Escolar Documentos
Profissional Documentos
Cultura Documentos
Trabalho Teste 1 Termo 2
Enviado por
DeysianeMartins0 notas0% acharam este documento útil (0 voto)
6 visualizações8 páginasTrabalho teste 1 termo 2
Título original
Trabalho teste 1 termo 2
Direitos autorais
© © All Rights Reserved
Formatos disponíveis
PDF ou leia online no Scribd
Compartilhar este documento
Compartilhar ou incorporar documento
Você considera este documento útil?
Este conteúdo é inapropriado?
Denunciar este documentoTrabalho teste 1 termo 2
Direitos autorais:
© All Rights Reserved
Formatos disponíveis
Baixe no formato PDF ou leia online no Scribd
0 notas0% acharam este documento útil (0 voto)
6 visualizações8 páginasTrabalho Teste 1 Termo 2
Enviado por
DeysianeMartinsTrabalho teste 1 termo 2
Direitos autorais:
© All Rights Reserved
Formatos disponíveis
Baixe no formato PDF ou leia online no Scribd
Você está na página 1de 8
PRESSURE-VOLUME-TEMPERATURE
{ELATIONSHIPS OF PURE GASES AND LIGUIDS
PVT RELATIONSINDS OF PURE GASES AND LI0UIDS 435
‘Tyn and Calus Method. With Eq, (4-10.2),
V, = 0.285 Vi = 0.285(209)¢* = 77.0 em* mol”
Enror = (17 ~ 77.6)/77.6 = ~ 0.008 or ~ 0.8%
Resulls for 35 substances with the method of Tyn and Calus ate shown in the fifth
column of Table 4-11. The average deviation for the three methods of this section
is 3 to 4%. Examination of Table 4-11 shows that theze is no pattern of error in
the Schroeder and Le Bas methods that would suggest either is to be preferred but
in any case, the Tyn and Calus method is more reliable than both, The simplicity,
Of the methods makes them altractive but none of them are as accurate as those
described in the next section (the last three columns of Table 4-11).
4-11 SATURATED LIQUID DENSITIES AS A
FUNCTION OF TEMPERATURE
‘A number of techniques are available to estimate pure saturated-liquid molar or
spevific volumes or densities as a function of temperature. Here, one group contri-
bbution technique and several corresponding states methods ate presented to estimate
saturated-liquid densities,
Rackett Equation
Rackett (1970) proposed that saturated liquid volumes be calculated by
V,= v.zggnrn @14)
where V, = saturated liquid volume, V, = critical volume, Z, = critical compress-
ibility factor, 7. ~ critical temperature. Eq. (4-11.1) is often written in the equiv-
alent form
RE. gu sary) '
aay a2)
While Eq. (4-11.1) is remarkably accurate for many substances, it underpredicts V,
when Z, = 0.22
Yamada and Gunn (1973) proposed that Z, in Bq, (4-11.1) be correlated with,
the acentrie factor:
y,
V.(0.29056 ~ 0.08775«)"-72"” 13)
If one experimental density, VF, is available at a reference temperature, T*, Eqs
(£1111) and (411.3) can be modified to give
V, = VH(0.29056 ~ 0.087750)¢ (4-114a)
V, = vize (4-11.40)
where
Downloaded trom Digtal Engineering Library @ MeGraw-til (oy digtalengineeringlbrary. com)
‘Copyright © 2004 The MoGraw-Hil Companies. al rights reserved
‘Any use is subject tothe Terms of Use as given at fhe webste
{ELATIONSHIPS OF PURE GASES AND LIGUIDS
&
2
3
g
vt or sie oo rs saeqstmomoyrpozoorc,
To To 60> 6 at
Lo z9 ve Lo Ly
* 80
ze vt st
vo sz me sie s si
To so eT 90 rz zr 298 TA
To st 00. vo- ro yeu 16274
et or Zo- ve pe
at ow Sse ve sr
vom TO 80> Ly 80
vom re er e- 6o- fra
To- ot Le ie ree ro-
vz Ts- er oI 50- fe
st re re Pee a
Lom so
vo vo rr 60 re 50
Lom oz atiagoeoe
so oo esawoqononty
zo ot 90> zo et eno
#0 rs Tr ¥o- s0- sein
Fo- re zr st sensor)
90- vs ror re suesapeis0-u
r0- 10 bo- a Suma,
zo ei ee L sedond
o> bie fs so 7
ery) OM sa wea HONS ug
okt
Te poueu &q pawmays oy OND HORA
amends, Smo4 aM
(OW PINbYT Jo suonwamne Jo eUORGOD Lip 3TOVL
436
Downloaded trom Digtal Engineering Library @ MeGraw-til (ony digtalengineeringlbrary. com)
‘Copyright © 2004 The MoGraw-Hil Companies. al rights reserved
‘Any use is subject tothe Terms of Use as given at fhe webste
3
3
6
5
ATU
PRESSURE-VOLUME-TEMI
ba uost) target 3
Pam.
¥0
so
ee
oe
oo
ro
oz
ow
xe
el
sb
v0
ve
ou
s0-
oss
ser
90¢
sar
rsP
ose
sou
os
est
ay
4
‘sprorp ms
spun w320mp S17
EA
sxn0p)
v
suodoud
puns orc,
vwedearses St
437
Downloaded trom Dighal Engineering Library @ Mera
w-ll yaw digtalengineeringlibrry.com)
‘Copyright © 2004 The MoGraw-Hil Companies. al rights reserved
‘Any use is subject tothe Terms of Use as given at fhe webste
PRESSURE-VOLUME-TEMPERATURE
{ELATIONSHIPS OF PURE GASES AND LIGUIDS
a3 CHAPTER FOUR
oa 0 TIT ~~ TT Gus)
Eq, (4-11.4a) is obtained ftom Eq. (4-11.3) by using the known reference volume
to eliminate V.. The same approach is used to obtain Eq. (4-11.40) from Eq. (4
11.1), It is also possible to eliminate Z, from Eq. (4-11.1), but then V. appears in
the final equation and itis generally known less accurately than the quantities that
appear in Eq, (4-11.4),
‘An often-used variation of Eq. (4-113) is
v,= Zoro -aosrsuyre-rm) tty
However, this form does not predict V, correctly unless the actual Z, ~ 0.29056 —
0.08775«, in which case it is identical to Eg. (411.2)
Equation (4-111) has been used as the starting point to develop a vasiety of
‘equations for corselating liquid densities. For example, Spencer and Danner (1972)
replaced Z, with an adjustable parameter, Zp, values of which are tabulated in
‘Spencer and Danner (1972), Spencer and Adler (1978), and the 4 Edition of this
book. Daubert, et al. (1997) changed the physical quantities and constants of Eq.
(4-111) into four adjustable parameters to give
V, = Beanery A aun
‘Values of the four constants A through D, are tabulated in Daubert, et al. (1997)
for approximately 1200 compounds. The value of C is generally equal to 7. while
A, B, and D are generally close to the values used in Eq. (4-11:3),
Of all the forms of the Rackett Equation shown above and including versions
of the 4th Edition, we recommend Bq, (4-1 4a). This form uses a known reference
value, VE, does not requite V_ of P., and is more accurate when Z, is low. Errors
associated with the assumption that a correlation in w applies to all substances is
mitigated by use of the reference value.
‘When various forms of the Rackett equation based on critical properties were
used to predict the liquid volumes tabulated in Appendix A, Eq. (4-11.3) performed
Detter than did either Fg. (4-11.1) or (4-11.6). Results of these calculations are
shown in Table 4-12. For w < 0.4, Eq. (4-11-3) gave an average deviation of 2.6%
for 225 substances. For w > 0.4, the average deviation was 6.1% for 65 substances,
lis likely that this conclusion would be valid al other conditions as well because
‘comparisons of reduced volumes at other reduced temperatures and acentzic Factors
all gave essentially the same results,
Another liquid volume correlation was proposed by Hankinson and Thomson,
(4979) and further developed in Thomson, et al. (1982). This correlation, herein
referred to as the HBT correlation, is
TABLE 412 Average Absolute Percent Deviations fr Predictions of Liquid Molae
Volumes Tabulated im Appendix A.
BILD EG HT
225 substances, © = 04 408) 256
05 substances, w > O4 17 506
Equation (118) 1 G11 10) wih ogg = w ane VO
Downloaded trom Digtal Engineering Library @ MeGraw-til (oy digtalengineeringlbrary. com)
‘Copyright © 2004 The MoGraw-Hil Companies. al rights reserved
‘Any use is subject tothe Terms of Use as given at fhe webste
PRESSURE-VOLUME-TEMPERATURE RELATIONSHIPS OF PURE GASES AND LIQUIDS
PVT RELATIONSINDS OF PURE GASES AND LI0UIDS 430
V, = VEVOLL — ery V%] arg)
VO = 1+ a0 - 7) + ba - TPP + ol 1) +a - 7) 4119)
4-11.10)
Equation (4-11.9) may be used in the range 0.25 < T, < 0.95 and Ey. (4-11.10)
may be used when 0.25 < T, < 1.0. Constants a through h are given by
a -152816 bL.43907
© =OsIHe = d—z90854
@ 0296173 f 0.386014
f 00127258 h — -0.0880645
In Eqs. (4-118) to (411.10), ogy is that value of the acenui factor that causes
the Soave equation of state to give the best fit to pore component vapor pressures,
and V* is a parameter whose value is close tothe critical volume. Values of day
and V* are labulated for a number of compounds in Hankinson and Thomson
(1979) and in the 4© Edition of this book. We have found that wzjq and V* can
be replaced with w and V. with litle loss in accuracy. Thus, we have used « and
V, for the comparisons shown in Table 4-12.
‘The dependence on temperature and acentic factor expressed by the HBT cor
relation is nearly identical to that described by Bq, (4-113). In fact for 7, < 0.96
and a < 04, the diference in these two sets of equations is always less than 1%
when V.- V* and «— tggy- Thus, can bo expected that any improversent seen
in the HBT contlation by using the empirical parameters wy and V* could be
reproduced with the same values used in place of w and V. in Eq, (4-11.3), The
errors shown in Table 4-12 suggest that the HBT method is marginally better than
Eg. (-11.3) when « > 0.4, The HBT correlation continues to be used with success
‘Aalto, 1997; Aalto etal, 1996; Nasrfar and Mosbleghian, 1999),
Example 45 Use various forms of the Rackett equation to caleulate the saturated
ligula volume of 1,1. T-tifuoroethane (R143) at 300 K. The literature value forthe
Tiguid volume at his esaperaite i 91.013 om” mol (Deibaugh and Moldover, 1997)
solution From Appendix A for 1,1 L-tiluoroethane, T, = 346.50 K, V, = 193,50,
fm mol”, P, = 3792 bat, Z, = 0255, and w ~ 0.259. Also from Appendix A, Va,
75.38 cm! mol"! at Ty, ~ 345 KT, = 300/346.3 = 0.8663, so 1 ~ 7, = 01337,
‘with Bq. (E11)
V, = (093.60)0.255)9"™"" = 99.726
not = (89.726 ~ 91,013)/91.013 = 00141
With Bags. (L11.4a) and (4115)
ysis) 245
ocsre —(1- 28) = ass
V, = (95.38)(0.29056 ~ 0.08775(0259)
Err - (9059 - 91.01)/91.01 - 0005 - 05%
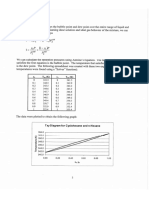
Figure 4-5 shows the percent deviation in liquid volume of 1,1-difluoroethane
Downloaded trom Digtal Engineering Library @ MeGraw-til (oy digtalengineeringlbrary. com)
‘Copyright © 2004 The MoGraw-Hil Companies. al rights reserved
‘Any use is subject tothe Terms of Use as given at fhe webste
PRESSURE-VOLUME-TEMPE!
sATURE,
{ELATIONSHIPS OF PURE GASES AND LIGUIDS
440 CHAPTER FOUR
Percent nor
Reduced Temperature
FIGURE 45 Percent deviation of liquid volumes af 1.1 diuoroethane (R152) calculated by
Yasious equations. Literature values fom Blanke and Weiss (1992) and Deflbaugh and Morrison
(0995), Lines: 28g, (11.6 B—Eg (113); eg. (lL ab): dB. CLT) Eg.
TD ta Ga,
(R152) when calculated by the different equations, The experimental data are best
reproduced by line d which is Eq. (4-11.7) with parameters A-D from Daubert, et
al. (1997). However, using Vjy ftom Appendix A as VF in Eg. (4-11.4a) is nearly
as accurate (line . Line c is Bg. (4-11.46); with the guanuty 029056 — 0.087750
0.266 replaced by Z. = 0.255, there is some loss of accuracy. Line b (Eq. 4-
11,3) and line © (Eq, 4411.1) are less accurate than Egs. (4-11.7) and (4-11 4ab)
bbut more accurate than Eq, (4-11.6) which is line a. This comparison among equa
tions is consistent with the results shown in Table 4-12 and suggests that among
the simpler models, Eq. (4-11.4a) is the most accurate.
Method of Elbro, et al. (1991)
Ebro, et al. (1991) have presented a group contribution method for the prediction
of liguid densities as a function of temperatuze from the tiple point to the normal
boiling point, In addition to being applicable to simple organic compounds, the
method can also be used for amorphous polymers from the glass transition tem-
perature to the degradation temperature. The method should not be used for cy-
Cloalkanes. To use the method, the volume is calculated by
V = 3mdv, @uap
‘where 1, is the number of group i in the substance and Ao, is a temperature depen-
dent group molar volume given by
Downloaded trom Digtal Engineering Library @ MeGraw-til (oy digtalengineeringlbrary. com)
‘Copyright © 2004 The MoGraw-Hil Companies. al rights reserved
‘Any use is subject tothe Terms of Use as given at fhe webste
PRESSURE-VOLUME-TEMPERATURE RELATIONSHIPS OF PURE GASES AND LIQUIDS
PVT RELATIONSINDS OF PURE GASES AND LI0UIDS an
Buy, AS) BT CT (4.112)
Values for the group volume temperature constants are given in Table 4-13. To
calculate the density of a polymer, only groups present in the repeat unit need be
considered. The technique first obtains the molar volume of the repeat unit and
then divides this into the repeat unit molecular weight to abtain the polymer density
‘The method is illustrated in Examples 4-6 and 4-7,
Example 46 Estimate V, of exadecane at 298.15 K with the method of Ebro, el
(1991). From Appendix A, Vi, 29411, cm mol at Ty, = 298.15 K
solution Using values from Table 4-13 in Bq. 4-11.12)
TABLE 4-13 Elbro Group Contibutons for Saturated Liquid Volume
A, 10%, 10,
No, Group em'/mol em/(01 K) em? fen K)
1 cH, 18.960 4558 0
2 cH 12520 1294 o
3 cH 6297 2192 o
4 ¢ 1296 59.66 °
5 ACH 10090 1137 °
6 acc, 23.580 mad °
1 CCH, 18.160 3589 °
8 accH $925 31.86 °
9 ace 71369 3360 o
10 cH 20630, sas o
n cH 6.761 2397 °
2 © 03971 -1410 o
B cH.on 39.460 110.60 2331
4 cHOH 40.920 = 19520 3221
18 ACOH, 41.20 A 198.20 278
16 cH,co 2180 61.17 25s
” cH.co 38560 =17040 w25
18 cco. 25.170 = 185.60 259
9 cHOR 12.090 4525 °
20 cH,coo. 42820, 2050 15.42
21 cH,coo 29.730 =154.10 33.19
2 cucoo 43.280 198.70 33.25
2 coo 14.230 1193 °
2 Accoo 33.060 14720 2095
25 cH 16.660 731 °
% cHO. 14.410 2854 0
n cHOR 35.070 =19970 4093
28 coo 30,120 28730 4059
» cue 25.29 49.11 °
30 uci 140 224 °
3 cet 3782 -1191 3247
2 cHcl, 3045, S431 °
2 co, 4874 6553 o
M4 Acc 2351 9.303 °
35 Si 8671 3555 9790
36 Sio ia =2018 °
Downloaded trom Digtal Engineering Library @ MeGraw-til (oy digtalengineeringlbrary. com)
‘Copyright © 2004 The MoGraw-Hil Companies. al rights reserved
‘Any use is subject tothe Terms of Use as given at fhe webste
PRESSURE-VOLUME-TEMPERATURE RELATIONSHIPS OF PURE GASES AND LIQUIDS
442 CHAPTER FOUR
ou number A 108 €
—cx, 2 18.960 4558 © 32550,
=cx, 4 12520 1294 0 16.378
With Bg. 11.1)
V, = 2.x 42.550 4 14 x 16.378 = 294.39 om? mol"!
Brror = (294.39 ~ 294.11)/294,L1 = 0001 or 0.1%
Example 4-7. Estimate the density of poly (methyl acrylate) at 298.15 K with the
‘method of Hitz, etal (1991). The value given in van Krevelea and Hoftyzer (1972)
i120 g em"
lution For poly (methyl acrylate), the repeat uni is
H,CHcH with M = $609
I
coocn,
Using values from Table 4-13 in Ba, 4-11.12):
opt ‘umber A 10° 10, a,
=n, 1 15360 4558 0 32.550,
=cut, 1 12.520 1298 0 16.378
=cHc00 1 43280-1080 sas 20839
Here,
V, = 32.550 + 16.78 + 22.539 = 71.4 cm'mol”
M _ $6.08
Yon grag 7 1208 gm
Brot = (1.205 ~ 1,220)/1.220 = 0012 = 1.2%
Discussion and Recommendations
For the saturated liquid volume at any temperature, including the normal boiling
point temperature if constants for the compound are availabe from Davbert, eta:
(1997), these should be used. If these constants are not available, but 7. 0, and
fone liquid density valve ate, then Eg. (4-11.4a) should be used. If only exitical
properties and w are availabe, Eq. 4-113) should be used. If citical properties
are not available, the Elbro method, Eq, (6-11.11) may be used at temperatures
below the normal boiling point when group coniribuion values are available in
Table 4-13. At the normal boiling point, the simple methods of Schroeder of Le
Bas can be used with errs generally less than 5%. Above the normal boiling
point. its possible to use estimated values of V, ftom these methods as VF values
In Bg, (14a)
Downloaded trom Digtal Engineering Library @ MeGraw-til (oy digtalengineeringlbrary. com)
‘Copyright © 2004 The MoGraw-Hil Companies. al rights reserved
‘Any use is subject tothe Terms of Use as given at fhe webste
Você também pode gostar
- Estudo Dirigido - O Meio Atmosferico2 PDFDocumento1 páginaEstudo Dirigido - O Meio Atmosferico2 PDFDeysianeMartinsAinda não há avaliações
- PeneiramentoDocumento3 páginasPeneiramentoDeysianeMartinsAinda não há avaliações
- TERMOELÉTRICASDocumento46 páginasTERMOELÉTRICASDeysianeMartins100% (1)
- Processos Químicos IndustriaisDocumento24 páginasProcessos Químicos IndustriaisKadima SantosAinda não há avaliações
- Valvula PDFDocumento180 páginasValvula PDFSergio Gomes Ferreira100% (40)
- Resolução Lista de Extração Líquido LíquidoDocumento25 páginasResolução Lista de Extração Líquido LíquidoDeysianeMartinsAinda não há avaliações
- Classificação dos Principais Tipos de Corrosão MetálicaDocumento47 páginasClassificação dos Principais Tipos de Corrosão MetálicaDeysianeMartinsAinda não há avaliações
- Resolução Da Lista de Destilação BateladaDocumento10 páginasResolução Da Lista de Destilação BateladaDeysianeMartinsAinda não há avaliações
- Potencial de Microalgas Na Biorremediação de Água Com Teor de CloretoDocumento5 páginasPotencial de Microalgas Na Biorremediação de Água Com Teor de CloretoDeysianeMartinsAinda não há avaliações
- Universidade Federal de São João del-Rei: Controlador Lógico ProgramávelDocumento35 páginasUniversidade Federal de São João del-Rei: Controlador Lógico ProgramávelDeysianeMartinsAinda não há avaliações
- Capítulo 4 - Leitos Fixo e FluidizadoDocumento9 páginasCapítulo 4 - Leitos Fixo e FluidizadoAdriana LopesAinda não há avaliações
- Questão 8 KorestkyDocumento2 páginasQuestão 8 KorestkyDeysianeMartinsAinda não há avaliações
- Upgrading Do BióleoDocumento68 páginasUpgrading Do BióleoDeysianeMartinsAinda não há avaliações
- Textos JurídicosDocumento52 páginasTextos JurídicosDeysianeMartinsAinda não há avaliações