage of the bond between phosphorous and the 5' oxygen generates one
2',3'-cyclic phosphate terminus and one 5'-OH. In the second step, water reacts with
the cyclic phosphate to yield a 3' phosphate. The 2',3' cyclic phosphate can be
isolated because it forms more rapidly than it hydrolyzes.
O
O
O
O 5'...
A
P
O
O
O
O OH
Base
3'...
O
O
O O
O 5'...
P O O
O
Pyri mi di ne
O
O OH
Base
3'...
H
Base abstracts
proton f rom 2' OH
NH HN
+
Hi s 119
Acid protonates
5' leaving group
NH :N
+
Hi s 12
Charge
stabilization
Trigonal bipyramidal transition state
O
O O
O
5'...
P
Pyri mi di ne
O
O OH
HO
Base
3'...
O O
-
Intermediate
H N
3
Lys 41
+
Transesterification
Nucleophilic attack
of 2' O on phosphate
First Step: 23 cyclic nucleotide produced. His 12 is general base, His 119 is general acid
Hydrolysis of 2',3' cyclic phosphate intermediate
O
O O
O 5'...
P
Pyri mi di ne
O O
-
H
O
H
NH :N
+
Hi s 119
Base abstracts
proton f rom H O
2
Nucleophilic attack of
H O on phosphate
2
NH HN
+
Hi s 12
Acid protonates
2' OH leaving group
O
O OH
P O O
O
Pyri mi di ne
O 5'...
3' phosphate
Second Step:
His 12 is general acid, His 119 is general base
Geometry of the pentacovalent transition state. The central
phosphorus atom is transiently bonded to 5 oxygen atoms. Three
oxygens are coplanar with the phosphorus. The oxygen atoms of the
leaving group is at one apex, and the oxygen atom of the attacking group
is at the other apex of the trigonal bipyramid (in-line attack).
Proposed mechanism of RNase A catalysis. The unionized form of His 12 accepts a
proton from the 2' OH which enhances its nucleophilicity. The protonated form of His 119
begins to donate its proton to the 5' O, and the 2'O begins to form a bond with P to form a
pentacoordinate transition state. The negative charge that develops is stabilized
electrostatically by the nearby positively charged side chain of lysine 41. The bond between
P and the 5'-O breaks when the proton from histidine 119 is completely transferred. At the
same time, a bond between P and the 2'-O becomes fully formed, producing the 2',3'-cyclic
intermediate. Hydrolysis of the cyclic intermediate is a reversal of the first stage with H
2
O
replacing the 5'-O component that was removed. Histidine 12 is now the proton donor and
histidine 119 is the proton acceptor.
Evidence for RNase A mechanism
pH dependence of Vmax/KM for RNase A catalyzed
hydrolysis of cytidine-2',3'-cyclic phosphate. Bell shaped
curve suggests a catalytic role for functional groups with
pK's of 5.4 and 6.4, consistent with histidines.
Crystal structure of RNase A complex with cytidine 2'3'-
cyclic phosphate intermediate. Shows histidines and lysine
appropriately positioned in the active site. Note hydrogen
bonding interactions between cytosine and threonine 45
that confer substrate specificity.
Chemical modification. Iodoacetate alkylates histidine 119 or histidine 12 but not both in the
same molecule. Alkylation of either histidine eliminates catalysis. Complex formation with
substrate or competitive inhibitors protects histidines from modification.
H O
2
2+
Mg
2+
Mg OH
-
+ H
+
pK
a
= 11.4
B. Metal ion catalysis
1. Water ionization. A metal ion's charge makes its bound water molecules more acidic than
free H
2
O and therefore a source of OH- ions even below neutral pH (Metal ions have been
called "Super acids").
2. Charge shielding - metal ions can have charge > +1.
3. Oxidation-Reduction
G
G
A
C
A
UCCUG
GGCC
CCGG
G
A
A
A
G
U
A
G
U
C
A
U
U
G
GG5'
5'
Ribozyme
C
C
U
G
U
C
AGGAU
Substrate
cleavage site
Hammerhead Catalytic RNA
The Hammerhead Catalytic RNA
The hammerhead ribozyme, like RNase A,
catalyzes a transesterification reaction to cleave
the phosphodiester backbone of substrate
RNAs yielding products with 5' hydroxyl and
2'3'cyclic phosphate termini. Unlike the RNase
A-catalyzed reaction, the hammerhead reaction
does not proceed through hydrolysis of the 2',3'
cyclic phosphate.
The hammerhead ribozyme obviously has no amino acid side chains to carry out proton
transfer and charge-shielding functions. RNAs are, however, capable of binding metal ions
with high specificity and affinity and the hammerhead ribozyme appears to make use of
metal ions to carry out both charge shielding and proton transfer functions.
C. Covalent catalysis - Transient formation of a catalyst-substrate covalent bond
-Provides an alternative reaction pathway, with two lower energy transition states
1. A nucleophile (electron-rich group with a strong tendency to donate electrons to an
electron-deficient nucleus) on the enzyme displaces a leaving group on the substrate,
forming a covalent bond.
2. The enzyme substrate bond decomposes to form product and free enzyme.
-Covalent catalyst must be a good nucleophile and a good leaving group - highly mobile
electrons (imidazole of His, thiol of Cys, carboxyl of Asp, hydroxyl of Ser).
Chymotrypsin, 25 kd serine protease, catalyzes hydrolysis of proteins in the small
intestine. Chymotrypsin catalyzes hydrolysis of esters as well as peptide bonds which has
been useful for analysis of the catalytic mechanism, although not physiologically relevant.
[
p
-
N
i
t
r
o
p
h
e
n
y
l
a
t
e
]
,
M
Time (min)
10
20
30
2 4 6 8 10 12
chymotrypsin
32M
24M
16M
8M
The plot at left shows the concentration of
p-nitrophenol produced as a function of time in
reactions containing different concentrations
of chymotrypsin and a large excess of
p-nirophenylacetate. An initial rapid phase
("burst") is followed by a slower phase. The
size of the initial burst is proportional to the
enzyme concentration. "Burst" kinetic s
provide evidence for a stable, enzyme-linked
intermediate.
C CH
3
NO
O
2
+ Chymotrypsin
p-Nitrophenylacetate
fast
O NO
2
-
p-Nitrophenylate
+
C CH
3
O
chymotrypsin
Acyl-enzyme intermediate
slow
H O
2
H
+
C CH
3
O
-
O
Acetate
+
chymotrypsin
Model reaction in which hydrolysis of acyl-enzyme intermediate is slow
Formation of the acyl-enzyme intermediate
occurs during the initial rapid phase and
slower hydrolysis (deacylation) of the acyl-
enzyme intermediate occurs during the
second, slower phase.
o
First stage in peptide bond hydrolysis: acylation. Hydrolysis of the peptide bond starts with an
attack by the oxygen atom of the Ser195 hydroxyl group on the carbonyl carbon atom of the
susceptible bond. The carbon-oxygen bond of this carbonyl group becomes a single bond, and
the oxygen atom acquires a net negative charge. The four atoms now bonded to the carbonyl
carbon are arranged as a tetrahedron. Transfer of a proton from Ser195 to His57 is facilitated
by Asp102 which (i) precisely orients the imidazole ring of His57 and (ii) partly neutralizes the
positive charge that develops on His57 during the transition state. The proton held by the
protonated form of His57 is then donated to the nitrogen atom of the peptide bond that is
cleaved. At this stage, the amine component is hydrogen bonded to His57, and the acid
component of the substrate is esterified to Ser195. The amine component diffuses away.
Oxyanion
hole
Second stage in peptide hydrolysis: deacylation. The acyl-enzyme intermediate
is hydrolyzed by water. Deacylation is essentially the reverse of acylation with
water playing the role as the attacking nucleophile, similar to Ser195 in the first
step. First, a proton is drawn away from water. The resulting OH- attacks the
carbonyl carbon of the acyl group that is attached to Ser195. As in acylation, a
transient tetrahedral intermediate is formed. His57 then donates a proton to the
oxygen atom of Ser195, which then releases the acid component of the substrate,
completing the reaction.
Oxyanion
hole
Chymotrypsin catalytic triad Ser195/His57/Asp102 located at the active site
by x-ray crystallography.
An important stabilizing feature of the interaction between enzymes and their
substrates, is transition state binding. In fact, most enzyme active sites are
organized such that binding to the transition state is preferred over binding to
either substrates or products. The active site of chymotrypsin is arranged to
stably interact with the negatively charged carbonyl oxygen of the tetrahedral
intermediate this part of the active site is referred to as the oxyanion hole.
Mechanism of Protein Splicing:
The protein splicing pathway
consists of four nucleophilic
displacements. X represents the S or
O atom of the Cys/Ser/Thr
sidechains.
From: Perler, FB (1998) Cell 92, 1-4
Você também pode gostar
- Mechanism of Enzyme CatalysisDocumento18 páginasMechanism of Enzyme CatalysisBuhlebenkosi Jackie BhebheAinda não há avaliações
- Chemical Energetics Notes EditedDocumento12 páginasChemical Energetics Notes EditedDaniel Png100% (1)
- Coursesaver (Chad) College Physics OutlinesDocumento40 páginasCoursesaver (Chad) College Physics Outlinescalong558Ainda não há avaliações
- CSIM2.24 - Signal TransductionDocumento6 páginasCSIM2.24 - Signal TransductionAinahMahaniAinda não há avaliações
- Hydrogenation of Ethylene On Cu CatalystDocumento7 páginasHydrogenation of Ethylene On Cu CatalystHillman WiraAinda não há avaliações
- Mechanism of Enzyme Catalysis MadhuDocumento9 páginasMechanism of Enzyme Catalysis MadhumengelhuAinda não há avaliações
- Catalytic Kinetics PDFDocumento47 páginasCatalytic Kinetics PDFCorby TranAinda não há avaliações
- 2 Bioenergetics and Oxidative Metabolism IDocumento3 páginas2 Bioenergetics and Oxidative Metabolism ILinus LiuAinda não há avaliações
- Acids and BasesDocumento26 páginasAcids and BasesGaayathiriAinda não há avaliações
- Hydrogen Bonds & Solubility in WaterDocumento8 páginasHydrogen Bonds & Solubility in WaternaifAinda não há avaliações
- CREII-Module-I - Lecture 4 PDFDocumento34 páginasCREII-Module-I - Lecture 4 PDFshubhamAinda não há avaliações
- Structure and Function of Bio-Molecules: 9 2. Proteins 13Documento62 páginasStructure and Function of Bio-Molecules: 9 2. Proteins 13Alex-Mihai CiubaraAinda não há avaliações
- Cleavable LinkersDocumento12 páginasCleavable LinkersSrinivasa Reddy Telukutla100% (1)
- 22 CH106 Metabolic Paths For Carbohydrates Timberlake 2ndDocumento70 páginas22 CH106 Metabolic Paths For Carbohydrates Timberlake 2ndEnrique LiKeAinda não há avaliações
- 1.1 Enzymology (Bravo)Documento11 páginas1.1 Enzymology (Bravo)Arman Carl DulayAinda não há avaliações
- 1 Chemical Kinetics and EquilibriumDocumento77 páginas1 Chemical Kinetics and EquilibriumApril Mergelle Lapuz100% (1)
- Why Chemical Kinetics MatterDocumento90 páginasWhy Chemical Kinetics MatterVikas MishraAinda não há avaliações
- Catalysis and Catalytic Reactions: A. Sarath BabuDocumento77 páginasCatalysis and Catalytic Reactions: A. Sarath Babuazmigalaxy8955Ainda não há avaliações
- Thermo II Exam II Cheat SheetDocumento1 páginaThermo II Exam II Cheat SheetbengtglaveAinda não há avaliações
- Chen 2007Documento9 páginasChen 2007Arisya JulvianaAinda não há avaliações
- Unit 5 ChemicChemical Kinetics and Equilibriumal Kinetics and Equilibrium Notes (Answers)Documento22 páginasUnit 5 ChemicChemical Kinetics and Equilibriumal Kinetics and Equilibrium Notes (Answers)Muhammad IrfanAinda não há avaliações
- Catalysis and Catalytic ReactorsDocumento59 páginasCatalysis and Catalytic ReactorssyedmuhammadtariqueAinda não há avaliações
- Fluid motion above a hot surface results from changes in density and buoyancyDocumento2 páginasFluid motion above a hot surface results from changes in density and buoyancytorance44Ainda não há avaliações
- Theories of Reaction RatesDocumento28 páginasTheories of Reaction RatesMay AlmogAinda não há avaliações
- Michaelis Menten EquationDocumento9 páginasMichaelis Menten Equationsadaf zaidiAinda não há avaliações
- Carbohydrates Aka Saccharides NumberDocumento2 páginasCarbohydrates Aka Saccharides NumberAngelica Llamas0% (1)
- CRE Notes PDFDocumento61 páginasCRE Notes PDFKrunal ThakarAinda não há avaliações
- Intro 2 MD SimulationDocumento20 páginasIntro 2 MD SimulationachsanuddinAinda não há avaliações
- Chem 40 Enzyme KineticsDocumento85 páginasChem 40 Enzyme KineticsJustine Grace Mariano100% (1)
- Unit 5 Chemical KineticsDocumento37 páginasUnit 5 Chemical KineticsSanjay SharmaAinda não há avaliações
- ChE426 Final Exam 2005Documento2 páginasChE426 Final Exam 2005احمد الدلالAinda não há avaliações
- Electrochemistry Lect Notes CambridgeDocumento4 páginasElectrochemistry Lect Notes Cambridgeavatar_75Ainda não há avaliações
- Principles of Catalysis: Part 1c: DiffusionDocumento29 páginasPrinciples of Catalysis: Part 1c: DiffusionSyahminazuhan PipimontelAinda não há avaliações
- 12 Chemistry Notes Ch04 Chemical KineticsDocumento4 páginas12 Chemistry Notes Ch04 Chemical KineticssrideviAinda não há avaliações
- Hidratación de Oxido de Etileno Cinética PDFDocumento5 páginasHidratación de Oxido de Etileno Cinética PDFangieAinda não há avaliações
- SN1 Vs SN2 ReactionsDocumento23 páginasSN1 Vs SN2 Reactionssamnas100Ainda não há avaliações
- Ch18 Lecture 6e FinalDocumento89 páginasCh18 Lecture 6e FinalSindi Yohana SitohangAinda não há avaliações
- 04 - Catalyst PreparationDocumento52 páginas04 - Catalyst PreparationArdhito SetiawanAinda não há avaliações
- Chapter 6. ThermodynamicsDocumento7 páginasChapter 6. Thermodynamicshoney1002Ainda não há avaliações
- Equilibrium Cheat Sheet InhouseDocumento2 páginasEquilibrium Cheat Sheet InhouseShirleyLinAinda não há avaliações
- HW1 Solns KineticsDocumento10 páginasHW1 Solns Kineticsapb91781Ainda não há avaliações
- Orgo Cheat Sheets 08 2019 PDFDocumento34 páginasOrgo Cheat Sheets 08 2019 PDFKobe AcobAinda não há avaliações
- Acids Bases and PHDocumento4 páginasAcids Bases and PHsatheeshAinda não há avaliações
- Enzymes: Biological Catalysts That Speed Up ReactionsDocumento9 páginasEnzymes: Biological Catalysts That Speed Up Reactionsibrahim khalilAinda não há avaliações
- Alcohols & Phenols:: GeneralizationsDocumento27 páginasAlcohols & Phenols:: GeneralizationsdoudoudoudouAinda não há avaliações
- Lecture Notes Enzyme 2 Enzyme Kinetics WebDocumento29 páginasLecture Notes Enzyme 2 Enzyme Kinetics WebAldren RebaLdeAinda não há avaliações
- Cre First Chapter Octave LevenspeilDocumento59 páginasCre First Chapter Octave LevenspeilManish PatilAinda não há avaliações
- Kinetics of Homogeneous Reactions Simple Reactor TypesDocumento9 páginasKinetics of Homogeneous Reactions Simple Reactor TypesNikai Hermawan AmrullahAinda não há avaliações
- Structure and Properties of Crystalline Solids and PolymersDocumento2 páginasStructure and Properties of Crystalline Solids and PolymersBabette FreyAinda não há avaliações
- Advanced and Modern MaterialsDocumento10 páginasAdvanced and Modern MaterialsSachin RaneAinda não há avaliações
- Catalysts: Role and Function of The CatalystDocumento20 páginasCatalysts: Role and Function of The Catalystyussra amerAinda não há avaliações
- Applications of Spectroscopic TechniquesDocumento20 páginasApplications of Spectroscopic Techniquesamanbioq1Ainda não há avaliações
- Kineticsss Notes PDFDocumento73 páginasKineticsss Notes PDFArun SharmaAinda não há avaliações
- Rate and Mechanism of Chemical ReactionsDocumento25 páginasRate and Mechanism of Chemical ReactionsShaurya100% (1)
- Collision TheoryDocumento10 páginasCollision TheoryAnonymous pgjIAZoAinda não há avaliações
- Physical Pharmacy q1 MidtermDocumento78 páginasPhysical Pharmacy q1 MidtermCatherine RiaAinda não há avaliações
- Bich411 Enzyme Catalysis PDFDocumento7 páginasBich411 Enzyme Catalysis PDFAn TranAinda não há avaliações
- Chemistry 206 Advanced Organic Chemistry: Olefin Addition Reactions: Part-2Documento17 páginasChemistry 206 Advanced Organic Chemistry: Olefin Addition Reactions: Part-2eraborAinda não há avaliações
- Properties and Reactions of Haloalkanes: Bimolecular Nucleophilic SubstitutionDocumento48 páginasProperties and Reactions of Haloalkanes: Bimolecular Nucleophilic SubstitutionKunjal100% (1)
- 9930 PTC by Using Phosphonium CompoundsDocumento9 páginas9930 PTC by Using Phosphonium Compoundsabrar hassanAinda não há avaliações
- Science8 Q2 Module3 (Week6)Documento30 páginasScience8 Q2 Module3 (Week6)Mary Grace Lemon100% (1)
- Fiziks: Basic Properties and Tools of ThermodynamicsDocumento28 páginasFiziks: Basic Properties and Tools of ThermodynamicsSURAJ PRATAP SINGHAinda não há avaliações
- CH 3Documento19 páginasCH 3Abhishek GiriAinda não há avaliações
- Kill Sheet CalculationsDocumento16 páginasKill Sheet CalculationsYash SinghAinda não há avaliações
- Richard A. Nyquist and Ronald O. Kagel (Auth.) - Handbook of Infrared and Raman Spectra of Inorganic Compounds and Organic Salts. Infrared Spectra of Inorganic Compounds-Academic Press (1971)Documento499 páginasRichard A. Nyquist and Ronald O. Kagel (Auth.) - Handbook of Infrared and Raman Spectra of Inorganic Compounds and Organic Salts. Infrared Spectra of Inorganic Compounds-Academic Press (1971)Patrícia Bodanese PratesAinda não há avaliações
- Methodology of Event StudiesDocumento4 páginasMethodology of Event Studieshaichellam5577Ainda não há avaliações
- Gas-Insulated Switchgear: Type 8DN8 Up To 170 KV, 63 Ka, 4000 ADocumento33 páginasGas-Insulated Switchgear: Type 8DN8 Up To 170 KV, 63 Ka, 4000 APélagie DAH SERETENONAinda não há avaliações
- Applications of Redox ReactionsDocumento50 páginasApplications of Redox ReactionsMlamuli MlarhAinda não há avaliações
- Unit 6 - Quantitative Analysis NotesDocumento53 páginasUnit 6 - Quantitative Analysis Notesapi-182809945Ainda não há avaliações
- Clone Steps RmanDocumento10 páginasClone Steps RmanKishore AdikarAinda não há avaliações
- State Standards: Common CoreDocumento24 páginasState Standards: Common CoreEddy R. VélezAinda não há avaliações
- QPCR Analysis DifferentlyDocumento12 páginasQPCR Analysis DifferentlyIan SaundersAinda não há avaliações
- Matrix Structural Analysis of BeamsDocumento28 páginasMatrix Structural Analysis of BeamsKristine May Maturan0% (1)
- Lubricants For Cement ProductionDocumento21 páginasLubricants For Cement Productiongrameshkreddy2013100% (1)
- Properties of Common Liquids Solids and Foods 2Documento2 páginasProperties of Common Liquids Solids and Foods 2Šhëënà de LeonAinda não há avaliações
- Expanding Wired Connectivity For SOHO Networks: Plus Gigabit Ethernet SwitchesDocumento4 páginasExpanding Wired Connectivity For SOHO Networks: Plus Gigabit Ethernet SwitchesAndré LinharesAinda não há avaliações
- MC0081Documento385 páginasMC0081Purushottam KumarAinda não há avaliações
- Welding Machine CatalogueDocumento12 páginasWelding Machine CatalogueRodney LanagAinda não há avaliações
- 03 Correcao Exercicios FixacaoDocumento3 páginas03 Correcao Exercicios FixacaoRodrigoAinda não há avaliações
- JACOB ThirdDocumento16 páginasJACOB ThirdWendell ReyesAinda não há avaliações
- Seminar ReportDocumento45 páginasSeminar Reportmanaskollam0% (1)
- Python Notes - 1Documento364 páginasPython Notes - 1hopefulantonelliAinda não há avaliações
- LsApi PDFDocumento347 páginasLsApi PDFEduardo Martin Vega100% (2)
- Design of Three Span Steel Composite FlyoverDocumento85 páginasDesign of Three Span Steel Composite FlyoverStructural SpreadsheetsAinda não há avaliações
- Influence of Ring-Stiffeners On Buckling Behavior of Pipelines UnderDocumento16 páginasInfluence of Ring-Stiffeners On Buckling Behavior of Pipelines UnderSUBHASHAinda não há avaliações
- BMW M5 ConfigurationDocumento12 páginasBMW M5 ConfigurationprasadAinda não há avaliações
- Solutions For Statistics and Probability For EngineersDocumento6 páginasSolutions For Statistics and Probability For EngineersPHAinda não há avaliações
- Mste 3.0 Plane Geometry Hand OutsDocumento8 páginasMste 3.0 Plane Geometry Hand OutsJasmine MartinezAinda não há avaliações
- List of Practical Cs With SolutionDocumento57 páginasList of Practical Cs With SolutionArjun KalaAinda não há avaliações
- Wi Cswip 3.1 Part 13Documento7 páginasWi Cswip 3.1 Part 13Ramakrishnan AmbiSubbiahAinda não há avaliações